Kinetic Hydrate Inhibition of Natural Gels in Complex Sediment Environments
Abstract
:1. Introduction
2. Results and Discussion
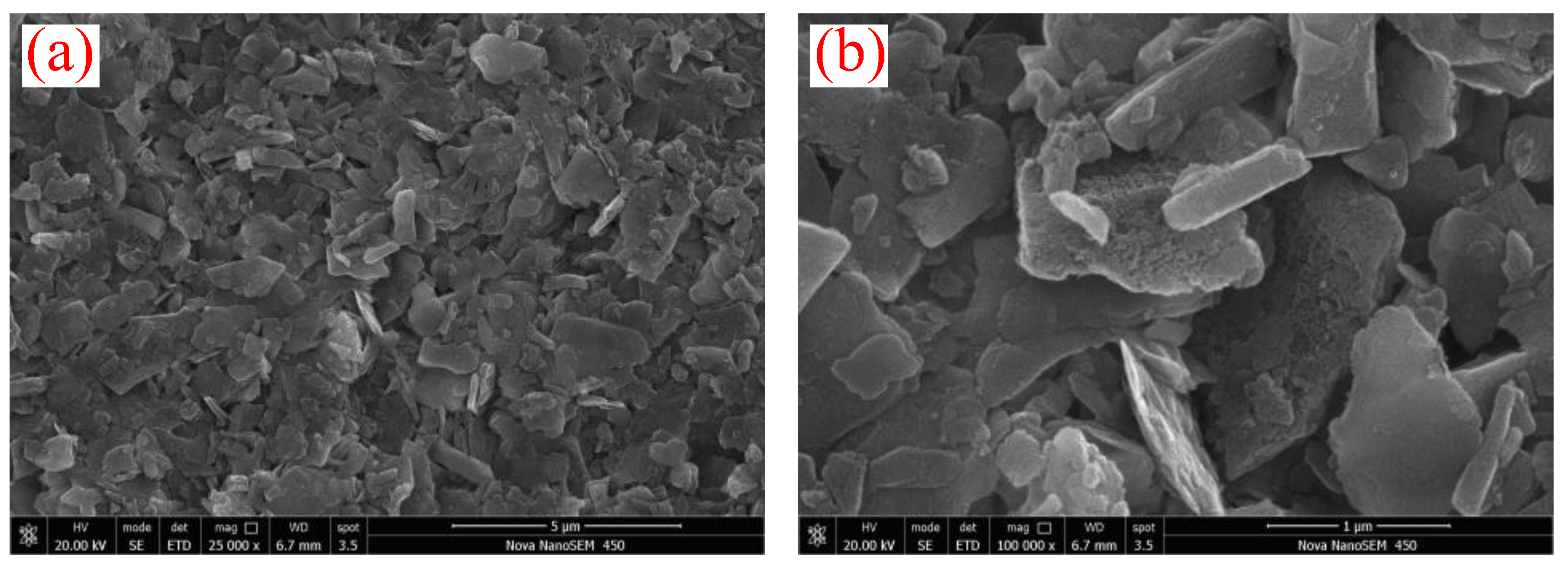
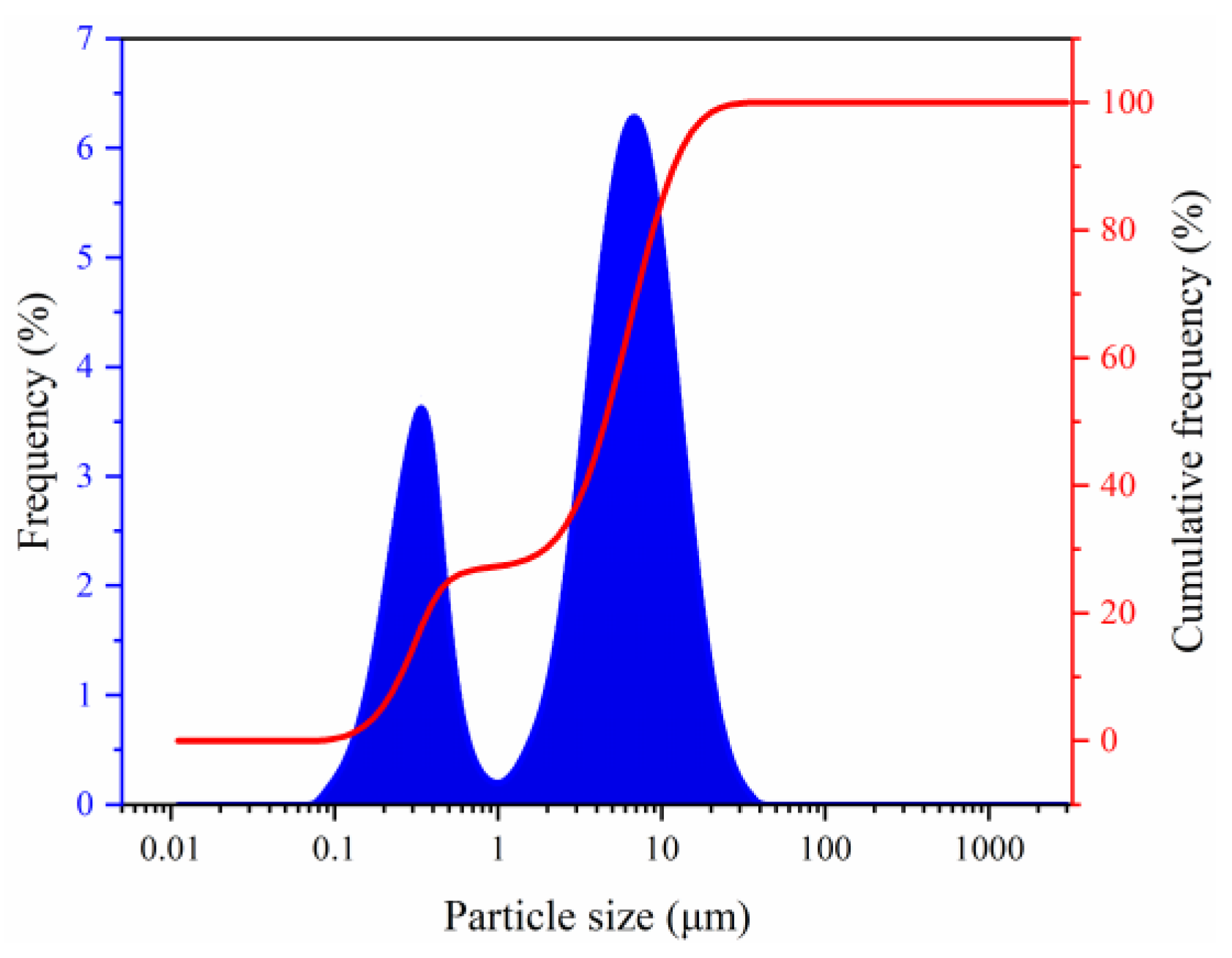
2.1. Basic Physical Properties of Sediments
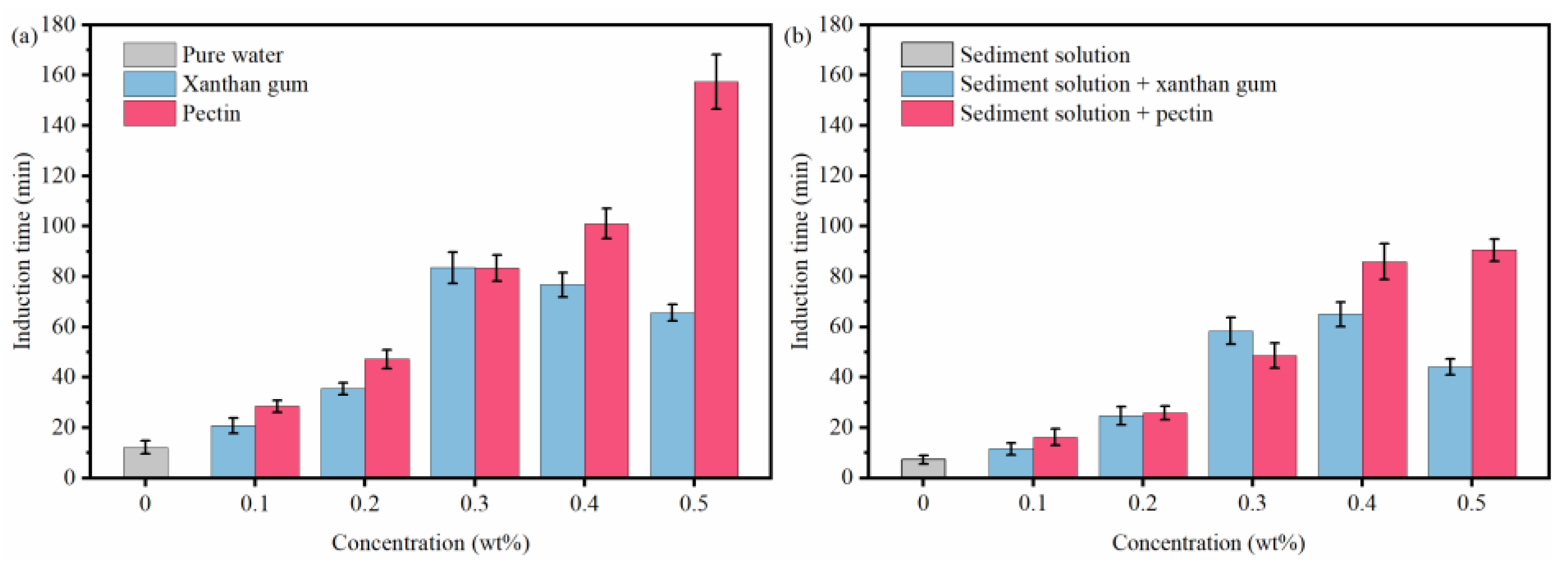
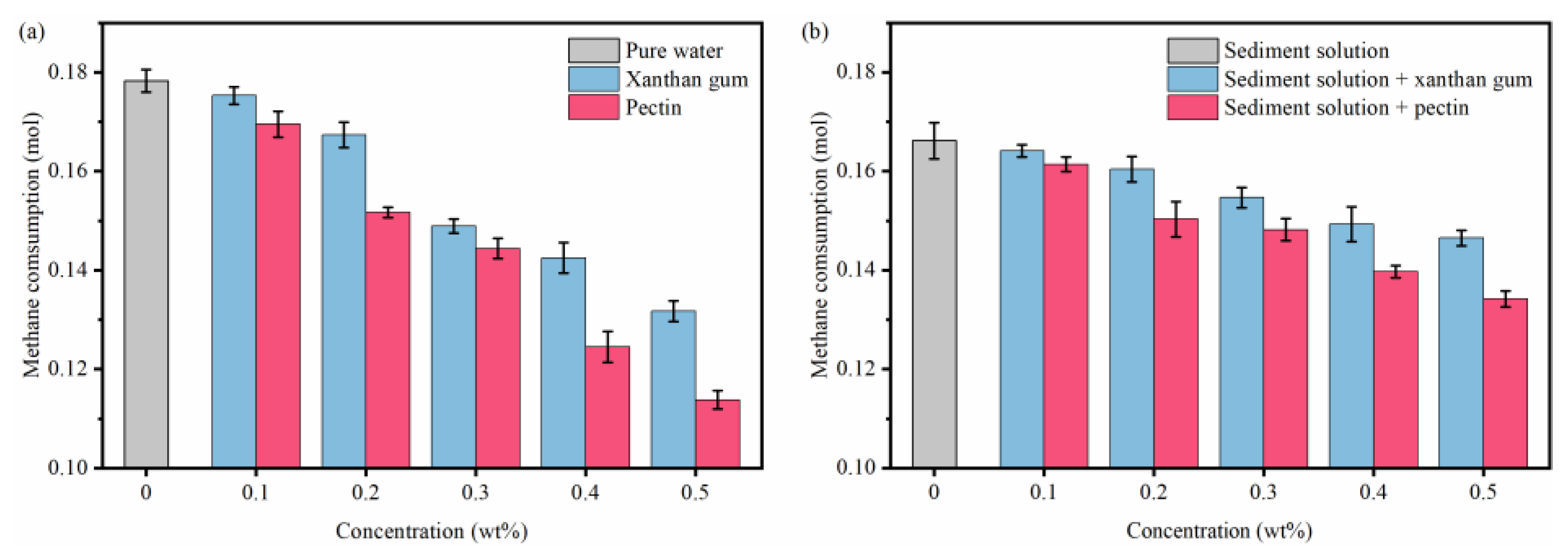
2.2. Effects of Natural Gels on Hydrate Formation
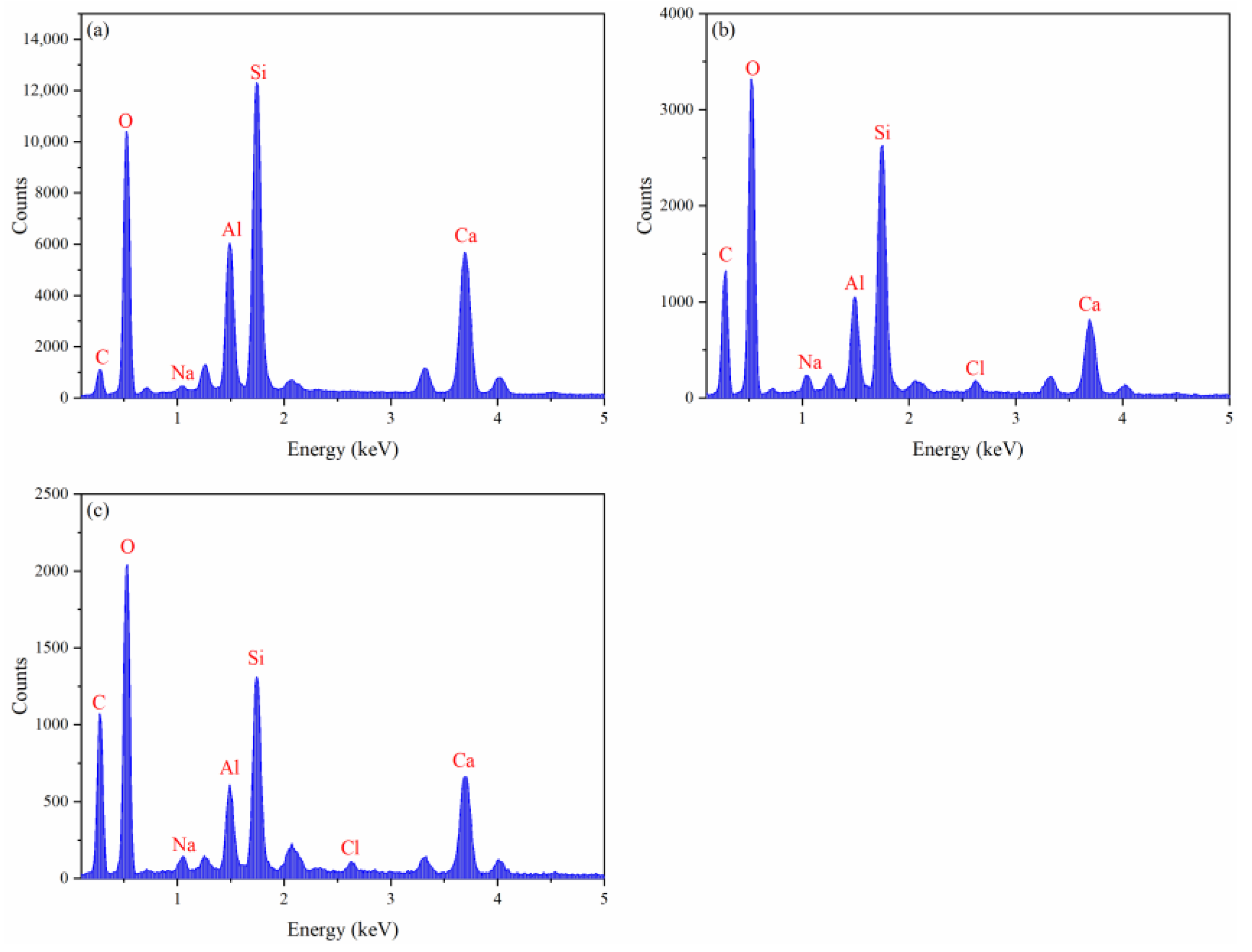
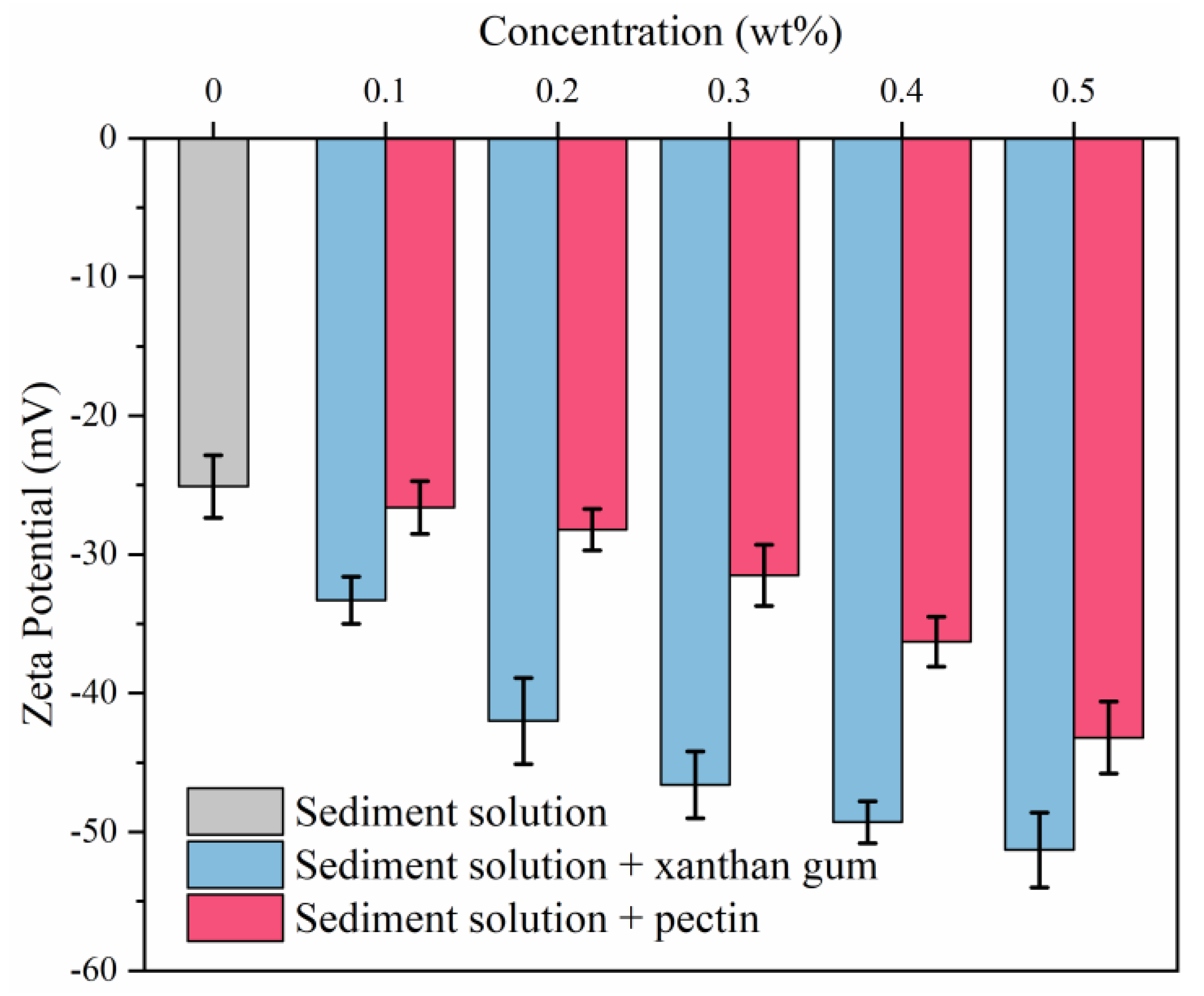
2.3. Analysis of Inhibition Mechanisms
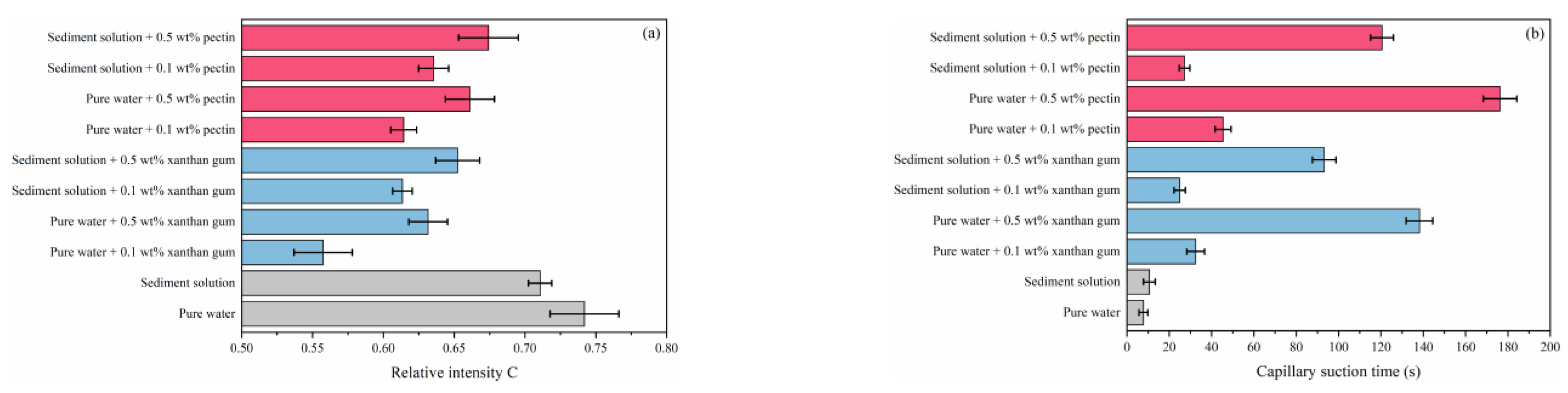
2.4. Effects of Natural Gels on Sediments
3. Conclusions
- (1)
- The natural gas hydrate-bearing (NGH-bearing) sediments in the South China Sea present an irregular laminated morphology. The sediment particles are micro–nano-scale, with a median particle size of 4.489 μm. The sediments are mainly composed of quartz and clay minerals, followed by feldspar and calcite. The clay minerals mainly include illite and illite–smectite mixed layers, with the contents of 41.6 wt% and 34.2 wt%, respectively.
- (2)
- By binding water molecules and disturbing their oriented arrangement, the resultant formation of clathrate structures, xanthan gum, and pectin prolong the induction time of hydrate nucleation. With the joint effects of the binding, disturbing, and interlayer mass transfer suppression processes, xanthan gum and pectin reduce the amount and rate of hydrate formation.
- (3)
- Sediments promote the hydrate nucleation yet inhibit the hydrate growth. Moreover, they have multiple effects on the hydrate inhibition performance of natural gels. On the one hand, the interaction between sediments and active groups of natural gels weakens the abilities of xanthan gum and pectin to inhibit hydrate nucleation and reduce hydrate formation. On the other hand, sediments significantly enhance the interlayer mass transfer suppression of xanthan gum and pectin and thus improve their effects on slowing down hydrate formation.
- (4)
- The comprehensive analysis pointed out the optimal gel concentration of 0.5 wt%, which delayed the induction time to 90.58 min, reduced the formed hydrates to 0.1342 mol, and decreased the hydrate formation rate to 1.492 × 10−4 mol/min. The hydrate formation was effectively inhibited, and the dispersion stability of sediments and the low-temperature rheology of fluids were considerably improved.
4. Experiments
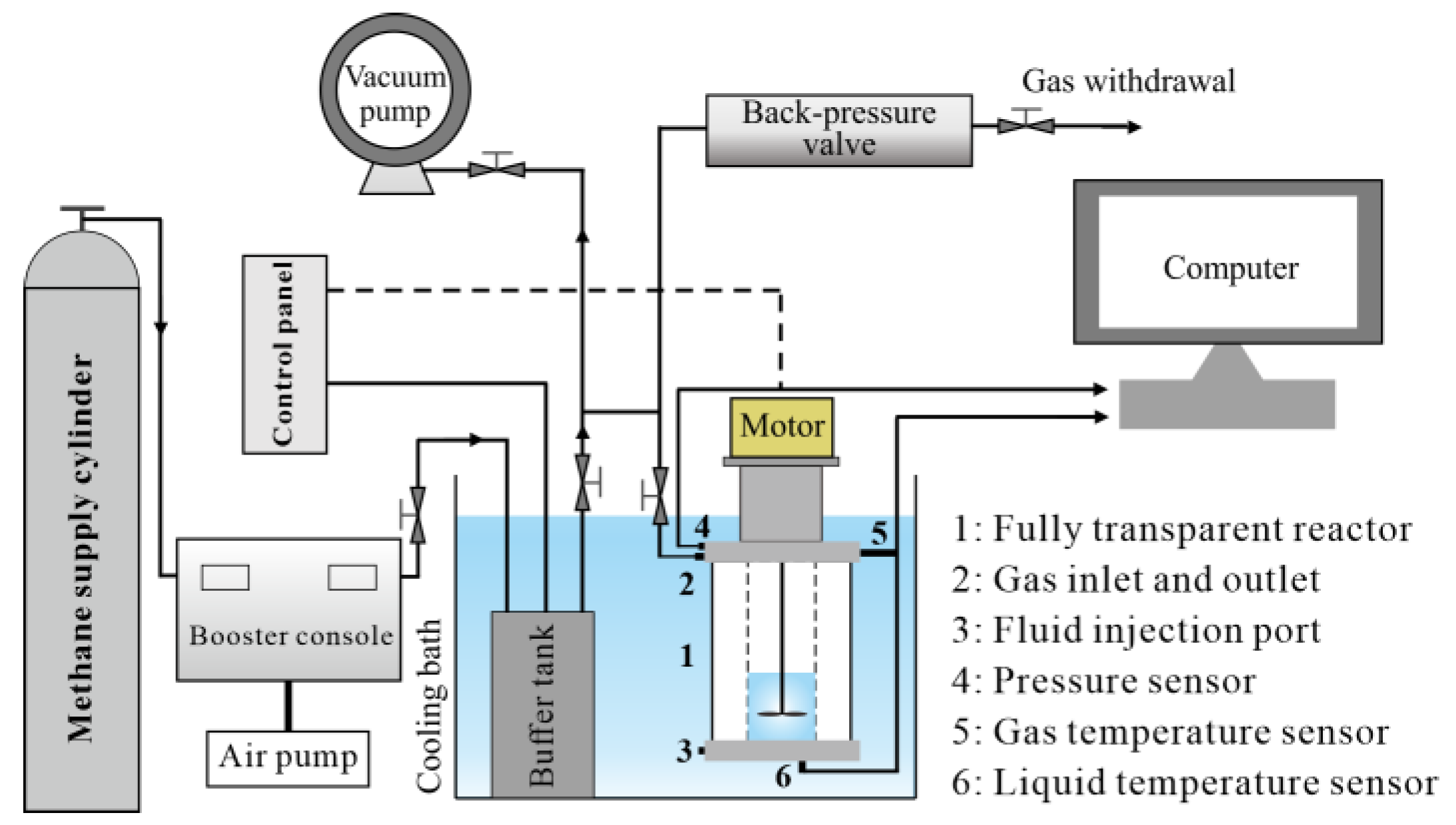
4.1. Materials and Apparatus
4.2. Methodology
4.2.1. Experimental Evaluation of Hydrate Formation Inhibition
4.2.2. Raman Spectroscopy
4.2.3. SEM/EDS Elemental Mapping
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Fundamentals and Applications of Gas Hydrates. Annu. Rev. Chem. Biomol. 2011, 2, 237. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, Z.; Zhang, L.; Wu, N.; Qin, Y.; Jiang, Z.; Geng, W.; Cao, H.; Zhang, X.; Zhai, B.; et al. Progress in Global Gas Hydrate Development and Production as a New Energy Resource. Acta Geol. Sin.-Engl. Ed. 2019, 93, 731–755. [Google Scholar] [CrossRef]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.-S. Review of natural gas hydrates as an energy resource: Prospects and challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Merey, S.; Longinos, S.N. Does the Mediterranean Sea have potential for producing gas hydrates? J. Nat. Gas Sci. Eng. 2018, 55, 113–134. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Wang, R.; Rui, Z.; Cheng, R.; Wang, Q.; Wang, J.; Lv, K. Impact of laponite on the formation of NGHs and its adaptability for use in NGH drilling fluids. J. Nat. Gas Sci. Eng. 2022, 107, 104799. [Google Scholar] [CrossRef]
- Liu, T.; Jiang, G.; Zhang, P.; Sun, J.; Sun, H.; Wang, R.; Zheng, M. A new low-cost drilling fluid for drilling in natural gas hydrate-bearing sediments. J. Nat. Gas Sci. Eng. 2016, 33, 934–941. [Google Scholar] [CrossRef]
- Liao, B.; Wang, J.; Han, X.; Wang, R.; Lv, K.; Bai, Y.; Jiang, H.; Shao, Z.; Wang, Y.; Sun, J. Microscopic molecular insights into clathrate methane hydrates dissociation in a flowing system. Chem. Eng. J. 2022, 430, 133098. [Google Scholar] [CrossRef]
- Ning, F.; Zhang, L.; Tu, Y.; Jiang, G.; Shi, M. Gas-hydrate formation, agglomeration and inhibition in oil-based drilling fluids for deep-water drilling. J. Nat. Gas Chem. 2010, 19, 234–240. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, Z.; Zhang, Z.; Zhang, Y. Relationship between the gas hydrate suppression temperature and water activity in the presence of thermodynamic hydrate inhibitor. Fuel 2020, 264, 116776. [Google Scholar] [CrossRef]
- Wang, R.; Sun, H.; Sun, J.; Qu, Y.; Zhang, J.; Shi, X.; Zhang, L.; Guo, D.; Lee, W. Effect of Dissolution and Dispersion Conditions of VC-713 on the Hydrate Inhibition. J. Chem. 2019, 2019, 3547518. [Google Scholar] [CrossRef]
- Xu, S.; Fan, S.; Fang, S.; Wang, Y.; Lang, X. Excellent synergy effect on preventing CH4 hydrate formation when glycine meets polyvinylcaprolactam. Fuel 2017, 206, 19–26. [Google Scholar] [CrossRef]
- Kelland, M.A. A Review of Kinetic Hydrate Inhibitors from an Environmental Perspective. Energy Fuels 2018, 32, 12001–12012. [Google Scholar] [CrossRef]
- Yaqub, S.; Lal, B.; Keong, L.K. Thermodynamic and kinetic effect of biodegradable polymers on carbondioxide hydrates. J. Ind. Eng. Chem. 2019, 79, 131–145. [Google Scholar] [CrossRef]
- Wang, R.; Sun, H.; Shi, X.; Xu, X.; Zhang, L.; Zhang, Z. Fundamental Investigation of the Effects of Modified Starch, Carboxymethylcellulose Sodium, and Xanthan Gum on Hydrate Formation under Different Driving Forces. Energies 2019, 12, 2026. [Google Scholar] [CrossRef] [Green Version]
- Effendi, A.D.; Sia, C.W.; Jasamai, M.; Hashmani, M.A. Investigation on esterified pectin as natural hydrate inhibitor on methane hydrate formation. J. Pet. Explor. Prod. Technol. 2022, 12, 3003–3019. [Google Scholar] [CrossRef]
- Kumar, A.; Sakpal, T.; Roy, S.; Kumar, R. Methane hydrate formation in a test sediment of sand and clay at various levels of water saturation. Can. J. Chem. 2015, 93, 874–881. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Yang, L.; Zhang, L.; Song, Y. Organics-Coated Nanoclays Further Promote Hydrate Formation Kinetics. J. Phys. Chem. Lett. 2021, 12, 3464–3467. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, R.; Sun, J.; Sun, J.; Lu, C.; Lv, K.; Wang, J.; Wang, J.; Yang, J.; Qu, Y. Effect of Drilling Fluid Invasion on Natural Gas Hydrate Near-Well Reservoirs Drilling in a Horizontal Well. Energies 2021, 14, 7075. [Google Scholar] [CrossRef]
- Xu, S.; Fan, S.; Fang, S.; Lang, X.; Wang, Y.; Chen, J. Pectin as an Extraordinary Natural Kinetic Hydrate Inhibitor. Sci. Rep. 2016, 6, 23220. [Google Scholar] [CrossRef]
- Xu, P.; Lang, X.; Fan, S.; Wang, Y.; Chen, J. Molecular dynamics simulation of methane hydrate growth in the presence of the natural product pectin. J. Phys. Chem. C 2016, 120, 5392–5397. [Google Scholar] [CrossRef]
- Yaqub, S.; bin Mohd Shariff, A.; Mellon, N.B. Unraveling the effect of sub-cooling temperatures on the kinetic performance of biopolymers for methane hydrate. J. Nat. Gas Sci. Eng. 2019, 65, 68–81. [Google Scholar] [CrossRef]
- Iijima, M.; Nakamura, K.; Hatakeyama, T.; Hatakeyama, H. Phase transition of pectin with sorbed water. Carbohydr. Polym. 2000, 41, 101–106. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Wang, R.; Lv, K.; Wang, J.; Liao, B.; Shi, X.; Wang, Q.; Qu, Y.; Huang, H. Mechanisms of synergistic inhibition of hydrophilic amino acids with kinetic inhibitors on hydrate formation. Fuel 2022, 321, 124012. [Google Scholar] [CrossRef]
- Ren, J.; Liu, X.; Niu, M.; Yin, Z. Effect of sodium montmorillonite clay on the kinetics of CH4 hydrate-implication for energy recovery. Chem. Eng. J. 2022, 437, 135368. [Google Scholar] [CrossRef]
- Wang, R.; Liao, B.; Wang, J.; Sun, J.; Wang, Y.; Wang, J.; Wang, Q.; Qu, Y.; Cheng, R. Microscopic molecular insights into methane hydrate growth on the surfaces of clay minerals: Experiments and molecular dynamics simulations. Chem. Eng. J. 2022, 451, 138757. [Google Scholar] [CrossRef]
- Jiang, G.; Ning, F.; Zhang, L.; Tu, Y. Effect of agents on hydrate formation and low-temperature rheology of polyalcohol drilling fluid. J. Earth Sci. 2011, 22, 652–657. [Google Scholar] [CrossRef]
- Yang, J.; Sun, J.; Wang, R.; Liu, F.; Wang, J.; Qu, Y.; Wang, P.; Huang, H.; Liu, L.; Zhao, Z. Laponite-polymer composite as a rheology modifier and filtration loss reducer for water-based drilling fluids at high temperature. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130261. [Google Scholar] [CrossRef]
- Jia, H.; Huang, P.; Wang, Q.; Han, Y.; Wang, S.; Zhang, F.; Pan, W.; Lv, K. Investigation of inhibition mechanism of three deep eutectic solvents as potential shale inhibitors in water-based drilling fluids. Fuel 2019, 244, 403–411. [Google Scholar] [CrossRef]
- Skovborg, P.; Ng, H.; Rasmussen, P.; Mohn, U. Measurement of induction times for the formation of methane and ethane gas hydrates. Chem. Eng. Sci. 1993, 48, 445–453. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Khan, M.S.; Lal, B.; Ghaniri, N.I.B.A.; Sabil, K.M. New methane hydrate phase boundary data in the presence of aqueous amino acids. Fluid Phase Equilibria 2018, 478, 129–133. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Q.; Zhang, B. Influence of super-absorbent polymer on the growth rate of gas hydrate. Saf. Sci. 2012, 50, 865–868. [Google Scholar] [CrossRef]
- Sa, J.-H.; Kwak, G.-H.; Han, K.; Ahn, D.; Lee, K.-H. Gas hydrate inhibition by perturbation of liquid water structure. Sci. Rep. 2015, 5, 11526. [Google Scholar] [CrossRef] [PubMed]





| Bulk Mineral Composition (wt%) | Clay Mineral Composition (wt%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clay | Quartz | Feldspar | Calcite | Dolomite | Pyrite | Illite | I/S | Kaolinite | Chlorite |
| 30.8 | 44.6 | 11.9 | 7.2 | 4.3 | 1.2 | 41.6 | 34.2 | 15.5 | 8.7 |
| Element | Element Content (wt%) | ||
|---|---|---|---|
| Sediment | Sediment + 0.5 wt% Xanthan Gum | Sediment + 0.5 wt% Pectin | |
| C | 10.90 | 31.31 | 32.96 |
| O | 51.32 | 48.40 | 48.24 |
| Na | 0.49 | 1.13 | 0.96 |
| Al | 7.58 | 3.67 | 3.09 |
| Si | 16.25 | 9.63 | 7.26 |
| Cl | 0.00 | 0.55 | 0.47 |
| Ca | 13.46 | 5.31 | 7.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Sun, J.; Bian, H.; Wang, Q.; Feng, Z.; Lu, C.; Ren, H.; Cheng, R.; Wang, J.; Wang, R. Kinetic Hydrate Inhibition of Natural Gels in Complex Sediment Environments. Gels 2022, 8, 758. https://doi.org/10.3390/gels8120758
Wang J, Sun J, Bian H, Wang Q, Feng Z, Lu C, Ren H, Cheng R, Wang J, Wang R. Kinetic Hydrate Inhibition of Natural Gels in Complex Sediment Environments. Gels. 2022; 8(12):758. https://doi.org/10.3390/gels8120758
Chicago/Turabian StyleWang, Jianlong, Jinsheng Sun, Hang Bian, Qibing Wang, Zhenbo Feng, Cheng Lu, Han Ren, Rongchao Cheng, Jintang Wang, and Ren Wang. 2022. "Kinetic Hydrate Inhibition of Natural Gels in Complex Sediment Environments" Gels 8, no. 12: 758. https://doi.org/10.3390/gels8120758
APA StyleWang, J., Sun, J., Bian, H., Wang, Q., Feng, Z., Lu, C., Ren, H., Cheng, R., Wang, J., & Wang, R. (2022). Kinetic Hydrate Inhibition of Natural Gels in Complex Sediment Environments. Gels, 8(12), 758. https://doi.org/10.3390/gels8120758






