Comparative Study of Physicochemical Properties of Alginate Composite Hydrogels Prepared by the Physical Blending and Electrostatic Assembly Methods
Abstract
1. Introduction
2. Results and Discussion
2.1. Fabrication of Homogeneous Alginate Composite Hydrogels
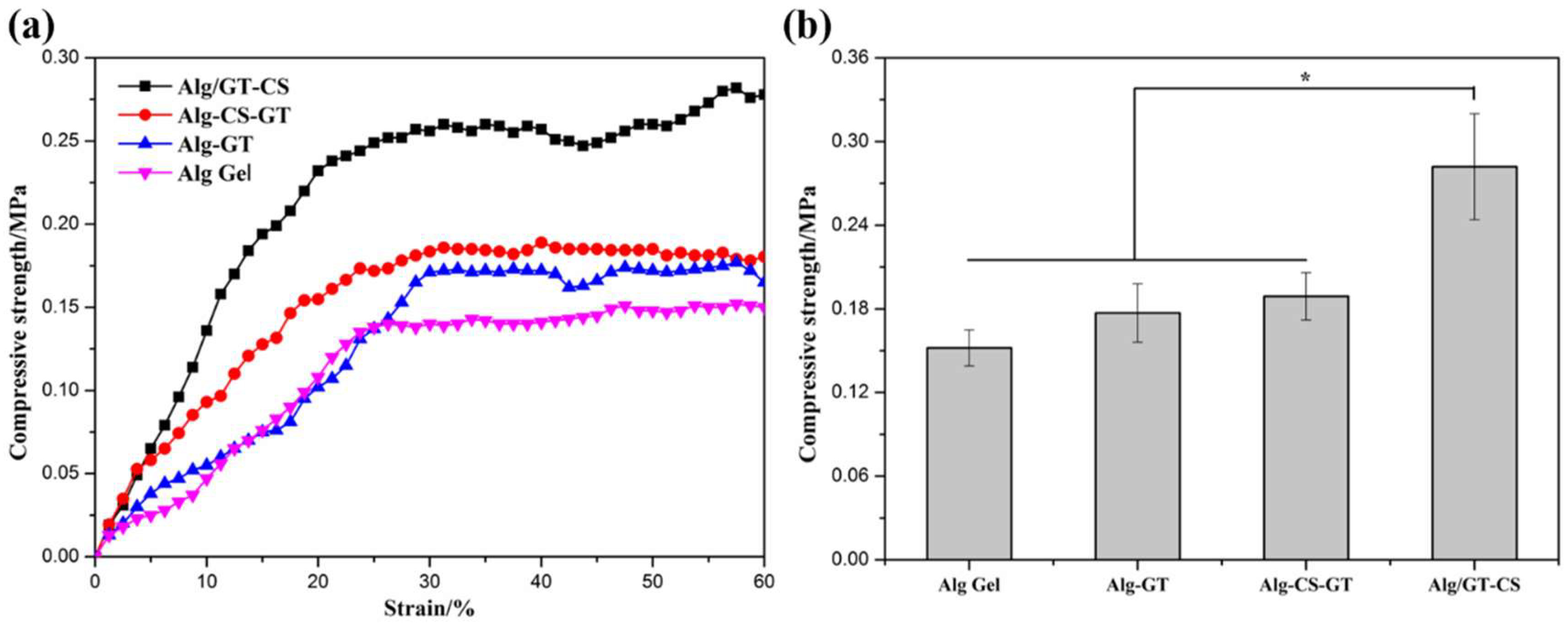
2.2. Pore Structure and Mechanical Properties of the Composite Hydrogels
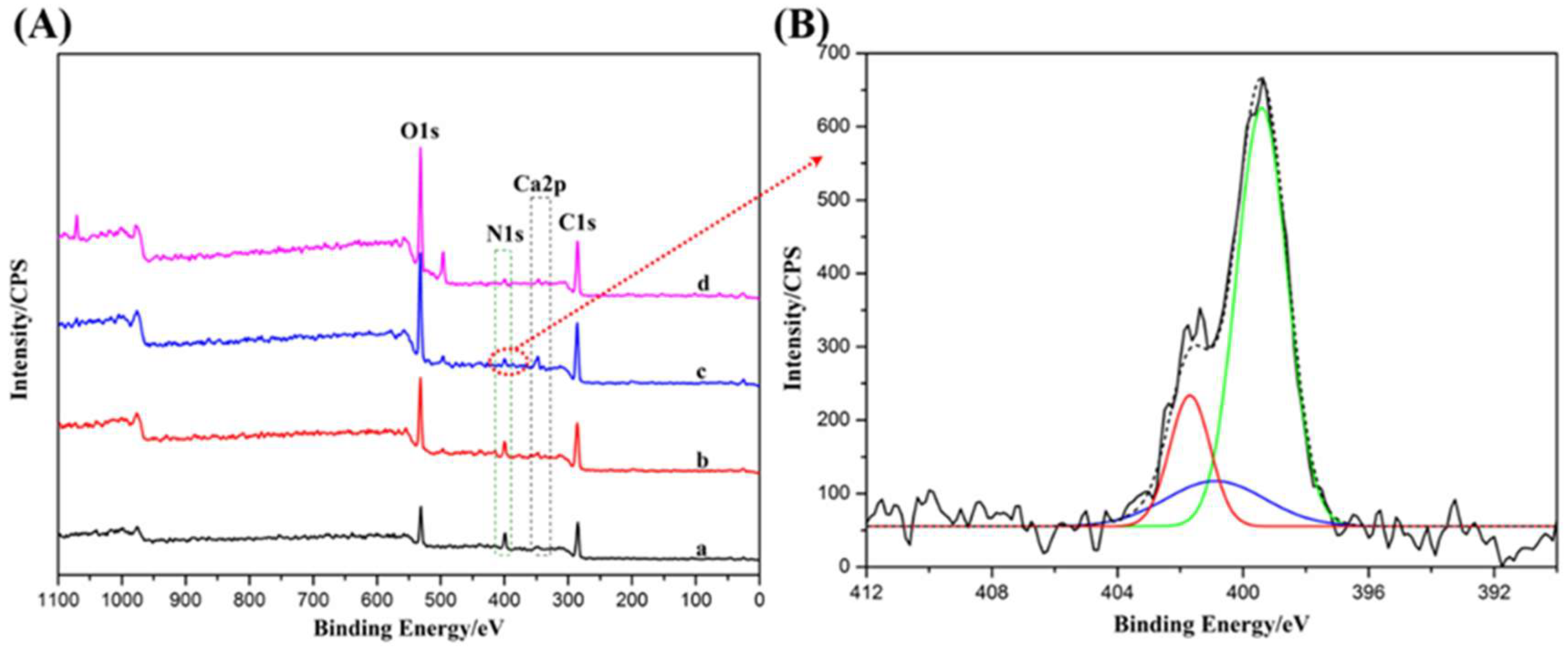
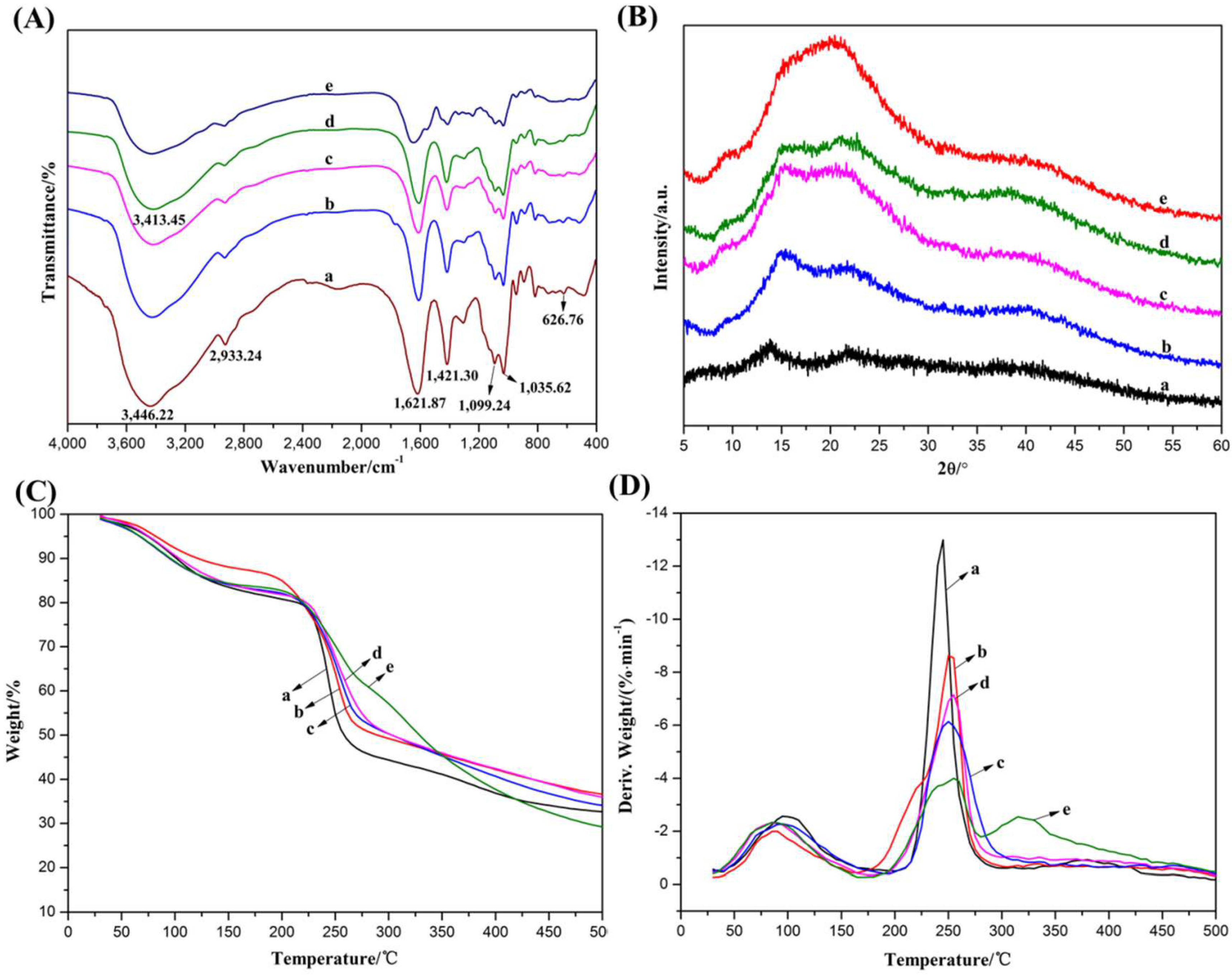
2.3. Interactions between Components of Alginate Composite Hydrogels
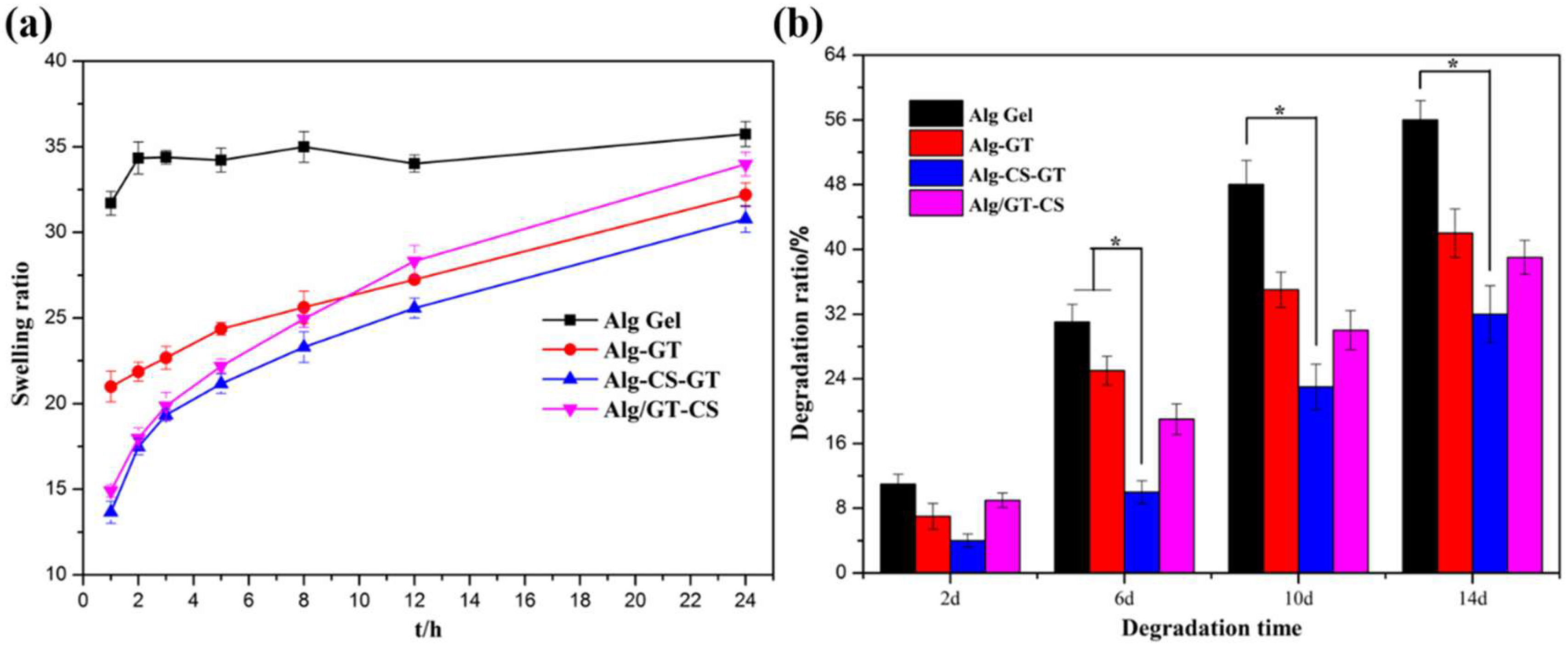
2.4. In Vitro Swelling and Biodegradation Analyses
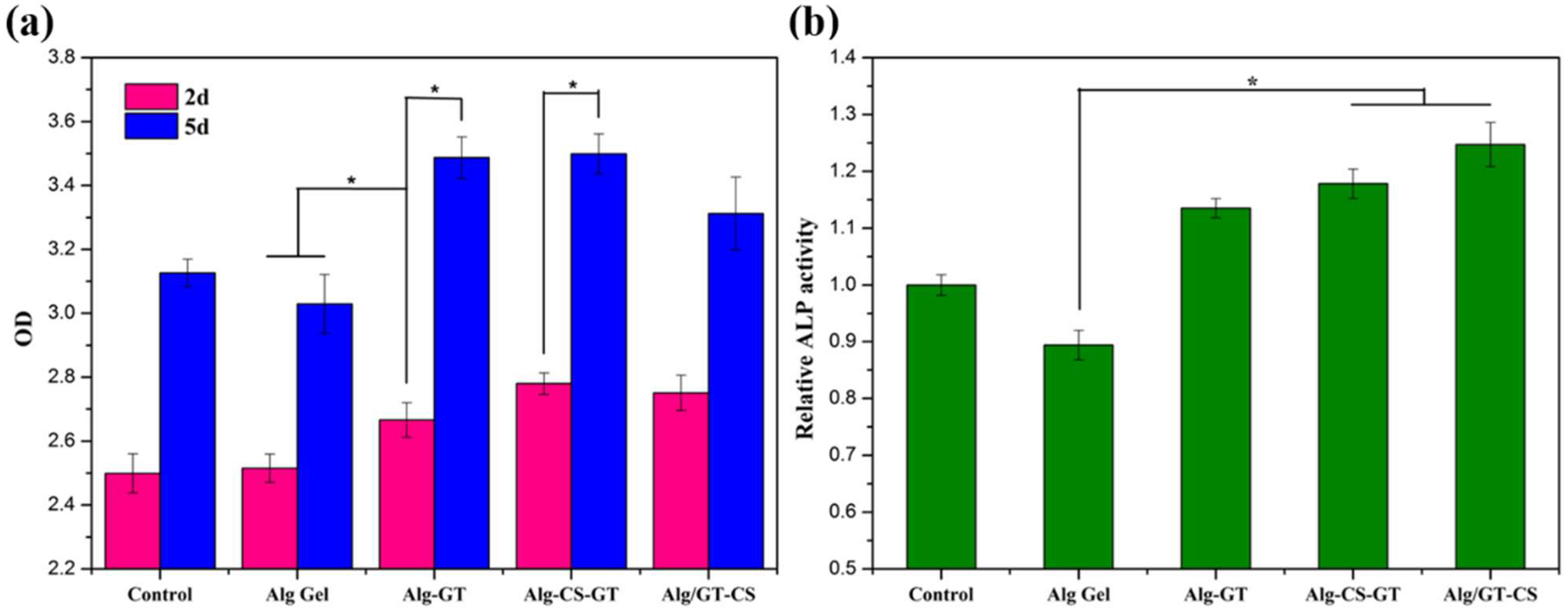
2.5. Cytocompatibility
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Fabrication of the Composite Hydrogels
4.3. Characterization of the Composite Hydrogels
4.4. Swelling and In Vitro Biodegradation Studies
4.5. Cytocompatibility of the Composite Hydrogels
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, O.D.; Weber, F.; Brunner, T.J.; Loher, S.; Ehrbar, M.; Schmidlin, P.R.; Stark, W.J. In vivo and in vitro evaluation of flexible, cotton wool-like nanocomposites as bone substitute material for complex defects. Acta Biomater. 2009, 5, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Bran, G.M.; Stern-Straeter, J.; Hörmann, K.; Riedel, F.; Goessler, U.R. Apoptosis in bone for tissue engineering. Arch. Med. Res. 2008, 39, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.Q.; Huang, D.G.; Chen, X.Q.; Liu, H.F.; Feng, Y.H.; Zhao, Z.D.; Dai, Z.H.; Zhang, X.Q.; Lin, Q. A novel and homogeneous scaffold material: Preparation and evaluation of alginate/bacterial cellulose nanocrystals/collagen composite hydrogel for tissue engineering. Polym. Bull. 2018, 75, 985–1000. [Google Scholar] [CrossRef]
- Dhand, A.P.; Galarraga, J.H.; Burdick, J.A. Enhancing biopolymer hydrogel functionality through nnterpenetrating networks. Trends Biotechnol. 2021, 39, 519–538. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef]
- Bidarra, S.J.; Barrias, C.C.; Granja, P.L. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014, 10, 1646–1662. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Yan, H.Q.; Chen, X.Q.; Li, J.C.; Feng, Y.H.; Shi, Z.F.; Wang, X.H.; Lin, Q. Synthesis of alginate derivative via the Ugi reaction and itscharacterization. Carbohydr. Polym. 2016, 136, 757–763. [Google Scholar] [CrossRef]
- Alsberg, E.; Anderson, K.W.; Albeiruti, A.; Franceschi, R.T.; Mooney, D.J. Cell-interactive alginate hydrogels for bone tissue engineering. J. Dent. Res. 2001, 80, 2025–2029. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.; Alsberg, E.; Hsiong, S.; Kim, W.J.; Mooney, D.J. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone 2004, 35, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Ionita, M.; Pandele, M.A.; Iovu, H. Sodium alginate/graphene oxide composite films with enhanced thermal and mechanical properties. Carbohydr. Polym. 2013, 94, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, C.G.; Huang, Z.H.; Xue, F.F. Preparation and characterization of nanoparticles based on hydrophobic alginate derivative as carriers for sustained release of vitamin D3. J. Agric. Food Chem. 2011, 59, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterial cellulose composites: A multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598. [Google Scholar] [CrossRef] [PubMed]
- Tanpichai, S.; Oksman, K. Cross-linked nanocomposite hydrogels based on cellulose nanocrystals and PVA: Mechanical properties and creep recovery. Compos. Part A-Appl. Sci. Manuf. 2016, 88, 226–233. [Google Scholar] [CrossRef]
- Devin, J.E.; Attawia, M.A.; Laurencin, C.T. Three-dimensional degradable porous polymer-ceramic matrices for use in bone repair. J. Biomater. Sci. Polym. Ed. 1996, 7, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Mosahebi, A.; Wiberg, M.; Terenghi, G. Addition of fibronectin to alginate matrix improves peripheral nerve regeneration in tissue-engineered conduits. Tissue Eng. 2003, 9, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Dhoot, N.O.; Tobias, C.A.; Fischer, I.; Wheatley, M.A. Peptide-modified alginate surfaces as a growth permissive substrate for neurite outgrowth. J. Biomed. Mater. Res. A 2004, 71, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Prang, P.; Muller, R.; Eljaouhari, A.; Heckmann, K.; Kunz, W.; Weber, T.; Faber, C.; Vroemen, M.; Bogdahn, U.; Weidner, N. The promotion of oriented axonal regrowth in the injured spinal cord by alginate based anisotropic capillary hydrogels. Biomaterials 2006, 27, 3560–3569. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef] [PubMed]
- Turco, G.; Marsich, E.; Bellomo, F.; Semeraro, S.; Donati, I.; Brun, F.; Grandolfo, M.; Accardo, A.; Paoletti, S. Alginate/hydroxyapatite biocomposite for bone ingrowth: A trabecular structure with high and isotropic connectivity. Biomacromolecules 2009, 10, 1575–1583. [Google Scholar] [CrossRef]
- Zhu, H.; Ji, J.; Shen, J. Biomacromolecules electrostatic self-assembly on 3-dimensional tissue engineering scaffold. Biomacromolecules 2004, 5, 1933–1939. [Google Scholar] [CrossRef]
- Chang, C.; Lin, Y.; Yeh, C.; Chen, Y.-C.; Chiou, S.-F.; Hsu, Y.-M.; Chen, Y.-S.; Wang, C.-C. Nanoparticles incorporated in pH-sensitive hydrogels as amoxicillin delivery for eradication of Helicobacter pylori. Biomacromolecules 2010, 11, 133–142. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef]
- Yan, H.Q.; Chen, X.Q.; Feng, M.X.; Shi, Z.F.; Zhang, D.S.; Lin, Q. Layer-by-layer assembly of 3D alginate-chitosan-gelatin composite scaffold incorporating bacterial cellulose nanocrystals for bone tissue engineering. Mater. Lett. 2017, 209, 492–496. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Bao, C.; Liu, C.; Liu, C.Y.; Li, D.; Yan, H.; Lin, Q. Fabrication and evaluation of alginate/bacterial cellulose nanocrystals-chitosan-gelatin composite scaffolds. Molecules 2021, 26, 5003. [Google Scholar] [CrossRef]
- Crow, B.B.; Nelson, K.D. Release of bovine serum albumin from a hydrogel-cored biodegradable polymer fiber. Biopolymers 2006, 81, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Wang, C. Recent advances in the use of gelatin in biomedical research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Ushaa, R.; Sreeramb, K.J.; Rajarama, A. Stabilization of collagen with EDC/NHS in the presence of l-lysine: A comprehensive study. Colloid. Surface. B 2012, 90, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mat. Sci. Eng. R 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Wen, Y.; Grøndahl, L.; Gallego, M.R.; Jorgensen, L.; Møller, E.H.; Nielsen, H.M. Delivery of dermatan sulfate from polyelectrolyte complex-containing alginate composite microspheres for tissue regeneration. Biomacromolecules 2012, 13, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Bernstein-Levi, O.; Ochbaum, G.; Bitton, R. The effect of covalently linked RGD peptide on the conformation of polysaccharides in aqueous solutions. Colloids Surf. B 2016, 137, 214–220. [Google Scholar] [CrossRef]
- Chen, X.; Yan, H.; Bao, C.; Zhu, Q.; Liu, Z.; Wen, Y.; Li, Z.; Zhang, T.; Lin, Q. Fabrication and evaluation of homogeneous alginate/polyacrylamide–chitosan–gelatin composite hydrogel scaffolds based on the interpenetrating networks for tissue engineering. Polym. Eng. Sci. 2022, 62, 116–128. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; Wen, Y.; Li, D.; Sun, X.; Liu, Z.; Yan, H.; Lin, Q. A study on the correlation between the oxidation degree of oxidized sodium alginate on its degradability and gelation. Polymers 2022, 14, 1679. [Google Scholar] [CrossRef]
- Kim, H.-L.; Jung, G.-Y.; Yoon, J.-H.; Han, J.-S.; Park, Y.-J.; Kim, D.-G.; Zhang, M.; Kim, D.-J. Preparation and characterization of nano-sized hydroxyapatite/alginate/chitosan composite scaffolds for bone tissue engineering. Mat. Sci. Eng. C 2015, 54, 20–25. [Google Scholar] [CrossRef]
- Jaikumar, D.; Sajesh, K.M.; Soumya, S.; Nimal, T.R.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Injectable alginate-O-carboxymethyl chitosan/nano fibrin compositehydrogels for adipose tissue engineering. Int. J. Biol. Macromol. 2015, 74, 318–326. [Google Scholar] [CrossRef]
- Liu, M.; Dai, L.; Shi, H.; Xiong, S.; Zhou, C. In vitro evaluation of alginate/halloysite nanotube composite scaffolds for tissue engineering. Mat. Sci. Eng. C 2015, 49, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.F.A.; Valente, T.A.M.; Alves, P.; Ferreira, P.; Silva, A.; Correia, I.J. Alginate based scaffolds for bone tissue engineering. Mat. Sci. Eng. C 2012, 32, 2596–2603. [Google Scholar] [CrossRef]
- Islam, M.S.; Karim, M.R. Fabrication and characterization of poly (vinyl alcohol)/alginate blend nanofibers by electrospinning method. Colloids Surf. A 2010, 366, 135–140. [Google Scholar] [CrossRef]
- Rafieian, S.; Mirzadeh, H.; Mahdav, H.; Masoumi, M.E. A review on nanocomposite hydrogels and their biomedical applications. Sci. Eng. Composite Mater. 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Kang, H.; Shu, Y.; Li, Z.; Guan, B.; Peng, S.; Huang, Y.; Liu, R. An effect of alginate on the stability of LDH nanosheets in aqueous solution and preparation of alginate/LDH nanocomposites. Carbohydr. Polym. 2014, 100, 158–165. [Google Scholar] [CrossRef]
- Eslahi, N.; Abdorahim, M.; Simchi, A. Smart polymeric hydrogels for cartilage tissue engineering: A review on the chemistry and biological functions. Biomacromolecules 2016, 17, 3441–3463. [Google Scholar] [CrossRef]
- Qin, Z.; Ji, L.; Yin, X.; Lin, Q.; Qin, J. Synthesis and characterization of bacterial cellulose sulfates usinga SO3/pyridine complex in DMAc/LiCl. Carbohydr. Polym. 2014, 101, 947–953. [Google Scholar] [CrossRef]
- Yan, H.Q.; Chen, X.Q.; Feng, M.X.; Shi, Z.F.; Zhang, W.; Wang, Y.; Ke, C.R.; Lin, Q. Entrapment of bacterial cellulose nanocrystals stabilized Pickering emulsions droplets in alginate beads for hydrophobic drug delivery. Colloids Surf. B 2019, 177, 112–120. [Google Scholar] [CrossRef]
- Yan, H.; Chen, X.; Bao, C.; Yi, J.; Lei, M.; Ke, C.; Zhang, W.; Lin, Q. Synthesis and assessment of CTAB and NPE modified organomontmorillonite for the fabrication of organo-montmorillonite/alginate based hydrophobic pharmaceutical controlled-release formulation. Colloids Surf. B 2020, 191, 110983. [Google Scholar] [CrossRef]
- Sowjanya, J.A.; Singh, J.; Mohita, T.; Sarvanan, S.; Moorthi, A.; Srinivasan, N.; Selvamurugan, N. Biocomposite scaffolds containing chitosan/alginate/nano-silica for bone tissue engineering. Colloids Surf. B 2013, 109, 294–300. [Google Scholar] [CrossRef]
- Tchobanian, A.; Oosterwyck, H.V.; Fardim, P. Polysaccharides for tissue engineering: Current landscape and future prospects. Carbohydr. Polym. 2019, 205, 601–625. [Google Scholar] [CrossRef] [PubMed]
- Neffe, A.T.; Loebus, A.; Zaupa, A.; Stoetzel, C.; Müller, F.A.; Lendlein, A. Gelatin functionalization with tyrosine derived moieties to increase the interaction with hydroxyapatite fillers. Acta Biomater. 2011, 7, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Donzelli, E.; Salvade, A.; Mimo, P.; Viganò, M.; Morrone, M.; Papagna, R.; Carini, F.; Zaopo, A.; Miloso, M.; Baldoni, M.; et al. Mesenchymal stem cells cultured on a collagen scaffold: In vitro osteogenic differentiation. Arch. Oral. Biol. 2007, 52, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Martino, A.; Sittinger, D.M.; Risbud, M.V. Chitosan: A versatile biopolymer fororthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]



| Sample | %C | %H | %O | %N |
|---|---|---|---|---|
| Alg Gel | 30.67 | 4.91 | 47.31 | 0.43 |
| Alg-GT | 34.29 | 5.47 | 42.39 | 4.33 |
| Alg-CS-GT | 31.62 | 5.55 | 46.87 | 2.13 |
| Alg/GT-CS | 38.51 | 6.27 | 40.63 | 8.24 |
| Sample | %C | %N | %O | %Ca |
|---|---|---|---|---|
| Alg Gel | 54.25 | 1.93 | 43.23 | 0.60 |
| Alg-GT | 58.98 | 9.51 | 30.56 | 0.95 |
| Alg-CS-GT | 57.68 | 3.79 | 36.77 | 1.76 |
| Alg/GT-CS | 63.46 | 12.49 | 23.06 | 0.70 |
| Samples | Porosity |
|---|---|
| ALG Gel | 94.17% |
| Alg-GT | 91.40% |
| Alg-CS-GT | 90.35% |
| ALG/GT-CS | 85.24% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Chen, X.; Yan, H.; Lin, Q. Comparative Study of Physicochemical Properties of Alginate Composite Hydrogels Prepared by the Physical Blending and Electrostatic Assembly Methods. Gels 2022, 8, 799. https://doi.org/10.3390/gels8120799
Wen Y, Chen X, Yan H, Lin Q. Comparative Study of Physicochemical Properties of Alginate Composite Hydrogels Prepared by the Physical Blending and Electrostatic Assembly Methods. Gels. 2022; 8(12):799. https://doi.org/10.3390/gels8120799
Chicago/Turabian StyleWen, Yanshi, Xiuqiong Chen, Huiqiong Yan, and Qiang Lin. 2022. "Comparative Study of Physicochemical Properties of Alginate Composite Hydrogels Prepared by the Physical Blending and Electrostatic Assembly Methods" Gels 8, no. 12: 799. https://doi.org/10.3390/gels8120799
APA StyleWen, Y., Chen, X., Yan, H., & Lin, Q. (2022). Comparative Study of Physicochemical Properties of Alginate Composite Hydrogels Prepared by the Physical Blending and Electrostatic Assembly Methods. Gels, 8(12), 799. https://doi.org/10.3390/gels8120799








