Formulation and Characterization of Ethyl Cellulose-Based Patches Containing Curcumin-Chitosan Nanoparticles for the Possible Management of Inflammation via Skin Delivery
Abstract
1. Introduction
2. Results and Discussion
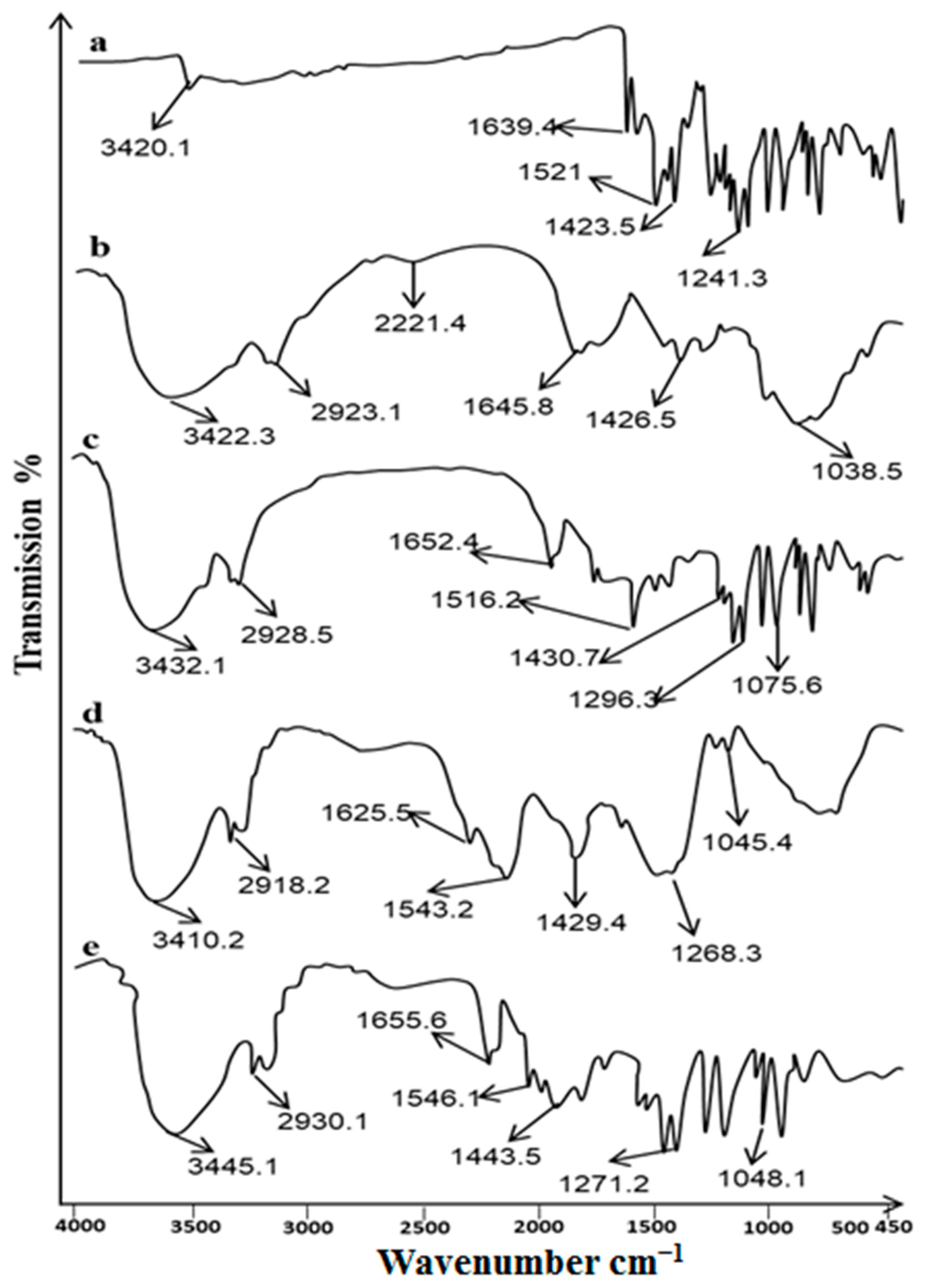
2.1. FTIR Analysis
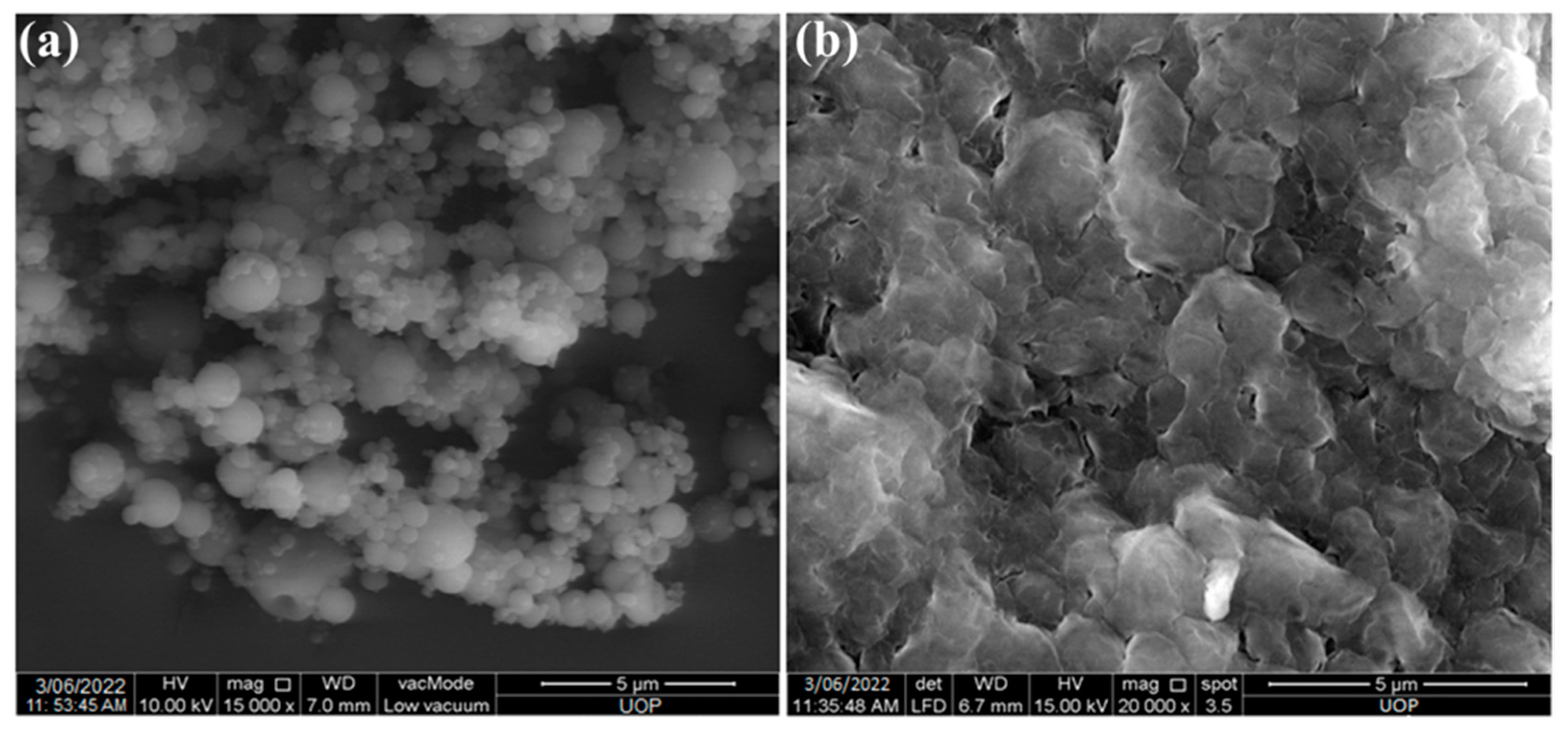
2.2. Preparation and Characterization of Nanoparticles
2.3. Preparation and Characterization of Patches
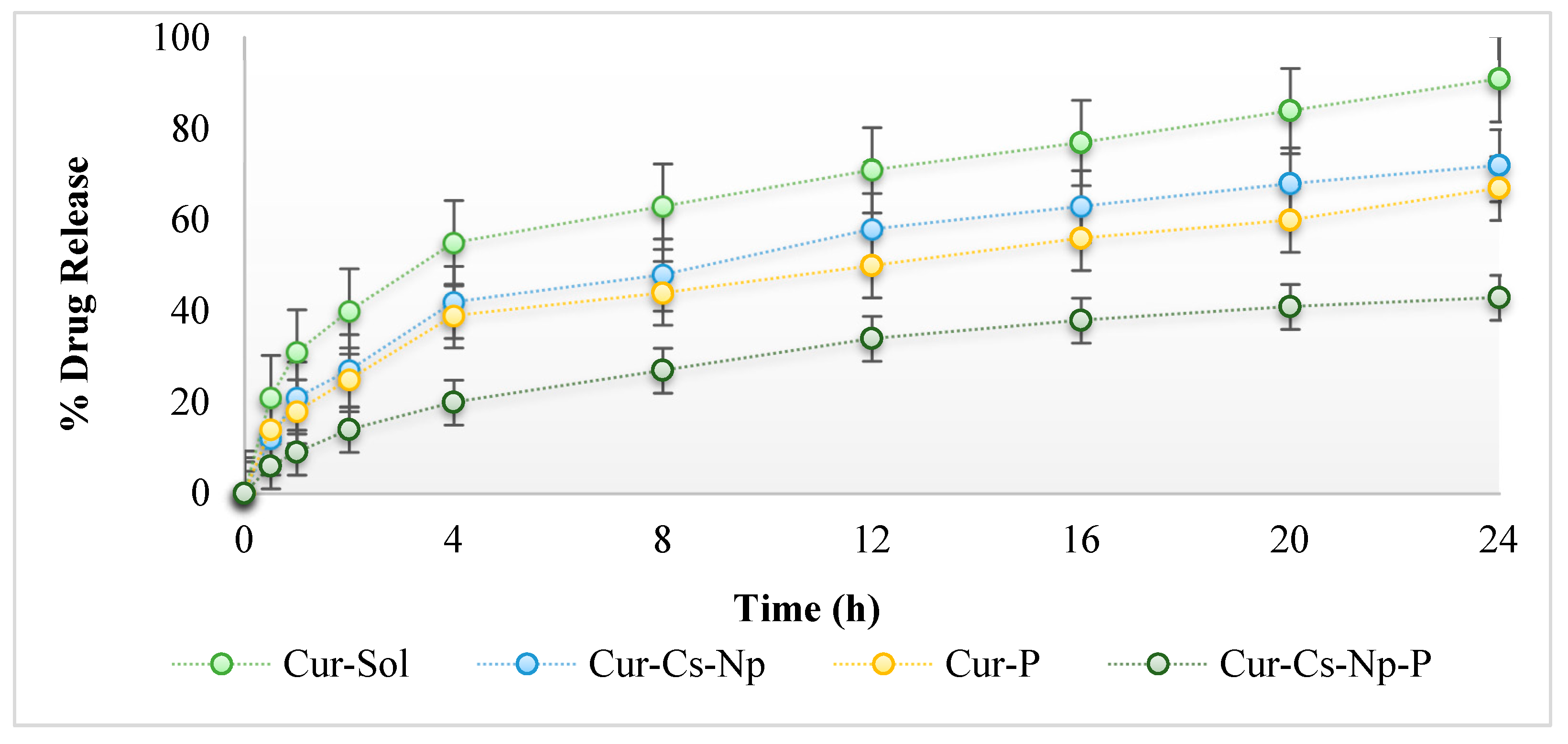
2.4. In Vitro Release
2.5. Ex Vivo Permeation and Skin Drug Retention
2.6. Anti-Inflammatory Studies
3. Conclusions
4. Materials and Methods
4.1. Material
4.2. ATR-FTIR Analysis
4.3. Synthesis of Curcumin-Loaded Chitosan Nanoparticles
4.3.1. Size and Zeta Potential Analysis
4.3.2. Surface Morphology
4.4. Preparation of Nanoparticles Loaded Patches
4.5. Characterization of Patches
4.5.1. Physicochemical Characterization of Patches
4.5.2. Drug Content
4.5.3. Tensile Strength and Percent Elongation
4.6. In Vitro Drug Release Study
4.7. Ex Vivo Drug Permeation and Retention Study
4.8. Anti-Inflammatory Activity
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safdari, F.; Gholipour, M.D.; Ghadami, A.; Saeed, M.; Zandi, M. Multi-antibacterial agent-based electrospun polycaprolactone for active wound dressing. Prog. Biomater. 2022, 11, 27–41. [Google Scholar] [CrossRef]
- Witika, B.A.; Makoni, P.A.; Matafwali, S.K.; Mweetwa, L.L.; Shandele, G.C.; Walker, R.B. Enhancement of biological and pharmacological properties of an encapsulated polyphenol: Curcumin. Molecules 2021, 26, 4244. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Mujtaba, M.A.; Lim, W.M.; Alhakamy, N.A.; Zeeshan, F.; Hosny, K.M. Nanoemulsions to Preserve/Process Bioactive and Nutritional Food Compounds: Contemporary Research and Applications. In Nanoemulsions in Food Technology; CRC Press: Boca Raton, FL, USA, 2021; pp. 247–278. [Google Scholar]
- Porro, C.; Cianciulli, A.; Trotta, T.; Lofrumento, D.D.; Panaro, M.A. Curcumin regulates anti-inflammatory responses by JAK/STAT/SOCS signaling pathway in BV-2 microglial cells. Biology 2019, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Kahkhaie, K.R.; Mirhosseini, A.; Aliabadi, A.; Mohammadi, A.; Mousavi, M.J.; Haftcheshmeh, S.M.; Sathyapalan, T.; Sahebkar, A. Curcumin: A modulator of inflammatory signaling pathways in the immune system. Inflammopharmacology 2019, 27, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Maleki Dizaj, S.; Alipour, M.; Dalir Abdolahinia, E.; Ahmadian, E.; Eftekhari, A.; Forouhandeh, H.; Rahbar Saadat, Y.; Sharifi, S.; Zununi Vahed, S. Curcumin nanoformulations: Beneficial nanomedicine against cancer. Phytother. Res. 2022, 36, 1156–1181. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Madureira, A.R.; Campos, D.; Sarmento, B.; Gomes, A.M.; Pintado, M.; Reis, F. Solid lipid nanoparticles as oral delivery systems of phenolic compounds: Overcoming pharmacokinetic limitations for nutraceutical applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 1863–1873. [Google Scholar] [CrossRef]
- Barnabas, J.; Raj, P. The human body: A digital twin of the cyber physical systems. In Advances in Computers; Elsevier: Amsterdam, The Netherlands, 2020; Volume 117, pp. 219–246. [Google Scholar]
- Chen, Z.; Lv, Y.; Qi, J.; Zhu, Q.; Lu, Y.; Wu, W. Overcoming or circumventing the stratum corneum barrier for efficient transcutaneous immunization. Drug Discov. Today 2018, 23, 181–186. [Google Scholar] [CrossRef]
- Ohta, S.; Mitsuhashi, K.; Chandel, A.K.S.; Qi, P.; Nakamura, N.; Nakamichi, A.; Yoshida, H.; Yamaguchi, G.; Hara, Y.; Sasaki, R. Silver-loaded carboxymethyl cellulose nonwoven sheet with controlled counterions for infected wound healing. Carbohydr. Polym. 2022, 286, 119289. [Google Scholar] [CrossRef]
- Yee Kuen, C.; Masarudin, M.J. Chitosan nanoparticle-based system: A new insight into the promising controlled release system for lung cancer treatment. Molecules 2022, 27, 473. [Google Scholar] [CrossRef]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C.-O. An evaluation of curcumin-encapsulated chitosan nanoparticles for transdermal delivery. AAPS PharmSciTech 2019, 20, 1–13. [Google Scholar] [CrossRef]
- Duse, L.; Baghdan, E.; Pinnapireddy, S.R.; Engelhardt, K.H.; Jedelská, J.; Schaefer, J.; Quendt, P.; Bakowsky, U. Preparation and characterization of curcumin loaded chitosan nanoparticles for photodynamic therapy. Phys. Status Solidi 2018, 215, 1700709. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, J.H.; Hong, Y.S. Development of chitosan/poly-γ-glutamic acid/pluronic/curcumin nanoparticles in chitosan dressings for wound regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Basit, H.M.; Mohd Amin, M.C.I.; Ng, S.-F.; Katas, H.; Shah, S.U.; Khan, N.R. Formulation and Evaluation of Microwave-Modified Chitosan-Curcumin Nanoparticles—A Promising Nanomaterials Platform for Skin Tissue Regeneration Applications Following Burn Wounds. Polymers 2020, 12, 2608. [Google Scholar] [CrossRef]
- Mashayekhi, K.; Zare Marzouni, H. Curcumin (extracted from tumeric) and its therapeutic effects. Jorjani Biomed. J. 2016, 4, 1–20. [Google Scholar]
- Latif, M.S.; Nawaz, A.; Rashid, S.A.; Akhlaq, M.; Iqbal, A.; Khan, M.J.; Khan, M.S.; Lim, V.; Alfatama, M. Formulation of Polymers-Based Methotrexate Patches and Investigation of the Effect of Various Penetration Enhancers: In Vitro, Ex Vivo and In Vivo Characterization. Polymers 2022, 14, 2211. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.S.; Al-Harbi, F.F.; Nawaz, A.; Rashid, S.A.; Farid, A.; Mohaini, M.A.; Alsalman, A.J.; Hawaj, M.A.A.; Alhashem, Y.N. Formulation and Evaluation of Hydrophilic Polymer Based Methotrexate Patches: In Vitro and In Vivo Characterization. Polymers 2022, 14, 1310. [Google Scholar] [CrossRef]
- Hassani, A.; Mahmood, S.; Enezei, H.H.; Hussain, S.A.; Hamad, H.A.; Aldoghachi, A.F.; Hagar, A.; Doolaanea, A.A.; Ibrahim, W.N. Formulation, characterization and biological activity screening of sodium alginate-gum arabic nanoparticles loaded with curcumin. Molecules 2020, 25, 2244. [Google Scholar] [CrossRef]
- Niranjan, R.; Kaushik, M.; Prakash, J.; Venkataprasanna, K.; Arpana, C.; Balashanmugam, P.; Venkatasubbu, G.D. Enhanced wound healing by PVA/Chitosan/Curcumin patches: In vitro and in vivo study. Colloids Surf. B Biointerfaces 2019, 182, 110339. [Google Scholar]
- Sabir, F.; Qindeel, M.; Rehman, A.U.; Ahmad, N.M.; Khan, G.M.; Csoka, I.; Ahmed, N. An efficient approach for development and optimisation of curcumin-loaded solid lipid nanoparticles’ patch for transdermal delivery. J. Microencapsul. 2021, 38, 233–248. [Google Scholar] [CrossRef]
- Nawaz, A.; Wong, T.W. Microwave as skin permeation enhancer for transdermal drug delivery of chitosan-5-fluorouracil nanoparticles. Carbohydr. Polym. 2017, 157, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-H.; Lim, S.-J.; Lee, M.-K. Chitosan-coated liposomes to stabilize and enhance transdermal delivery of indocyanine green for photodynamic therapy of melanoma. Carbohydr. Polym. 2019, 224, 115143. [Google Scholar] [CrossRef]
- Murdock, R.C.; Braydich-Stolle, L.; Schrand, A.M.; Schlager, J.J.; Hussain, S.M. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol. Sci. 2008, 101, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, A.; Jose, J.; Kumar, L.; Salwa, S.; Vijay Kumar, M.; Nabavi, S.M. Transdermal Delivery of Curcumin-Loaded Solid Lipid Nanoparticles as Microneedle Patch: An In Vitro and In Vivo Study. AAPS PharmSciTech 2022, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.S.; Azad, A.K.; Nawaz, A.; Rashid, S.A.; Rahman, M.H.; Al Omar, S.Y.; Bungau, S.G.; Aleya, L.; Abdel-Daim, M.M. Ethyl Cellulose and Hydroxypropyl Methyl Cellulose Blended Methotrexate-Loaded Transdermal Patches: In Vitro and Ex Vivo. Polymers 2021, 13, 3455. [Google Scholar] [CrossRef]
- Ullah, W.; Nawaz, A.; Akhlaq, M.; Shah, K.U.; Latif, M.S.; Alfatama, M. Transdermal delivery of gatifloxacin carboxymethyl cellulose-based patches: Preparation and characterization. J. Drug Deliv. Sci. Technol. 2021, 66, 102783. [Google Scholar] [CrossRef]
- Putri, F.R.; Adjeng, A.N.T.; Yuniar, N.; Handoyo, M.; Sahumena, M.A. Formulation and physical characterization of curcumin nanoparticle transdermal patch. Int. J. Appl. Pharm. 2019, 11, 217–221. [Google Scholar]
- Suksaeree, J.; Waiprib, R.; Pichayakorn, W. Improving the hydrophilic properties of deproteinized natural rubber latex films for lidocaine transdermal patches by starch blending. J. Polym. Environ. 2022, 30, 1574–1586. [Google Scholar] [CrossRef]
- Nawaz, A.; Latif, M.S.; Alnuwaiser, M.A.; Ullah, S.; Iqbal, M.; Alfatama, M.; Lim, V. Synthesis and Characterization of Chitosan-Decorated Nanoemulsion Gel of 5-Fluorouracil for Topical Delivery. Gels 2022, 8, 412. [Google Scholar] [CrossRef]
- Nawaz, A.; Farid, A.; Safdar, M.; Latif, M.S.; Ghazanfar, S.; Akhtar, N.; Al Jaouni, S.K.; Selim, S.; Khan, M.W. Formulation Development and Ex-Vivo Permeability of Curcumin Hydrogels under the Influence of Natural Chemical Enhancers. Gels 2022, 8, 384. [Google Scholar] [CrossRef]
- Pillai, M.M.; Dandia, H.; Checker, R.; Rokade, S.; Sharma, D.; Tayalia, P. Novel combination of bioactive agents in bilayered dermal patches provides superior wound healing. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102495. [Google Scholar] [CrossRef] [PubMed]

| F. Code | Size [20] | Zeta Potential (mV) | PDI | Drug Content (%) | %EE |
|---|---|---|---|---|---|
| Cs-Np | 203.1 ± 6.95 | +36.3 ± 1.25 | 0.27 ± 0.12 | ---- | ----- |
| Cur-Cs-Np | 229.4 ± 9.53 | +25.8 ± 1.56 | 0.29 ± 0.11 | 53.2 ± 3.32 | 59.3 ± 2.98 |
| F. Codes | pH | Thickness (mm) | Weight Variation (mg) | Folding Endurance | Tensile Strength kg/cm2 | % Moisture Content | % Drug Content |
|---|---|---|---|---|---|---|---|
| Blank-P | 5.9 | 0.73 ± 0.14 | 85.67 ± 0.12 | 67 ± 2.35 | 11.23 ± 0.56 | 8.38 ± 0.71 | ----- |
| Cur-P | 6.2 | 0.78 ± 0.21 | 88.54 ± 0.25 | 60 ± 2.67 | 11.13 ± 0.72 | 9.76 ± 0.86 | 83.51 ± 2.92 |
| Cur-Cs-Np-P | 6.3 | 0.84 ± 0.27 | 91.98 ± 0.29 | 58 ± 2.14 | 12.65 ± 0.86 | 10.32 ± 0.95 | 85.73 ± 2.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, A.; Latif, M.S.; Shah, M.K.A.; Elsayed, T.M.; Ahmad, S.; Khan, H.A. Formulation and Characterization of Ethyl Cellulose-Based Patches Containing Curcumin-Chitosan Nanoparticles for the Possible Management of Inflammation via Skin Delivery. Gels 2023, 9, 201. https://doi.org/10.3390/gels9030201
Nawaz A, Latif MS, Shah MKA, Elsayed TM, Ahmad S, Khan HA. Formulation and Characterization of Ethyl Cellulose-Based Patches Containing Curcumin-Chitosan Nanoparticles for the Possible Management of Inflammation via Skin Delivery. Gels. 2023; 9(3):201. https://doi.org/10.3390/gels9030201
Chicago/Turabian StyleNawaz, Asif, Muhammad Shahid Latif, Muhammad Khurshid Alam Shah, Tarek M. Elsayed, Saeed Ahmad, and Hamid Ali Khan. 2023. "Formulation and Characterization of Ethyl Cellulose-Based Patches Containing Curcumin-Chitosan Nanoparticles for the Possible Management of Inflammation via Skin Delivery" Gels 9, no. 3: 201. https://doi.org/10.3390/gels9030201
APA StyleNawaz, A., Latif, M. S., Shah, M. K. A., Elsayed, T. M., Ahmad, S., & Khan, H. A. (2023). Formulation and Characterization of Ethyl Cellulose-Based Patches Containing Curcumin-Chitosan Nanoparticles for the Possible Management of Inflammation via Skin Delivery. Gels, 9(3), 201. https://doi.org/10.3390/gels9030201








