Kiwi Fruits Preservation Using Novel Edible Active Coatings Based on Rich Thymol Halloysite Nanostructures and Chitosan/Polyvinyl Alcohol Gels
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Structural Characterization of TO@HNT Hybrid Nanostructure
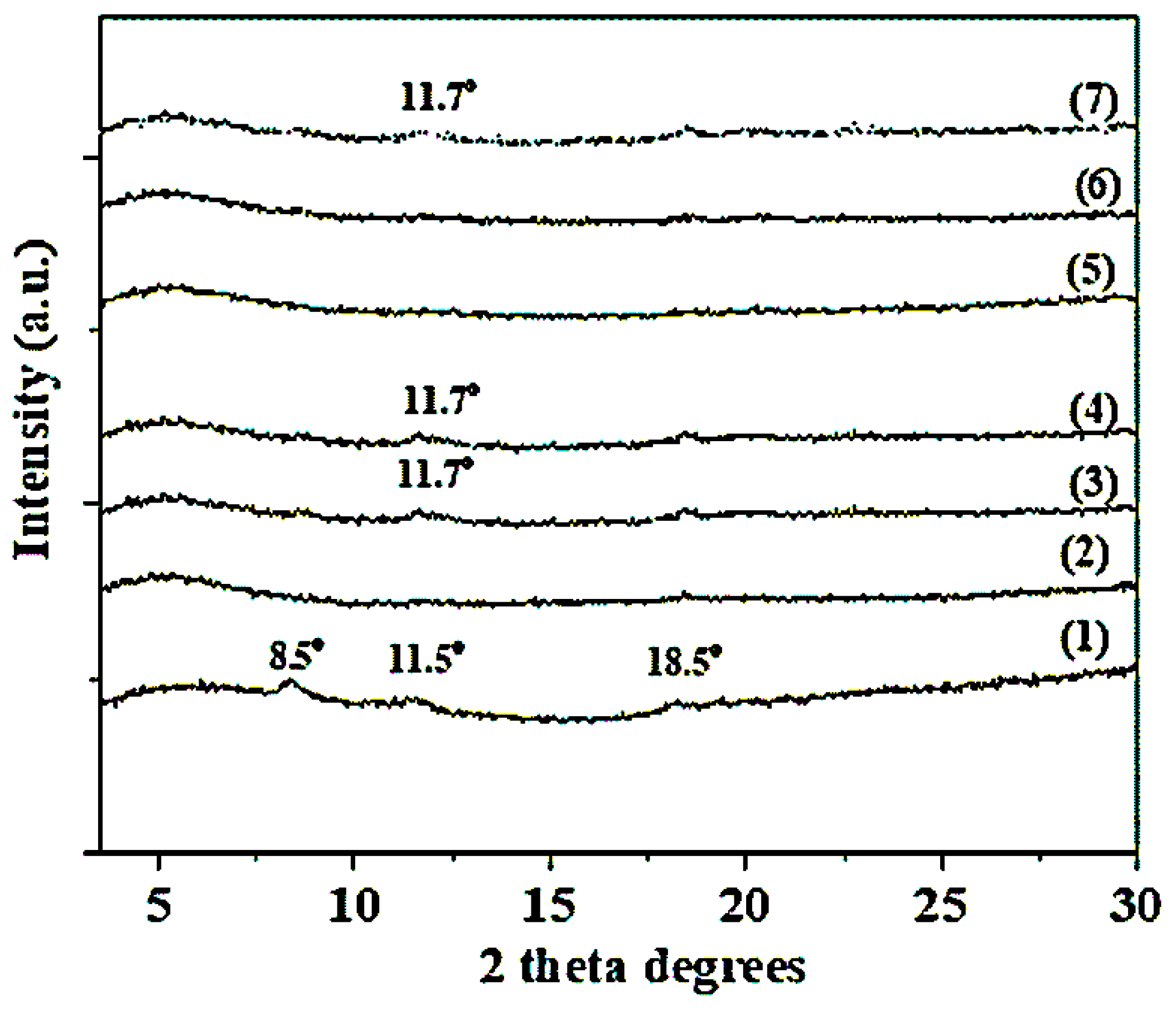
2.2. XRD Analysis of CS/PVOH/HNT and CS/PVOH/TO@HNT Films
2.3. FTIR Spectroscopy of CS/PVOH/HNT and CS/PVOH/TO@HNT Films
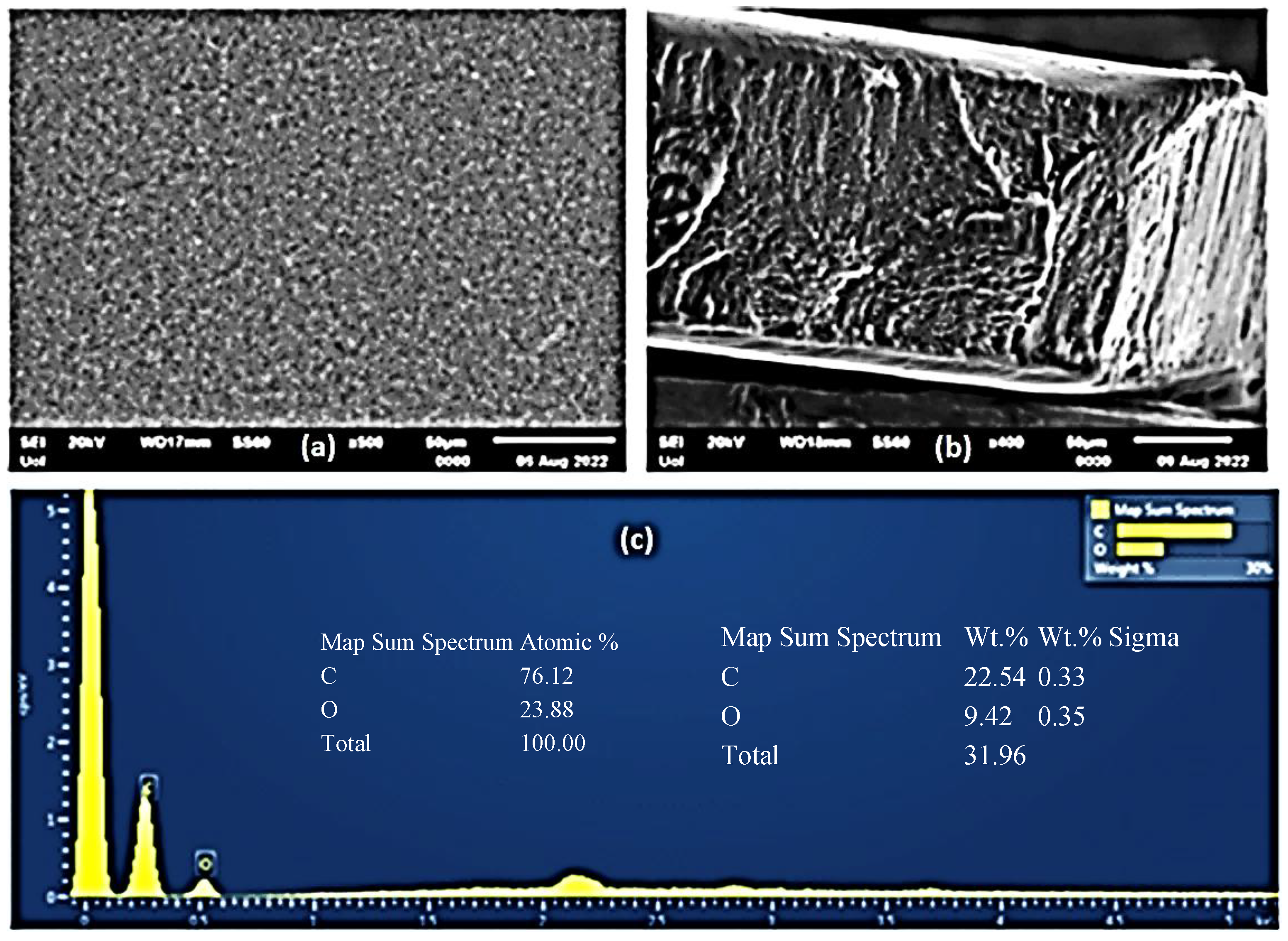
2.4. SEM Images of CS/PVOH/HNT and CS/PVOH/TO@HNT Films
2.5. Tensile Properties of CS/PVOH/HNT and CS/PVOH/TO@HNT Films
2.6. UV-vis Transmittance of CS/PVOH/HNT and CS/PVOH/TO@HNT Films
2.7. Water-Oxygen Barrier Properties of CS/PVOH/HNT and CS/PVOH/TO@HNT Films
2.8. Antioxidant Activity of CS/PVOH/HNT and CS/PVOH/TO@HNT Films
2.9. Antibacterial Activity of CS/PVOH/HNT and CS/PVOH/TO@HNT Films
2.10. Packaging Test-Application of CS/PVOH/HNT and CS/PVOH/TO@HNT Films as Coating on Fresh Kiwifruits
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of TO@HNT Hybrid Nanostructure
4.3. Preparation of CS/PVOH/HNT and CS/PVOH/TO@HNT Films/Coatings
4.4. Characterization of TO@HNT Hybrid Nanostructure
4.5. XRD Analysis of CS/PVOH/HNT and CS/PVOH/TO@HNT Films
4.6. FTIR Analysis of CS/PVOH/HNT and CS/PVOH/TO@HNT Films
4.7. SEM Images
4.8. Tensile Properties of CS/PVOH/HNT and CS/PVOH/TO@HNT Films
4.9. UV-vis Transparency of CS/PVOH/HNT and CS/PVOH/TO@HNT Films
4.10. Water and Oxygen Barrier Properties of CS/PVOH/HNT and CS/PVOH/TO@HNT Films
4.11. Oxygen Permeability of CS/PVOH/HNT and CS/PVOH/TO@HNT Films
4.12. Antioxidant Activity of CS/PVOH/HNT and CS/PVOH/TO@HNT Films
4.13. Antibacterial Activity of CS/PVOH/HNT and CS/PVOH/TO@HNT Films
4.14. Packaging Test of CS/PVOH/HNT and CS/PVOH/TO@HNT Coatings in Preservation of Kiwifruits
4.15. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hamam, M.; Chinnici, G.; Di Vita, G.; Pappalardo, G.; Pecorino, B.; Maesano, G.; D’Amico, M. Circular Economy Models in Agro-Food Systems: A Review. Sustainability 2021, 13, 3453. [Google Scholar] [CrossRef]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The Next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front. Nutr. 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, N.; Majid, I.; Nayik, G.A. Bioplastics and Food Packaging: A Review. Cogent Food Agric. 2015, 1, 1117749. [Google Scholar] [CrossRef]
- Taherimehr, M.; Yousefnia Pasha, H.; Tabatabaeekoloor, R.; Pesaranhajiabbas, E. Trends and Challenges of Biopolymer-Based Nanocomposites in Food Packaging. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5321–5344. [Google Scholar] [CrossRef]
- Sid, S.; Mor, R.S.; Kishore, A.; Sharanagat, V.S. Bio-Sourced Polymers as Alternatives to Conventional Food Packaging Materials: A Review. Trends Food Sci. Technol. 2021, 115, 87–104. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A Review of Cellulose and Its Derivatives in Biopolymer-Based for Food Packaging Application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for Food Packaging: Recent Advances in Active and Intelligent Films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Mahmud, N.; Islam, J.; Tahergorabi, R. Marine Biopolymers: Applications in Food Packaging. Processes 2021, 9, 2245. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan Based Edible Films and Coatings: A Review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Yang, Y.; Khan, H.; Gao, S.; Khalil, A.K.; Ali, N.; Khan, A.; Show, P.L.; Bilal, M.; Khan, H. Fabrication, Characterization, and Photocatalytic Degradation Potential of Chitosan-Conjugated Manganese Magnetic Nano-Biocomposite for Emerging Dye Pollutants. Chemosphere 2022, 306, 135647. [Google Scholar] [CrossRef]
- Cai, D.-L.; Thanh, D.T.H.; Show, P.-L.; How, S.-C.; Chiu, C.-Y.; Hsu, M.; Chia, S.R.; Chen, K.-H.; Chang, Y.-K. Studies of Protein Wastes Adsorption by Chitosan-Modified Nanofibers Decorated with Dye Wastes in Batch and Continuous Flow Processes: Potential Environmental Applications. Membranes 2022, 12, 759. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Ooi, C.W.; Show, P.L.; Hoe, B.C.; Chai, W.S.; Chiu, C.-Y.; Wang, S.S.-S.; Chang, Y.-K. Removal of Ionic Dyes by Nanofiber Membrane Functionalized with Chitosan and Egg White Proteins: Membrane Preparation and Adsorption Efficiency. Membranes 2022, 12, 63. [Google Scholar] [CrossRef]
- Ortiz-Duarte, G.; Pérez-Cabrera, L.E.; Artés-Hernández, F.; Martínez-Hernández, G.B. Ag-Chitosan Nanocomposites in Edible Coatings Affect the Quality of Fresh-Cut Melon. Postharvest Biol. Technol. 2019, 147, 174–184. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure—Activity Relationship|Biomacromolecules. Available online: https://pubs.acs.org/doi/10.1021/acs.biomac.7b01058 (accessed on 20 May 2022).
- Giannakas, A.E.; Salmas, C.E.; Leontiou, A.; Baikousi, M.; Moschovas, D.; Asimakopoulos, G.; Zafeiropoulos, N.E.; Avgeropoulos, A. Synthesis of a Novel Chitosan/Basil Oil Blend and Development of Novel Low Density Poly Ethylene/Chitosan/Basil Oil Active Packaging Films Following a Melt-Extrusion Process for Enhancing Chicken Breast Fillets Shelf-Life. Molecules 2021, 26, 1585. [Google Scholar] [CrossRef]
- Giannakas, A.; Vlacha, M.; Salmas, C.; Leontiou, A.; Katapodis, P.; Stamatis, H.; Barkoula, N.-M.; Ladavos, A. Preparation, Characterization, Mechanical, Barrier and Antimicrobial Properties of Chitosan/PVOH/Clay Nanocomposites. Carbohydr. Polym. 2016, 140, 408–415. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Leontiou, A.; Siasou, Z.; Karakassides, M.A. Development of Poly(L-Lactic Acid)/Chitosan/Basil Oil Active Packaging Films via a Melt-Extrusion Process Using Novel Chitosan/Basil Oil Blends. Processes 2021, 9, 88. [Google Scholar] [CrossRef]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Martínez-Hernández, A.L.; Tijerina-Ramos, B.I.; García-Hernández, M.; Rivera-Armenta, J.L.; Páramo-García, U.; Reyes-de la Torre, A.I. Antimicrobial, Optical and Mechanical Properties of Chitosan–Starch Films with Natural Extracts. Int. J. Mol. Sci. 2017, 18, 997. [Google Scholar] [CrossRef]
- Meng, W.; Shi, J.; Zhang, X.; Lian, H.; Wang, Q.; Peng, Y. Effects of Peanut Shell and Skin Extracts on the Antioxidant Ability, Physical and Structure Properties of Starch-Chitosan Active Packaging Films. Int. J. Biol. Macromol. 2020, 152, 137–146. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y. A Review on the Preparation and Characterization of Chitosan-Clay Nanocomposite Films and Coatings for Food Packaging Applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100102. [Google Scholar] [CrossRef]
- Wiles, J.L.; Vergano, P.J.; Barron, F.H.; Bunn, J.M.; Testin, R.F. Water Vapor Transmission Rates and Sorption Behavior of Chitosan Films. J. Food Sci. 2000, 65, 1175–1179. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and Intelligent Biodegradable Packaging Films Using Food and Food Waste-Derived Bioactive Compounds: A Review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- De Carvalho, A.P.A.; Conte Junior, C.A. Green Strategies for Active Food Packagings: A Systematic Review on Active Properties of Graphene-Based Nanomaterials and Biodegradable Polymers. Trends Food Sci. Technol. 2020, 103, 130–143. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active Packaging Films with Natural Antioxidants to Be Used in Meat Industry: A Review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Giannakas, A. Na-Montmorillonite vs. Organically Modified Montmorillonite as Essential Oil Nanocarriers for Melt-Extruded Low-Density Poly-Ethylene Nanocomposite Active Packaging Films with a Controllable and Long-Life Antioxidant Activity. Nanomaterials 2020, 10, 1027. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Leontiou, A.A. Montmorillonite Composite Materials and Food Packaging. In Composites Materials for Food Packaging; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 1–71. ISBN 978-1-119-16024-3. [Google Scholar]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of Essential Oils in Bioactive Edible Coatings: A Review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef]
- Maurya, A.; Singh, V.K.; Das, S.; Prasad, J.; Kedia, A.; Upadhyay, N.; Dubey, N.K.; Dwivedy, A.K. Essential Oil Nanoemulsion as Eco-Friendly and Safe Preservative: Bioefficacy against Microbial Food Deterioration and Toxin Secretion, Mode of Action, and Future Opportunities. Front. Microbiol. 2021, 12, 751062. [Google Scholar] [CrossRef]
- Full Article: Microemulsions: A Potential Delivery System for Bioactives in Food. Available online: https://www.tandfonline.com/doi/full/10.1080/10408690590956710 (accessed on 20 May 2022).
- De Oliveira, L.H.; Trigueiro, P.; Souza, J.S.N.; de Carvalho, M.S.; Osajima, J.A.; da Silva-Filho, E.C.; Fonseca, M.G. Montmorillonite with Essential Oils as Antimicrobial Agents, Packaging, Repellents, and Insecticides: An Overview. Colloids Surf. B Biointerfaces 2022, 209, 112186. [Google Scholar] [CrossRef]
- Li, Q.; Ren, T.; Perkins, P.; Hu, X.; Wang, X. Applications of Halloysite Nanotubes in Food Packaging for Improving Film Performance and Food Preservation. Food Control 2021, 124, 107876. [Google Scholar] [CrossRef]
- Villa, C.C.; Valencia, G.A.; López Córdoba, A.; Ortega-Toro, R.; Ahmed, S.; Gutiérrez, T.J. Zeolites for Food Applications: A Review. Food Biosci. 2022, 46, 101577. [Google Scholar] [CrossRef]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A Novel Method for the Preparation of Inorganic and Organo-Modified Montmorillonite Essential Oil Hybrids. Appl. Clay Sci. 2017, 146, 362–370. [Google Scholar] [CrossRef]
- Shiekh, K.A.; Ngiwngam, K.; Tongdeesoontorn, W. Polysaccharide-Based Active Coatings Incorporated with Bioactive Compounds for Reducing Postharvest Losses of Fresh Fruits. Coatings 2022, 12, 8. [Google Scholar] [CrossRef]
- Kiwifruit Production and Research in Greece|International Society for Horticultural Science. Available online: http://www.actahort.org/books/444/444_3.htm (accessed on 27 September 2022).
- Göksel, Z.; Atak, A. Kiwifruit processing studies. Acta Hortic. 2015, 1096, 99–107. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods 2021, 10, 3038. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Karydis-Messinis, A.; Moschovas, D.; Kollia, E.; Tsigkou, V.; Proestos, C.; Avgeropoulos, A.; Zafeiropoulos, N.E. Nanoclay and Polystyrene Type Efficiency on the Development of Polystyrene/Montmorillonite/Oregano Oil Antioxidant Active Packaging Nanocomposite Films. Appl. Sci. 2021, 11, 9364. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Leontiou, A.; Moschovas, D.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Karakassides, A.; Avgeropoulos, A.; Proestos, C. Performance of Thyme Oil@Na-Montmorillonite and Thyme Oil@Organo-Modified Montmorillonite Nanostructures on the Development of Melt-Extruded Poly-L-Lactic Acid Antioxidant Active Packaging Films. Molecules 2022, 27, 1231. [Google Scholar] [CrossRef]
- Tsagkalias, I.S.; Loukidi, A.; Chatzimichailidou, S.; Salmas, C.E.; Giannakas, A.E.; Achilias, D.S. Effect of Na- and Organo-Modified Montmorillonite/Essential Oil Nanohybrids on the Kinetics of the In Situ Radical Polymerization of Styrene. Nanomaterials 2021, 11, 474. [Google Scholar] [CrossRef]
- Saucedo-Zuñiga, J.N.; Sánchez-Valdes, S.; Ramírez-Vargas, E.; Guillen, L.; Ramos-de Valle, L.F.; Graciano-Verdugo, A.; Uribe-Calderón, J.A.; Valera-Zaragoza, M.; Lozano-Ramírez, T.; Rodríguez-González, J.A.; et al. Controlled Release of Essential Oils Using Laminar Nanoclay and Porous Halloysite/Essential Oil Composites in a Multilayer Film Reservoir. Microporous Mesoporous Mater. 2021, 316, 110882. [Google Scholar] [CrossRef]
- Jang, S.; Jang, S.; Lee, G.; Ryu, J.; Park, S.; Park, N. Halloysite Nanocapsules Containing Thyme Essential Oil: Preparation, Characterization, and Application in Packaging Materials. J. Food Sci. 2017, 82, 2113–2120. [Google Scholar] [CrossRef]
- Lee, M.H.; Seo, H.-S.; Park, H.J. Thyme Oil Encapsulated in Halloysite Nanotubes for Antimicrobial Packaging System. J. Food Sci. 2017, 82, 922–932. [Google Scholar] [CrossRef]
- Le Ba, T.; Alkurdi, A.Q.; Lukács, I.E.; Molnár, J.; Wongwises, S.; Gróf, G.; Szilágyi, I.M. A Novel Experimental Study on the Rheological Properties and Thermal Conductivity of Halloysite Nanofluids. Nanomaterials 2020, 10, 1834. [Google Scholar] [CrossRef]
- Shemesh, R.; Krepker, M.; Natan, M.; Danin-Poleg, Y.; Banin, E.; Kashi, Y.; Nitzan, N.; Vaxman, A.; Segal, E. Novel LDPE/Halloysite Nanotube Films with Sustained Carvacrol Release for Broad-Spectrum Antimicrobial Activity. RSC Adv. 2015, 5, 87108–87117. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Zaharioudakis, K.; Georgopoulos, S.; Asimakopoulos, G.; Aktypis, A.; Proestos, C.; Karakassides, A.; Avgeropoulos, A.; et al. The Increase of Soft Cheese Shelf-Life Packaged with Edible Films Based on Novel Hybrid Nanostructures. Gels 2022, 8, 539. [Google Scholar] [CrossRef]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G.G. Study of the Combined Effect of Both Clay and Glycerol Plasticizer on the Properties of Chitosan Films. Carbohydr. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- Giannakas, A.; Grigoriadi, K.; Leontiou, A.; Barkoula, N.-M.; Ladavos, A. Preparation, Characterization, Mechanical and Barrier Properties Investigation of Chitosan–Clay Nanocomposites. Carbohydr. Polym. 2014, 108, 103–111. [Google Scholar] [CrossRef]
- Grigoriadi, K.; Giannakas, A.; Ladavos, A.K.; Barkoula, N.-M. Interplay between Processing and Performance in Chitosan-Based Clay Nanocomposite Films. Polym. Bull. 2015, 72, 1145–1161. [Google Scholar] [CrossRef]
- A Chitosan/Poly(Vinyl Alcohol) Nanocomposite Film Reinforced with Natural Halloysite Nanotubes—Huang—2012—Polymer Composites—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/pc.22302 (accessed on 14 June 2022).
- Suflet, D.M.; Popescu, I.; Pelin, I.M.; Ichim, D.L.; Daraba, O.M.; Constantin, M.; Fundueanu, G. Dual Cross-Linked Chitosan/PVA Hydrogels Containing Silver Nanoparticles with Antimicrobial Properties. Pharmaceutics 2021, 13, 1461. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, Structural and Antimicrobial Properties of Poly Vinyl Alcohol–Chitosan Biodegradable Films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Karakassides, A.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Nanocomposite Film Development Based on Chitosan/Polyvinyl Alcohol Using ZnO@Montmorillonite and ZnO@Halloysite Hybrid Nanostructures for Active Food Packaging Applications. Nanomaterials 2022, 12, 1843. [Google Scholar] [CrossRef]
- Unuabonah, E.I.; Ugwuja, C.G.; Omorogie, M.O.; Adewuyi, A.; Oladoja, N.A. Clays for Efficient Disinfection of Bacteria in Water. Appl. Clay Sci. 2018, 151, 211–223. [Google Scholar] [CrossRef]
- What Makes a Natural Clay Antibacterial? Environmental Science & Technology. Available online: https://pubs.acs.org/doi/10.1021/es1040688 (accessed on 27 September 2022).
- Abhinayaa, R.; Jeevitha, G.; Mangalaraj, D.; Ponpandian, N.; Meena, P. Toxic Influence of Pristine and Surfactant Modified Halloysite Nanotubes on Phytopathogenic Bacteria. Appl. Clay Sci. 2019, 174, 57–68. [Google Scholar] [CrossRef]
- Duan, L.; Zhao, Q.; Liu, J.; Zhang, Y. Antibacterial Behavior of Halloysite Nanotubes Decorated with Copper Nanoparticles in a Novel Mixed Matrix Membrane for Water Purification. Environ. Sci. Water Res. Technol. 2015, 1, 874–881. [Google Scholar] [CrossRef]
- Wang, L.-F.; Rhim, J.-W. Functionalization of Halloysite Nanotubes for the Preparation of Carboxymethyl Cellulose-Based Nanocomposite Films. Appl. Clay Sci. 2017, 150, 138–146. [Google Scholar] [CrossRef]
- Taylor, A.A.; Aron, G.M.; Beall, G.W.; Dharmasiri, N.; Zhang, Y.; McLean, R.J.C. Carbon and Clay Nanoparticles Induce Minimal Stress Responses in Gram Negative Bacteria and Eukaryotic Fish Cells. Environ. Toxicol. 2014, 29, 961–968. [Google Scholar] [CrossRef]
- Boelter, J.F.; Brandelli, A.; Meira, S.M.M.; Göethel, G.; Garcia, S.C. Toxicology Study of Nanoclays Adsorbed with the Antimicrobial Peptide Nisin on Caenorhabditis Elegans. Appl. Clay Sci. 2020, 188, 105490. [Google Scholar] [CrossRef]
- Krepker, M.; Shemesh, R.; Danin Poleg, Y.; Kashi, Y.; Vaxman, A.; Segal, E. Active Food Packaging Films with Synergistic Antimicrobial Activity. Food Control 2017, 76, 117–126. [Google Scholar] [CrossRef]
- Maruthupandy, M.; Seo, J. Allyl Isothiocyanate Encapsulated Halloysite Covered with Polyacrylate as a Potential Antibacterial Agent against Food Spoilage Bacteria. Mater. Sci. Eng. C 2019, 105, 110016. [Google Scholar] [CrossRef]
- Giannakas, A.; Giannakas, A.; Ladavos, A. Preparation and Characterization of Polystyrene/Organolaponite Nanocomposites. Polym. Plast. Technol. Eng. 2012, 51, 1411–1415. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering Properties of Polymeric-Based Antimicrobial Films for Food Packaging: A Review. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Units of Gas Permeability Constants—Yasuda—1975—Journal of Applied Polymer Science—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/app.1975.070190915 (accessed on 11 November 2021).










| Sample Name | E-Elastic Modulus (MPa) | σ uts (MPa) | ε%-Elongation at Break |
|---|---|---|---|
| CS/PVOH | 2249.3 ± 20.0 | 71.2 ± 1.8 | 14.8 ± 0.9 |
| CS/PVOH/5HNT | 2552.0 ± 21.3 | 74.1 ± 2.1 | 7.0 ± 1.6 |
| CS/PVOH/10HNT | 2766.0 ± 35.1 | 79.8 ± 1.7 | 5.7 ± 0.8 |
| CS/PVOH/15HNT | 2865.0 ± 27.4 | 96.0 ± 1.2 | 6.7 ± 0.6 |
| CS/PVOH/5TO@HNT | 2644.5 ± 13.4 | 74.9 ± 2.3 | 7.2 ± 2.1 |
| CS/PVOH/10TO@HNT | 2993.7 ± 27.6 | 103.7 ± 1.4 | 7.1 ± 0.2 |
| CS/PVOH/15TO@HNT | 2965.0 ± 29.4 | 98.5 ± 1.5 | 6.8 ± 0.7 |
| Sample Name | Film Thickness (mm) | WVTR × 10−6 (gr × cm−2 × s−1) | D × 10−4 (cm2 × s−1) | OTR (mL × m−2 × day−1) | PeO2 × 10−7 (cm2 × s−1) |
|---|---|---|---|---|---|
| CS/PVOH | 0.140 ± 0.010 | 1.15 ± 0.13 | 3.65 ± 0.31 | 49,577 ± 2478 | 8.03 ± 0.40 |
| CS/PVOH/5HNT | 0.113 ± 0.025 | 1.42 ± 0.18 | 3.67 ± 0.72 | 57,345 ± 2867 | 7.52 ± 0.38 |
| CS/PVOH/10HNT | 0.123 ± 0.015 | 1.02 ± 0.13 | 2.83 ± 0.62 | 32,785 ± 1639 | 4.68 ± 0.23 |
| CS/PVOH/15HNT | 0.117 ± 0.012 | 1.03 ± 0.20 | 2.75 ± 0.17 | 44,234 ± 2211 | 5.97 ± 0.30 |
| CS/PVOH/5TO@HNT | 0.140 ± 0.010 | 1.14 ± 0.13 | 3.59 ± 0.14 | 43,345 ± 2167 | 7.02 ± 0.35 |
| CS/PVOH/10TO@HNT | 0.117 ± 0.021 | 1.02 ± 0.21 | 2.62 ± 0.17 | 28,974 ± 1449 | 3.91 ± 0.20 |
| CS/PVOH/15TO@HNT | 0.170 ± 0.017 | 1.01 ± 0.67 | 3.64 ± 0.14 | 30,434 ± 1521 | 5.64 ± 0.28 |
| Sample Name | Antioxidant Activity after 24 h 1 (%) |
|---|---|
| CS/PVOH | 3.5 ± 1.6 |
| CS/PVOH/5HNT | 5.4 ± 2.6 |
| CS/PVOH/10HNT | 5.5 ± 2.2 |
| CS/PVOH/15HNT | 6.1 ± 3.1 |
| CS/PVOH/5TO@HNT | 18.1 ± 5.0 |
| CS/PVOH/10TO@HNT | 25.5 ± 3.6 |
| CS/PVOH/15TO@HNT | 32.2 ± 5.1 |
| Film Material | E. coli | S. enterica | S. aureus | L. monocytogenes | ||||
|---|---|---|---|---|---|---|---|---|
| Inhibition a | Contact b | Inhibition a | Contact b | Inhibition a | Contact b | Inhibition a | Contact b | |
| CS | 0.00 | - | 0.00 | - | 0.00 | - | 0.00 9 | + |
| CS/20PVOH | 3.50 ± 0.87 | - | 3.83 ± 0.76 5,6,8 | - | 4.47 ± 0.81 3 | - | 0.00 9 | + |
| CS/20PVOH/5HNT | 4.83 ± 0.29 1 | - | 5.00 ± 0.00 5,6,7,8 | - | 5.40 ± 0.66 3,4 | - | 5.00 ± 0.00 10 | - |
| CS/20PVOH/10HNT | 5.00 ± 0.00 1 | - | 5.33 ± 0.76 6,7,8 | - | 5.77 ± 0.75 3,4 | - | 5.00 ± 0.00 10 | - |
| CS/20PVOH/15HNT | 7.00 ± 0.50 2 | - | 6.33 ± 0.29 6,7,8 | - | 6.00 ± 0.00 4 | - | 6.57 ± 0.51 | - |
| CS/20PVOH/5TO@HNT | 7.50 ± 0.50 2 | - | 6.83 ± 0.298 | - | 9.00± 0.00 | - | 8.00 ± 0.50 | - |
| CS/20PVOH/10TO@HNT | 7.80 ± 0.20 | - | 8.00 ± 0.00 | - | 9.50 ± 0.50 | - | 9.03 ± 0.45 | - |
| CS/20PVOH/15TO@HNT | 8.00 ± 0.50 | - | 9.00 ± 0.87 | - | 10.00 ± 0.00 | - | 9.00 ± 0.50 | - |
| E | σuts | ε% | WVTR | OTR | Antiox. | E. coli | Saur. | Senter. | L. monoc. | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sig. < 0.05 | 0–0.0110 | 0–0.0185 | 0–0.014 | 0–0.0220 | 0–0.0120 | 0–0.0400 | 0–0.037 | 0–0.024 | 0–0.039 | 0–0.002 |
| IA (%) | 78–100 | 63–100 | 72–100 | 56–100 | 76–100 | 20–100 | 26–100 | 52–100 | 22–100 | 96–100 |
| Sig. > 0.05 | - | - | - | - | - | - | 0.862–1 | 0.071–0.999 | 0.081–0.991 | 1 |
| EA (%) | - | - | - | - | - | - | 85–100 | 2–100 | 3–99 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmas, C.E.; Giannakas, A.E.; Moschovas, D.; Kollia, E.; Georgopoulos, S.; Gioti, C.; Leontiou, A.; Avgeropoulos, A.; Kopsacheili, A.; Avdylaj, L.; et al. Kiwi Fruits Preservation Using Novel Edible Active Coatings Based on Rich Thymol Halloysite Nanostructures and Chitosan/Polyvinyl Alcohol Gels. Gels 2022, 8, 823. https://doi.org/10.3390/gels8120823
Salmas CE, Giannakas AE, Moschovas D, Kollia E, Georgopoulos S, Gioti C, Leontiou A, Avgeropoulos A, Kopsacheili A, Avdylaj L, et al. Kiwi Fruits Preservation Using Novel Edible Active Coatings Based on Rich Thymol Halloysite Nanostructures and Chitosan/Polyvinyl Alcohol Gels. Gels. 2022; 8(12):823. https://doi.org/10.3390/gels8120823
Chicago/Turabian StyleSalmas, Constantinos E., Aris E. Giannakas, Dimitrios Moschovas, Eleni Kollia, Stavros Georgopoulos, Christina Gioti, Areti Leontiou, Apostolos Avgeropoulos, Anna Kopsacheili, Learda Avdylaj, and et al. 2022. "Kiwi Fruits Preservation Using Novel Edible Active Coatings Based on Rich Thymol Halloysite Nanostructures and Chitosan/Polyvinyl Alcohol Gels" Gels 8, no. 12: 823. https://doi.org/10.3390/gels8120823
APA StyleSalmas, C. E., Giannakas, A. E., Moschovas, D., Kollia, E., Georgopoulos, S., Gioti, C., Leontiou, A., Avgeropoulos, A., Kopsacheili, A., Avdylaj, L., & Proestos, C. (2022). Kiwi Fruits Preservation Using Novel Edible Active Coatings Based on Rich Thymol Halloysite Nanostructures and Chitosan/Polyvinyl Alcohol Gels. Gels, 8(12), 823. https://doi.org/10.3390/gels8120823












