Chitosan-Based Green Pea (Pisum sativum L.) Pod Extract Gel Film: Characterization and Application in Food Packaging
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical Properties
2.1.1. Film Thickness and Density
2.1.2. Film Water Vapour Permeability (WVP)
2.1.3. Film Water Solubility (WS)
2.1.4. Film Oil Resistance Ability
2.2. Film Colour and Opacity
2.3. Mechanical Properties
2.4. Bioactivities of CH-EPPE Film
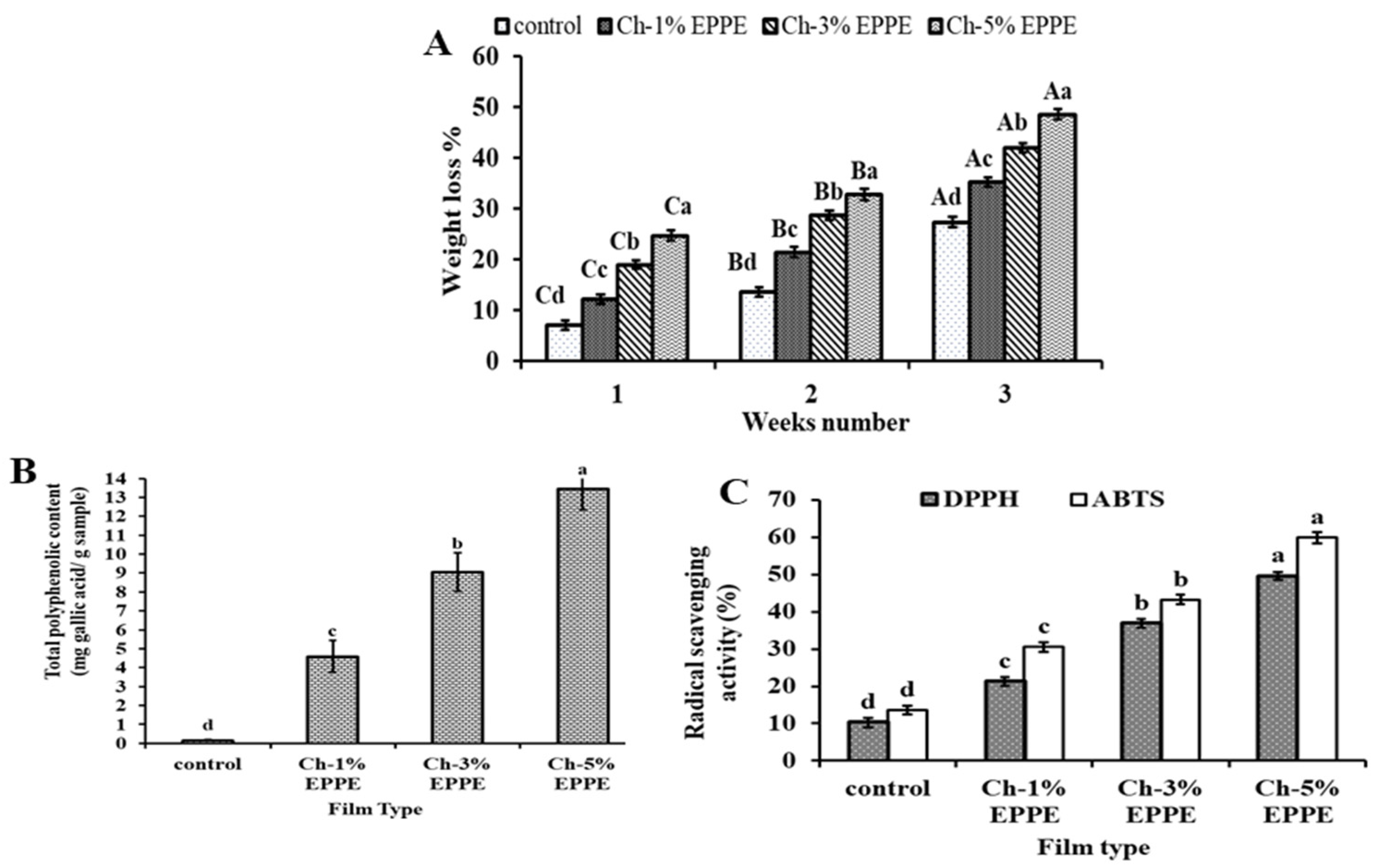
2.4.1. Biodegradation Evaluation
2.4.2. Antioxidant Properties
2.4.3. Migration Test
2.4.4. Antimicrobial Activity
2.5. Films SEM Photographs
2.6. Application of Films in Packaging Edible Oil
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Empty Pea Pods Extract (EPPE)
4.3. Gel Film Preparation
4.4. Physical Properties
4.4.1. Film Thickness (T)
4.4.2. Film Density (D)
4.4.3. Film WVP
4.4.4. Film Water Solubility(S)
4.4.5. Films’ ORA
4.5. Optical Characteristics
4.6. Mechanical Properties
4.7. Bioactivities of CH-EPPE Film
4.7.1. Biodegradation Test
4.7.2. Antioxidant Properties
TPC Measurement
Antioxidant Activity Measurement
Migration Test
Antimicrobial Activity
4.8. SEM Scanning
4.9. Film Application in Corn Oil Packaging
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pauer, E.; Wohner, B.; Heinrich, V.; Tacker, M. Assessing the environmental sustainability of food packaging: An extended life cycle assessment including packaging-related food losses and waste and circularity assessment. Sustainability 2019, 11, 925. [Google Scholar] [CrossRef]
- Wohner, B.; Pauer, E.; Heinrich, V.; Tacker, M. Packaging-related food losses and waste: An overview of drivers and issues. Sustainability 2019, 11, 264. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Ko, S. Nanoclays in food and beverage packaging. J. Nanomater. 2019, 2019, 8927167. [Google Scholar] [CrossRef]
- Horodytska, O.; Valdés, F.J.; Fullana, A. Plastic flexible films waste management—A state of art review. Waste Manag. 2018, 77, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, M.; Nath, O.; Deb, P. Enhancing barrier properties of biodegradable film by reinforcing with 2D heterostructure. Appl. Surf. Sci. 2021, 541, 148464. [Google Scholar] [CrossRef]
- Yao, Q.; Song, Z.; Li, J.; Zhang, L. Micromorphology, mechanical, crystallization and permeability properties analysis of HA/PBAT/PLA (HA, hydroxyapatite; PBAT, poly (butylene adipate-co-butylene terephthalate); PLA, polylactide) degradability packaging films. Polym. Int. 2020, 69, 301–307. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun functional materials toward food packaging applications: A review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Manigandan, V.; Karthik, R.; Ramachandran, S.; Rajagopal, S. Chitosan applications in food industry. In Biopolymers for Food Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 469–491. [Google Scholar]
- Bourtoom, T. Edible films and coatings: Characteristics and properties. Int. Food Res. J. 2008, 15, 237–248. [Google Scholar]
- Nura, A. Advances in food packaging technology-a review. J. Postharvest Technol. 2018, 6, 55–64. [Google Scholar]
- Quezada-Gallo, J. Delivery of food additives and antimicrobials using edible films and coatings. In Edible Films and Coatings for Food Applications; Embuscado, M.E., Huber, K.C., Eds.; Springer: New York, NY, USA, 2009; pp. 315–333. [Google Scholar]
- Pokorný, J. Are natural antioxidants better–and safer–than synthetic antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 629–642. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Antioxidative activity and properties of fish skin gelatin films incorporated with BHT and α-tocopherol. Food Hydrocoll. 2008, 22, 449–458. [Google Scholar] [CrossRef]
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Dao, U.T.T.; Bui, Q.P.T.; Bach, G.L.; Thuc, C.H.; Thuc, H.H. Enhanced antimicrobial activities and physiochemical properties of edible film based on chitosan incorporated with Sonneratia caseolaris (L.) Engl. leaf extract. Prog. Org. Coat. 2020, 140, 105487. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Sogut, E.; Seydim, A.C. The effects of Chitosan and grape seed extract-based edible films on the quality of vacuum packaged chicken breast fillets. Food Packag. Shelf Life 2018, 18, 13–20. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Jiang, W. Development of antioxidant chitosan film with banana peels extract and its application as coating in maintaining the storage quality of apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Cui, H.; Surendhiran, D.; Li, C.; Lin, L. Biodegradable zein active film containing chitosan nanoparticle encapsulated with pomegranate peel extract for food packaging. Food Packag. Shelf Life 2020, 24, 100511. [Google Scholar] [CrossRef]
- Kõrge, K.; Bajić, M.; Likozar, B.; Novak, U. Active chitosan–chestnut extract films used for packaging and storage of fresh pasta. Int. J. Food Sci. Technol. 2020, 55, 3043–3052. [Google Scholar] [CrossRef]
- El Shaer, S.; El-Sharkawy, H.; El-Salehein, A. Efficiency of transmission pea enation mosaic virus (PEMV) by the pea aphid in Dakhlia governorate, Egypt. J. Product. Dev. 2022, 27, 189–200. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Redondo-Cuenca, A.; Villanueva-Suárez, M.-J.; Zapata-Revilla, M.-A.; Tenorio-Sanz, M.-D. Pea pod, broad bean pod and okara, potential sources of functional compounds. LWT-Food Sci. Technol. 2010, 43, 1467–1470. [Google Scholar] [CrossRef]
- Awad, K.; Abdel-Nabey, A.; Awney, H. Phenolic Composition and Antioxidant Activity of Some Agro-industrial Wastes. Alex. J. Fd. Sci. Technol 2018, 15, 33–44. [Google Scholar]
- Hadjout, L.; Dahmoune, F.; Hentabli, M.; Spigno, G.; Madani, K. Physicochemical, Functional and Bioactive Properties of Pea (Pisum sativum L.) Pods Microwave and Convective Dried Powders. 2021. Available online: https://assets.researchsquare.com/files/rs-951507/v1_covered.pdf?c=1635862935 (accessed on 3 March 2021).
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wu, Y.; Li, Y. Development of tea extracts and chitosan composite films for active packaging materials. Int. J. Biol. Macromol. 2013, 59, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Kurek, M.; Garofulić, I.E.; Bakić, M.T.; Ščetar, M.; Uzelac, V.D. Development and evaluation of a novel antioxidant and pH indicator film based on chitosan and food waste sources of antioxidants. Food Hydrocoll. 2018, 84, 238–246. [Google Scholar] [CrossRef]
- Uranga, J.; Puertas, A.; Etxabide, A.; Dueñas, M.; Guerrero, P.; De La Caba, K. Citric acid-incorporated fish gelatin/chitosan composite films. Food Hydrocoll. 2019, 86, 95–103. [Google Scholar] [CrossRef]
- Riaz, A.; Lagnika, C.; Luo, H.; Dai, Z.; Nie, M.; Hashim, M.M.; Liu, C.; Song, J.; Li, D. Chitosan-based biodegradable active food packaging film containing Chinese chive (Allium tuberosum) root extract for food application. Int. J. Biol. Macromol. 2020, 150, 595–604. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Chen, Y.; Xia, W.; Xiong, Y.L.; Wang, H. Enhanced physicochemical properties of chitosan/whey protein isolate composite film by sodium laurate-modified TiO2 nanoparticles. Carbohydr. Polym. 2016, 138, 59–65. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Vicente, A.A. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll. 2012, 27, 220–227. [Google Scholar] [CrossRef]
- Hopkins, E.J.; Chang, C.; Lam, R.S.; Nickerson, M.T. Effects of flaxseed oil concentration on the performance of a soy protein isolate-based emulsion-type film. Food Res. Int. 2015, 67, 418–425. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.G.; Wu, N.F.; Fan, M.M.; Shen, X.L.; Chen, M.T.; Jiang, A.M.; Lai, L.-S. Effect of hsian-tsao gum (HG) content upon rheological properties of film-forming solutions (FFS) and physical properties of soy protein/hsian-tsao gum films. Food Hydrocoll. 2015, 50, 211–218. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar]
- Laohakunjit, N.; Noomhorm, A. Effect of plasticizers on mechanical and barrier properties of rice starch film. Starch-Stärke 2004, 56, 348–356. [Google Scholar] [CrossRef]
- Pastor, C.; Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and antifungal properties of hydroxypropylmethylcellulose based films containing propolis as affected by moisture content. Carbohydr. Polym. 2010, 82, 1174–1183. [Google Scholar] [CrossRef]
- Balti, R.; Mansour, M.B.; Sayari, N.; Yacoubi, L.; Rabaoui, L.; Brodu, N.; Massé, A. Development and characterization of bioactive edible films from spider crab (Maja crispata) chitosan incorporated with Spirulina extract. Int. J. Biol. Macromol. 2017, 105, 1464–1472. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, L.; Pérez-Álvarez, J.; Fernandez-Lopez, J.; Muñoz, L.; Viuda-Martos, M. Assessment of antibacterial and antioxidant properties of chitosan edible films incorporated with maqui berry (Aristotelia chilensis). LWT-Food Sci. Technol. 2015, 64, 1057–1062. [Google Scholar] [CrossRef]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar]
- Moradi, M.; Tajik, H.; Rohani, S.M.R.; Oromiehie, A.R.; Malekinejad, H.; Aliakbarlu, J.; Hadian, M. Characterization of antioxidant chitosan film incorporated with Zataria multiflora Boiss essential oil and grape seed extract. LWT-Food Sci. Technol. 2012, 46, 477–484. [Google Scholar] [CrossRef]
- Atarés, L.; Pérez-Masiá, R.; Chiralt, A. The role of some antioxidants in the HPMC film properties and lipid protection in coated toasted almonds. J. Food Eng. 2011, 104, 649–656. [Google Scholar] [CrossRef]
- Colín-Chávez, C.; Soto-Valdez, H.; Peralta, E.; Lizardi-Mendoza, J.; Balandrán-Quintana, R. Diffusion of natural astaxanthin from polyethylene active packaging films into a fatty food simulant. Food Res. Int. 2013, 54, 873–880. [Google Scholar] [CrossRef]
- Kurek, M.; Guinault, A.; Voilley, A.; Debeaufort, F. Effect of relative humidity on carvacrol release and permeation properties of chitosan based films and coatings. Food Chem. 2014, 144, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Adilah, Z.M.; Jamilah, B.; Hanani, Z.N. Functional and antioxidant properties of protein-based films incorporated with mango kernel extract for active packaging. Food Hydrocoll. 2018, 74, 207–218. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, X.; Li, W.; Song, R.; Li, J.; Li, Y.; Li, B.; Liu, S. Green and biodegradable composite films with novel antimicrobial performance based on cellulose. Food Chem. 2016, 197, 250–256. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Jiang, Y.; Chai, Z.; Li, P.; Cheng, Y.; Jing, H.; Leng, X. Synergistic antimicrobial activities of natural essential oils with chitosan films. J. Agric. Food Chem. 2011, 59, 12411–12419. [Google Scholar] [CrossRef] [PubMed]
- Ouattara, B.; Simard, R.E.; Holley, R.A.; Piette, G.J.-P.; Bégin, A. Antibacterial activity of selected fatty acids and essential oils against six meat spoilage organisms. Int. J. Food Microbiol. 1997, 37, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Rezaei, M.; Farzi, G. Improvement of active chitosan film properties with rosemary essential oil for food packaging. Int. J. Food Sci. Technol. 2012, 47, 847–853. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Mariniello, L.; Giosafatto, C.; Di Pierro, P.; Sorrentino, A.; Porta, R. Swelling, mechanical, and barrier properties of albedo-based films prepared in the presence of phaseolin cross-linked or not by transglutaminase. Biomacromolecules 2010, 11, 2394–2398. [Google Scholar] [CrossRef] [PubMed]
- Pinchao-Pinchao, Y.A.; Ordoñez-Santos, L.E.; Osorio-Mora, O. Evaluation of the effect of different factors on the ultrasound assisted extraction of phenolic compounds of the pea pod. Dyna 2019, 86, 211–215. [Google Scholar]
- Zhang, P.; Zhao, Y.; Shi, Q. Characterization of a novel edible film based on gum ghatti: Effect of plasticizer type and concentration. Carbohydr. Polym. 2016, 153, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A. Physical, barrier and antioxidant properties of a novel plasticized edible film from quince seed mucilage. Int. J. Biol. Macromol. 2013, 62, 500–507. [Google Scholar] [CrossRef] [PubMed]
- ASTM. Annual Book of ASTM Standards; American Society for Testing and Materials: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Liu, J.; Meng, C.-g.; Liu, S.; Kan, J.; Jin, C.-h. Preparation and characterization of protocatechuic acid grafted chitosan films with antioxidant activity. Food Hydrocoll. 2017, 63, 457–466. [Google Scholar] [CrossRef]
- Oliveira, V.; Monteiro, M.; Silva, K.; Leite, R.; Aroucha, E. Analysis of the Barrier and Thermogravimetric Properties of Cassava Starch Biopolymeric Films with Addition of Beeswax. J. Food Process. Technol. 2018, 10, 2. [Google Scholar]
- Elsebaie, E.M.; Essa, R.Y. Application of barnûf (Pluchea dioscoridis) leaves extract as a natural antioxidant and antimicrobial agent for eggs quality and safety improvement during storage. J. Food Process. Preserv. 2022, 46, e16061. [Google Scholar] [CrossRef]
- Elsebaie, E.M.; Kassem, M.M.; Mousa, M.M.; Basuony, M.A.M.; Zeima, N.M.; Essa, R.Y. Cod Liver Oil’s Encapsulation into Sodium Alginate/Lupin Protein Beads and Its Application in Functional Meatballs’ Preparation. Foods 2022, 11, 1328. [Google Scholar] [CrossRef]


| Film Samples | Film Properties | ||||
|---|---|---|---|---|---|
| T (mm) | D (g/cm3) | WVP (×10−10 g−1 s−1 pa−1) | S (%) | ORA (%) | |
| Control (Ch-0% EPPE) | 0.132 ± 0.08 d | 1.13 ± 0.02 d | 2.34 ± 0.04 a | 29.40 ± 1.23 a | 0.31 ± 0.006 a |
| Ch-1% EPPE | 0.167 ± 0.09 c | 1.52 ± 0.03 c | 1.58 ± 0.09 b | 28.17 ± 1.78 a | 0.26 ± 0.004 b |
| Ch-3% EPPE | 0.189 ± 0.06 b | 1.75 ± 0.02 b | 1.32 ± 0.07 b | 22.43 ± 2.11 b | 0.15 ± 0.003 c |
| Ch-5% EPPE | 0.216 ± 0.08 a | 1.94 ± 0.02 a | 1.08 ± 0.06 c | 18.75 ± 1.94 c | 0.08 ± 0.001 d |
| Film Samples | L* | b* | a* | Whiteness Index | Opacity |
|---|---|---|---|---|---|
| Control (Ch-0% EPPE) | 88.17 ± 0.55 a | 1.03 ± 0.01 a | 0.84 ± 0.01 a | 88.10 ± 0.43 a | 0.71 ± 0.02 a |
| Ch-1% EPPE | 85.40 ± 0.72 b | 1.19 ± 0.07 b | 0.87 ± 0.01 a | 85.33 ± 0.51 b | 0.80 ± 0.03 b |
| Ch-3% EPPE | 80.35 ± 0.61 c | 1.47 ± 0.05 c | 0.94 ± 0.03 a | 80.27 ± 0.60 c | 0.97 ± 0.03 c |
| Ch-5% EPPE | 77.64 ± 0.66 d | 1.98 ± 0.04 d | 1.12 ± 0.03 b | 77.53 ± 0.48 d | 1.23 ± 0.04 d |
| Film Samples | Tensile Strength (MPa) | Elongation at Break (EB) % |
|---|---|---|
| Control (Ch-0% EPPE) | 21.30 ± 1.19 d | 53.42 ± 3.02 d |
| Ch-1% EPPE | 23.16 ± 1.23 c | 54.17 ± 2.98 c |
| Ch-3% EPPE | 25.92 ± 1.40 b | 56.83 ± 2.77 b |
| Ch-5% EPPE | 26.87 ± 1.38 a | 58.64 ± 3.00 a |
| Film Samples | Simulant Type | |
|---|---|---|
| Total Phenolic Content (mg gallic acid/mL Water) | Total Phenolic Content (mg gallic acid/mL Ethanol) | |
| Control (Ch-0% EPPE) | 0.002 ± 0.000 a | 0.004 ± 0.001 a |
| Ch-1% EPPE | 1.04 ± 0.05 c | 1.92 ± 0.09 c |
| Ch-3% EPPE | 3.19 ± 0.03 b | 5.06 ± 0.06 b |
| Ch-5% EPPE | 5.09 ± 0.06 a | 9.12 ± 0.08 a |
| Film Samples | Inhibition Zone Diameter (mm) | |||
|---|---|---|---|---|
| Salmonella typhimurium | E. coli | Bacillus subtilis | Pseudomonas aeruginosa | |
| Control (Ch-0% EPPE) | NA | NA | NA | NA |
| Ch-1% EPPE | 7.89 ± 0.10 c | 8.12 ± 0.15 c | 10.67 ± 0.13 c | 10.35 ± 0.16 c |
| Ch-3% EPPE | 11.38 ± 0.17 b | 12.63 ± 0.12 b | 15.94 ± 0.14 b | 15.41 ± 0.11 b |
| Ch-5% EPPE | 15.66 ± 0.14 a | 16.25 ± 0.10 a | 19.42 ± 0.20 a | 18.98 ± 0.18 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsebaie, E.M.; Mousa, M.M.; Abulmeaty, S.A.; Shaat, H.A.Y.; Elmeslamy, S.A.-E.; Asker, G.A.; Faramawy, A.A.; Shaat, H.A.Y.; Abd Elrahman, W.M.; Eldamaty, H.S.E.; et al. Chitosan-Based Green Pea (Pisum sativum L.) Pod Extract Gel Film: Characterization and Application in Food Packaging. Gels 2023, 9, 77. https://doi.org/10.3390/gels9020077
Elsebaie EM, Mousa MM, Abulmeaty SA, Shaat HAY, Elmeslamy SA-E, Asker GA, Faramawy AA, Shaat HAY, Abd Elrahman WM, Eldamaty HSE, et al. Chitosan-Based Green Pea (Pisum sativum L.) Pod Extract Gel Film: Characterization and Application in Food Packaging. Gels. 2023; 9(2):77. https://doi.org/10.3390/gels9020077
Chicago/Turabian StyleElsebaie, Essam Mohamed, Mona Metwally Mousa, Samah Amin Abulmeaty, Heba Ali Yousef Shaat, Soher Abd-Elfttah Elmeslamy, Galila Ali Asker, Asmaa Antar Faramawy, Hala Ali Yousef Shaat, Wesam Mohammed Abd Elrahman, Hanan Salah Eldeen Eldamaty, and et al. 2023. "Chitosan-Based Green Pea (Pisum sativum L.) Pod Extract Gel Film: Characterization and Application in Food Packaging" Gels 9, no. 2: 77. https://doi.org/10.3390/gels9020077
APA StyleElsebaie, E. M., Mousa, M. M., Abulmeaty, S. A., Shaat, H. A. Y., Elmeslamy, S. A.-E., Asker, G. A., Faramawy, A. A., Shaat, H. A. Y., Abd Elrahman, W. M., Eldamaty, H. S. E., Abd Allah, A. L., & Badr, M. R. (2023). Chitosan-Based Green Pea (Pisum sativum L.) Pod Extract Gel Film: Characterization and Application in Food Packaging. Gels, 9(2), 77. https://doi.org/10.3390/gels9020077







