Chitosan Based Aerogels with Low Shrinkage by Chemical Cross-Linking and Supramolecular Interaction
Abstract
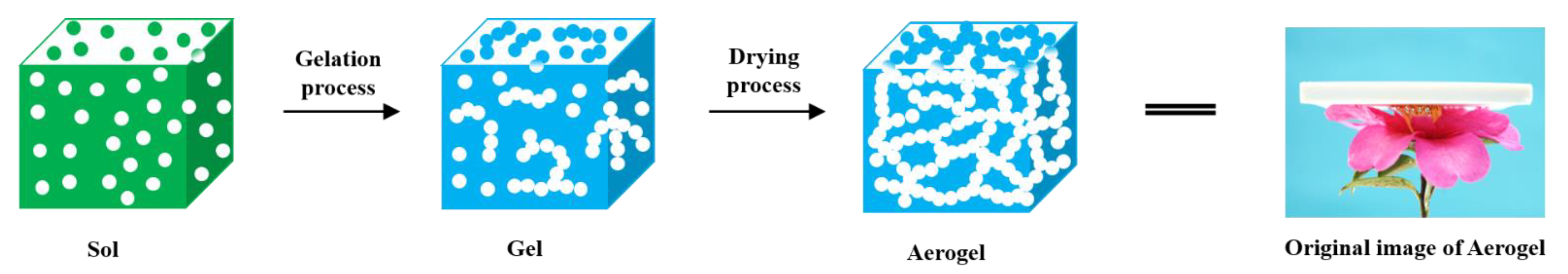
:1. Introduction
2. Results and Discussion
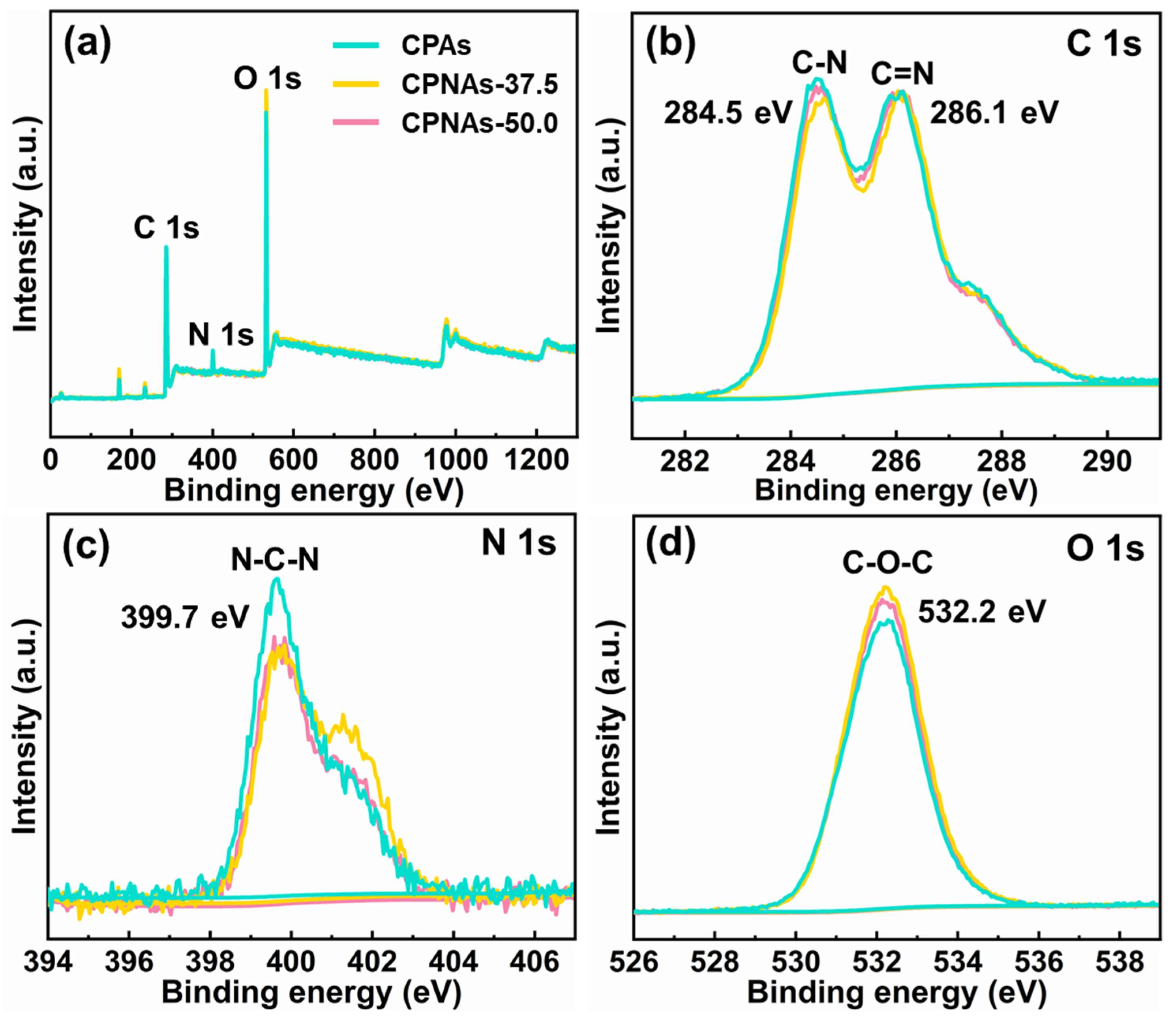
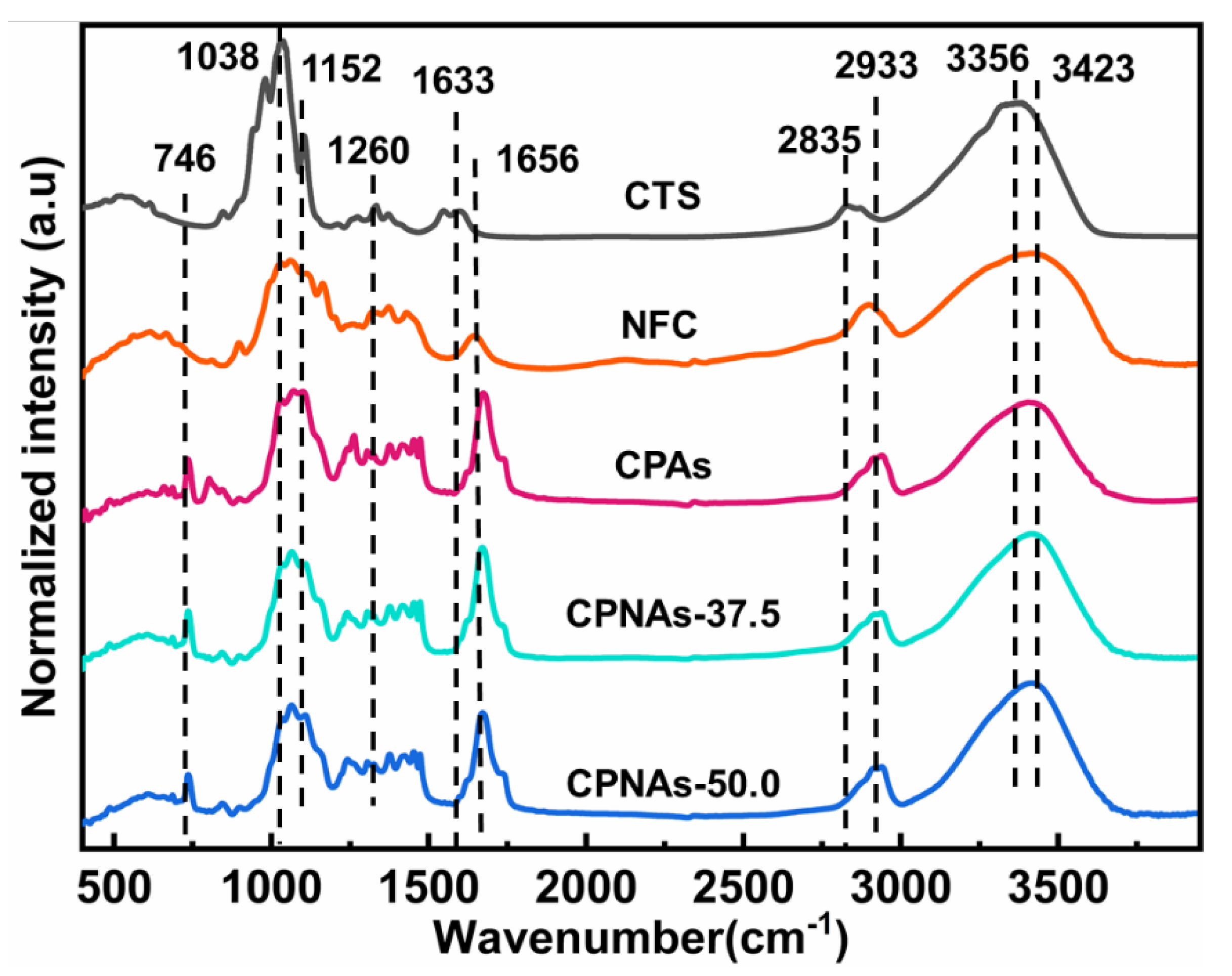
2.1. Proposed Reaction Process and Evidence Confirmation
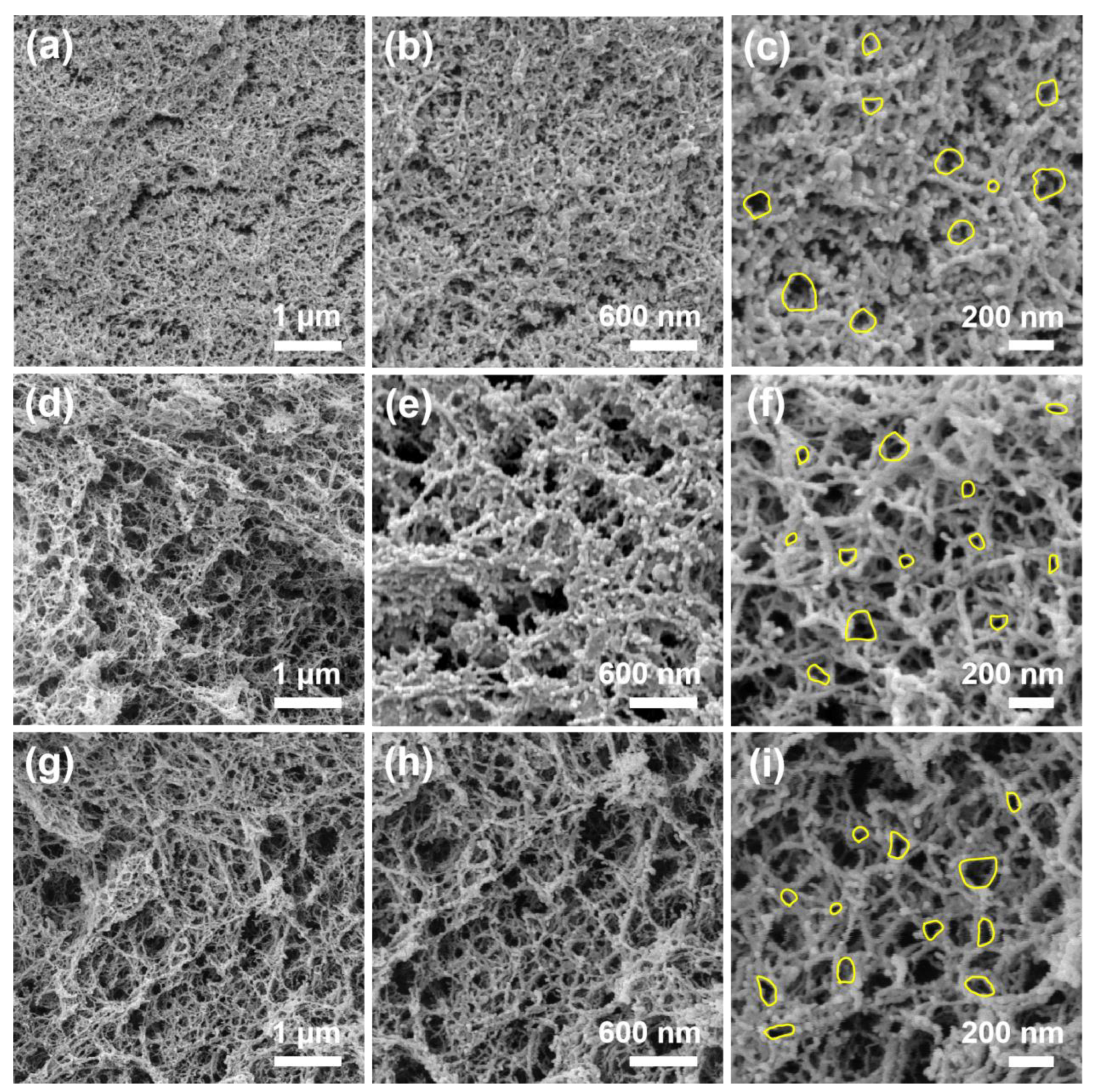
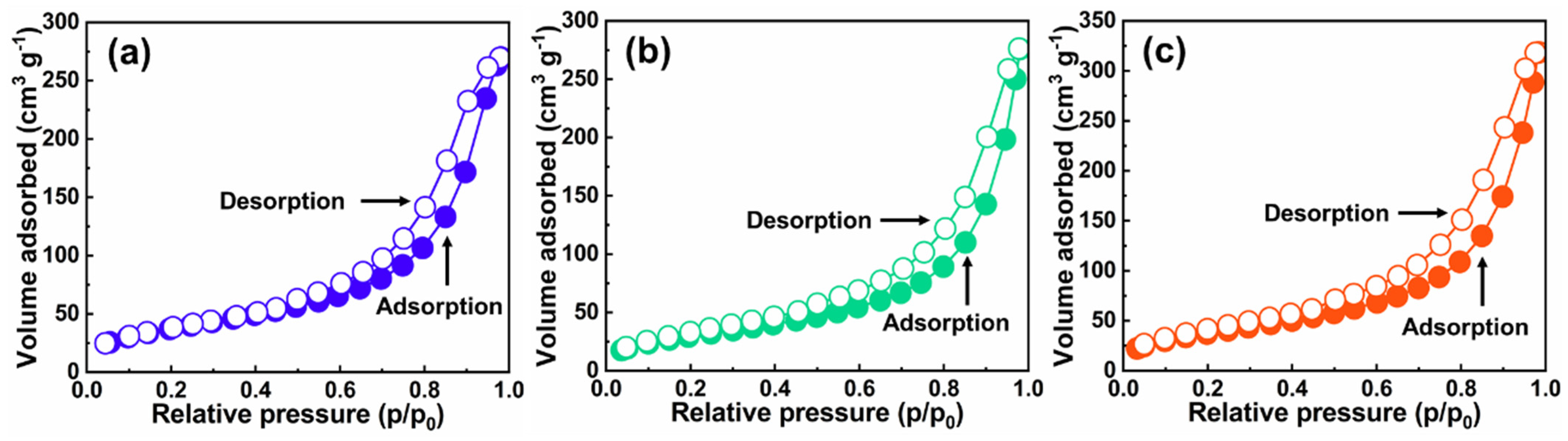
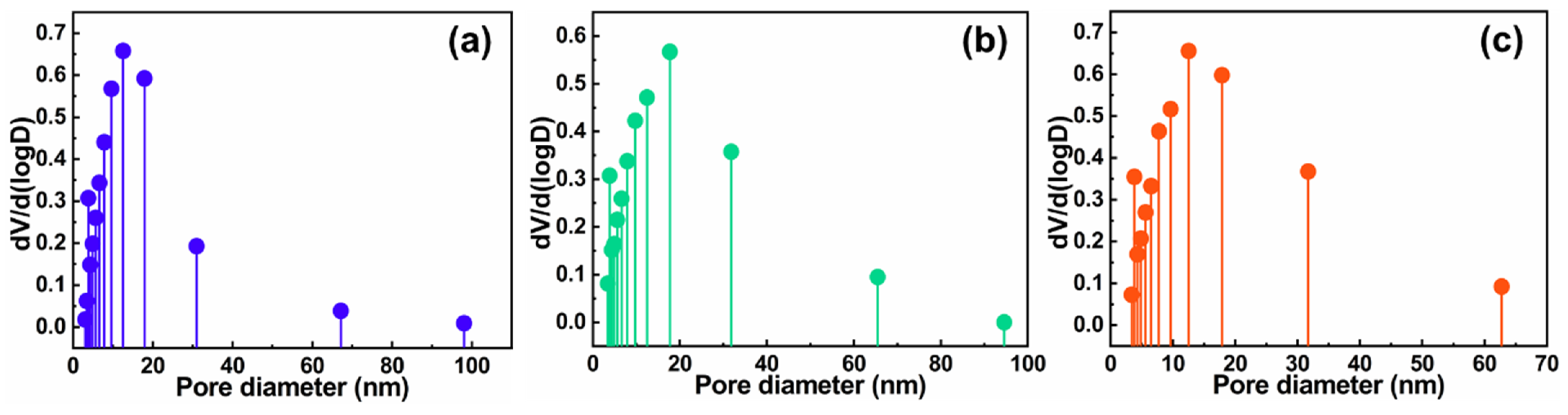
2.2. Nanostructure Textural Evaluation
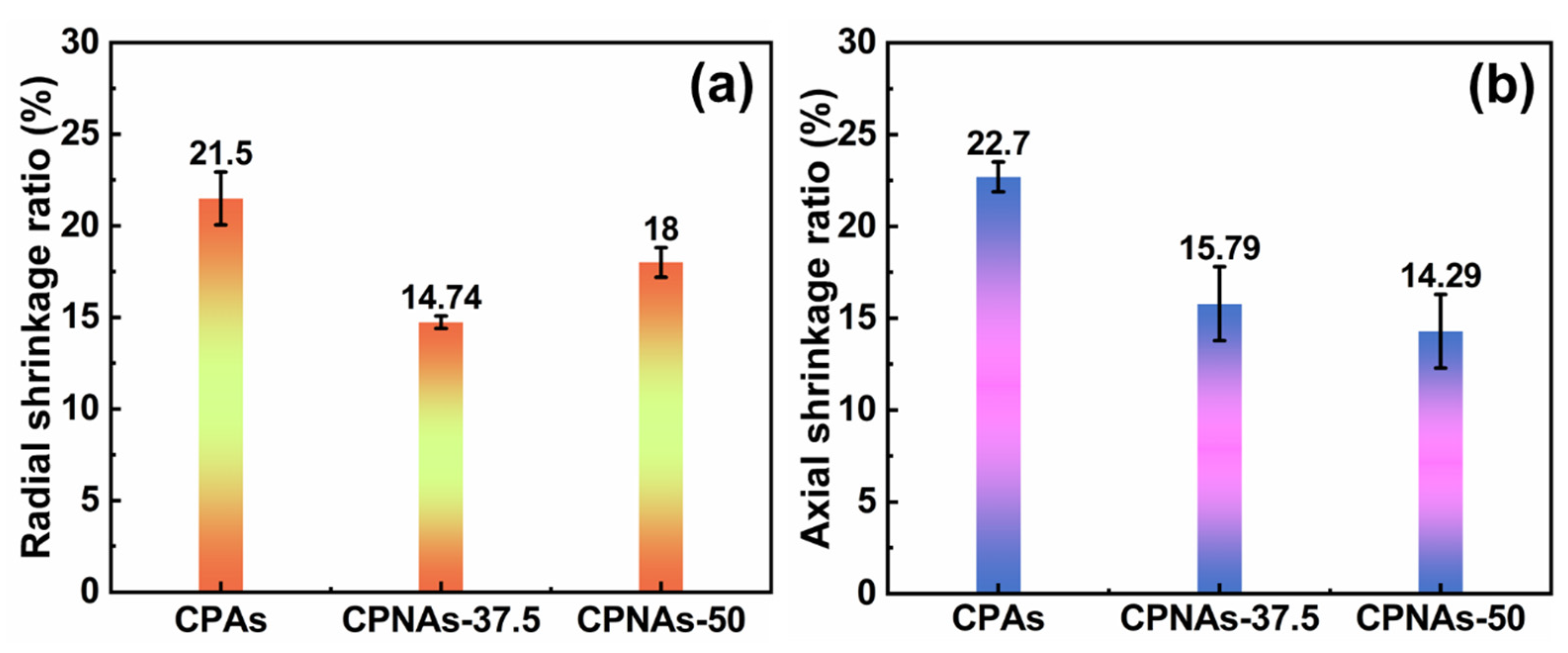
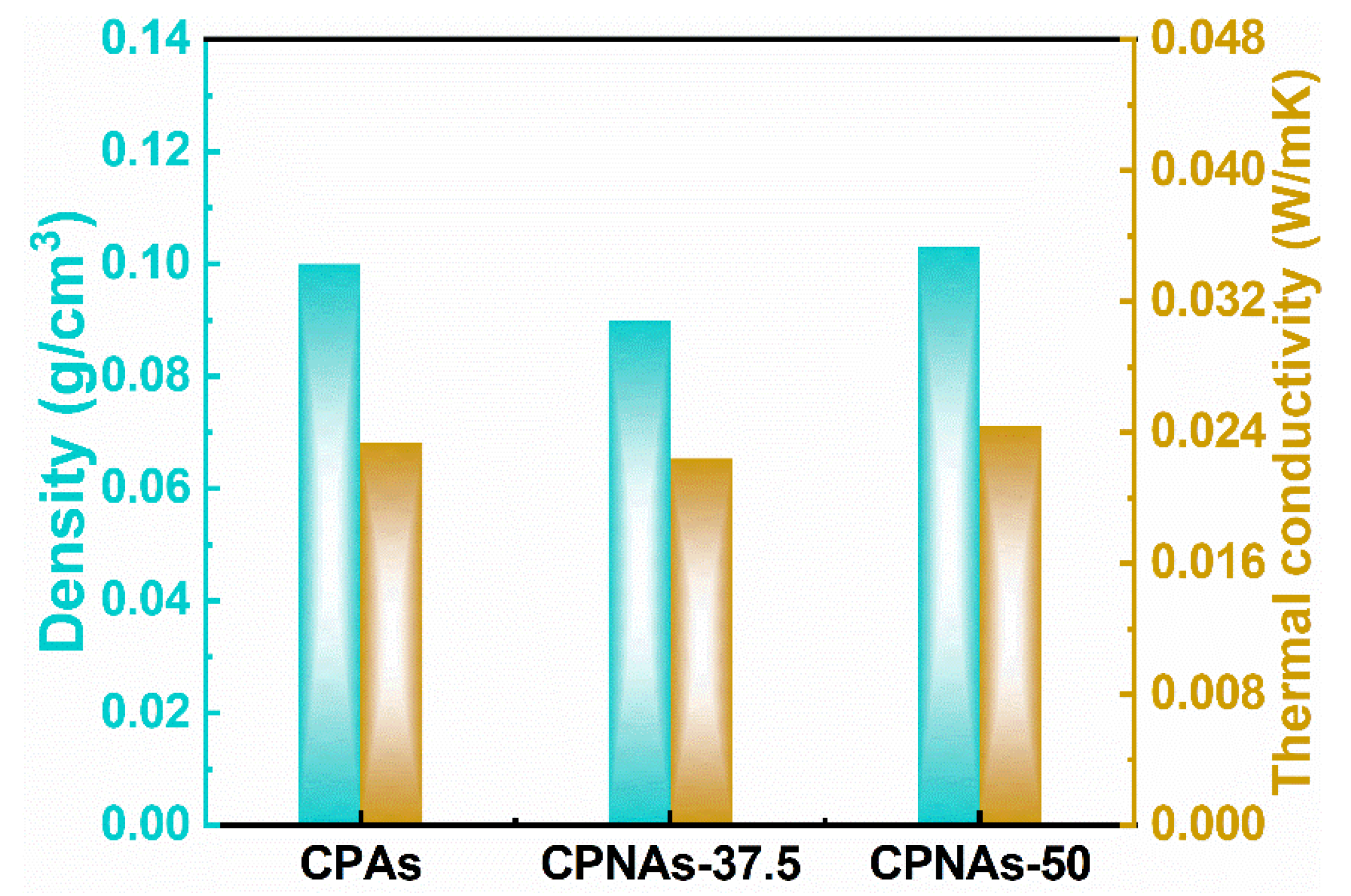
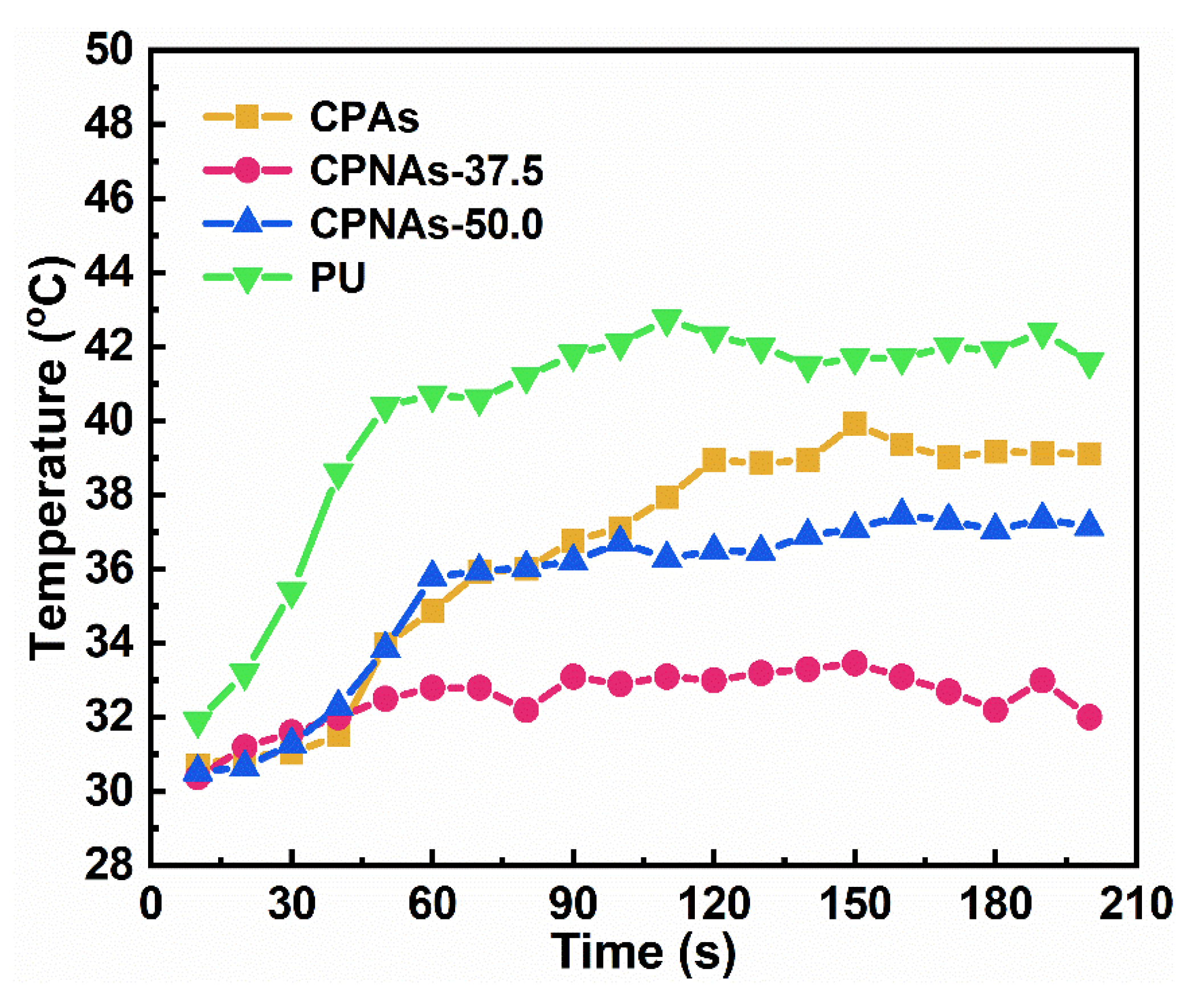
2.3. Physical Feature Assessment
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Fabrication of CPNAs
4.3. Characterization Measurements
4.3.1. Chemical Characteristic Evaluation
4.3.2. Surface Morphology
4.3.3. Nitrogen Adsorption-Desorption Test
4.3.4. Bulk Shrinkage and Density
4.3.5. Compression Measurement
4.3.6. Thermal Conductivity Measurement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Z.; Xu, W.; Wang, D.; Li, L.; Niu, L.; Wang, W.; Wang, B.; Song, Y. A Study of City-Level Building Energy Efficiency Benchmarking System for China. Energy Build. 2018, 179, 1–14. [Google Scholar] [CrossRef]
- Economidou, M.; Todeschi, V.; Bertoldi, P.; D’Agostino, D.; Zangheri, P.; Castellazzi, L. Review of 50 years of EU energy efficiency policies for buildings. Energy Build. 2020, 225, 110322. [Google Scholar] [CrossRef]
- Looney, B.; Dale, S. World Energy Outlook Special Report on Energy and Climate Change; International Energy Agency: Paris, France, 2021. [Google Scholar]
- Abu-Jdayil, B.; Mourad, A.H.; Hittini, W.; Hassan, M.; Hameedi, S. Traditional, State of the Art and Renewable Thermal Building Insulation Materials: An Overview. Constr. Build. Mater. 2019, 214, 709–735. [Google Scholar] [CrossRef]
- Xiong, X.; Yuan, Y.; Niu, Y.; Zhang, L. Development of a Cornstarch Adhesive for Laminated Veneer Lumber Bonding for Use in Engineered Wood Flooring. Int. J. Adhes. Adhes. 2020, 98, 102534. [Google Scholar]
- Muthuraj, R.; Lacoste, C.; Lacroix, P.; Bergeret, A. Sustainable thermal insulation biocomposites from rice husk, wheat husk, wood fibers and textile waste fibers: Elaboration and performances evaluation. Ind. Crop. Prod. 2019, 135, 238–245. [Google Scholar] [CrossRef]
- Smirnova, E.; Kot, S.; Kolpak, E.; Shestak, V. Governmental support and renewable energy production: A cross-country review. Energy 2021, 230, 120903. [Google Scholar] [CrossRef]
- Tran, D.T.; Nguyen, S.T.; Do, N.D.; Thai, N.N.T.; Thai, Q.B.; Huynh, H.K.P.; Nguyen, V.T.T.; Phan, A.N. Green aerogels from rice straw for thermal, acoustic insulation and oil spill cleaning applications. Mater. Chem. Phys. 2020, 253, 123363. [Google Scholar] [CrossRef]
- Zou, S.; Li, H.; Wang, S.; Jiang, R.; Zou, J.; Zhang, X.; Liu, L.; Zhang, G. Experimental research on an innovative sawdust biomass-based insulation material for buildings. J. Clean. Prod. 2020, 260, 121029. [Google Scholar] [CrossRef]
- Engelhardt, S.; Sarsour, J. Solar heat harvesting and transparent insulation in textile architecture inspired by polar bear fur. Energy Build. 2015, 103, 96–106. [Google Scholar] [CrossRef]
- Wi, S.; Park, J.H.; Kim, Y.U.; Yang, S.; Kim, S. Thermal, hygric, and environmental performance evaluation of thermal insulation materials for their sustainable utilization in buildings. Environ. Pollut. 2020, 272, 116033. [Google Scholar] [CrossRef]
- Mastali, M.; Zahra, A.; Hugo, K.; Faraz, R. Utilization of mineral wools in production of alkali activated materials. Constr. Build. Mater. 2021, 283, 122790. [Google Scholar] [CrossRef]
- Cho, H.M.; Park, J.H.; Wi, S.; Chang, S.; Yun, G.Y.; Kim, S. Energy Retrofit Analysis of Cross-Laminated Timber Residential Buildings in Seoul, Korea: Insights from a Case Study of Packages. Energy Build. 2019, 202, 109329. [Google Scholar] [CrossRef]
- Adhikary, S.K.; Ashish, D.K.; Rudžionis, Ž. Aerogel based thermal insulating cementitious composites: A review. Energy Build. 2021, 245, 111058. [Google Scholar] [CrossRef]
- Gao, H.; Liu, H.; Liao, L.; Mei, L.; Shuai, P.; Xi, Z.; Lv, G. A novel inorganic thermal insulation material utilizing perlite tailings. Energy Build. 2019, 190, 25–33. [Google Scholar] [CrossRef]
- Streimikiene, D.; Skulskis, V.; Balezentis, T.; Agnusdei, G.P. Uncertain multi-criteria sustainability assessment of green building insulation materials. Energy Build. 2020, 219, 110021. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, M.; Jiang, W.; Xu, Q.; Yu, J.; Liu, L.; Liu, L. Multiscale nanocelluloses hybrid aerogels for thermal insulation: The study on mechanical and thermal properties. Carbohydr. Polym. 2020, 247, 116701. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, Á. Investigation of the Thermal Insulation Performance of Fibrous Aerogel Samples Under Various Hygrothermal Environment: Laboratory Tests Completed with Calculations and Theory. Energy Build. 2020, 214, 109902. [Google Scholar] [CrossRef]
- Gonçalves, M.; Simões, N.; Serra, C.; Flores-Colen, I. A review of the challenges posed by the use of vacuum panels in external insulation finishing systems. Appl. Energy 2019, 257, 114028. [Google Scholar] [CrossRef]
- Mao, S.; Kan, A.; Wang, N. Numerical analysis and experimental investigation on thermal bridge effect of vacuum insulation panel. Appl. Therm. Eng. 2020, 169, 114980. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, G.; Xia, X.; Setunge, S.; Shi, L. A Review of Internal and External Influencing Factors on Energy Efficiency Design of Buildings. Energy Build. 2020, 216, 109944. [Google Scholar] [CrossRef]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohydr. Polym. 2019, 231, 115744. [Google Scholar] [CrossRef] [PubMed]
- Rizal, S.; Yahya, E.; Khalil, H.; Abdullah, C.; Marwan, M.; Ikramullah, I.; Muksin, U. Preparation and Characterization of Nanocellulose/Chitosan Aerogel Scaffolds Using Chemical-Free Approach. Gels 2021, 7, 246. [Google Scholar] [CrossRef]
- Guastaferro, M.; Reverchon, E.; Baldino, L. Polysaccharide-Based Aerogel Production for Biomedical Applications: A Comparative Review. Materials 2021, 14, 1631. [Google Scholar] [CrossRef] [PubMed]
- Esam, B.; Amirul, A.; Abdul, K.; Niyi, G.; Muhammad, O.; Fauziah, J.; Atty, S.; Adnan, A. Insights into the Role of Biopolymer Aerogel Scaffolds in Tissue Engineering and Regenerative Medicine. Polymers 2021, 13, 1612. [Google Scholar]
- Guerrero-Alburquerque, N.; Zhao, S.; Adilien, N.; Koebel, M.M.; Lattuada, M.; Malfait, W.J. Strong, Machinable, and Insulating Chitosan−Urea Aerogels: Toward Ambient Pressure Drying of Biopolymer Aerogel Monoliths. ACS Appl. Mater. Interfaces 2020, 12, 22037–22049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Feng, J.; Feng, J.; Jiang, Y. Formation of Enhanced Gelatum Using Ethanol/Water Binary Medium for Fabricating Chitosan Aerogels with High Specific Surface Area. Chem. Eng. J. 2017, 309, 700–707. [Google Scholar] [CrossRef]
- Robitzer, M.; Di Renzo, F.; Quignard, F. Natural materials with high surface area. Physisorption methods for the characterization of the texture and surface of polysaccharide aerogels. Microporous Mesoporous Mater. 2011, 140, 9–16. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, J.; Feng, J.; Jiang, Y.; Li, L. Ultra-low shrinkage chitosan aerogels trussed with polyvinyl alcohol. Mater. Des. 2018, 156, 398–406. [Google Scholar] [CrossRef]
- Zhang, S.; He, J.; Xiong, S.; Xiao, Q.; Xiao, Y.; Ding, F.; Ji, H.; Yang, Z.; Li, Z. Construction and Nanostructure of Chitosan/Nanocellulose Hybrid Aerogels. Biomacromolecules 2021, 22, 3216–3222. [Google Scholar] [CrossRef]
- Yousefi, K.; Manesh, H.D.; Khalifeh, A.; Moazami, F.; Sanaee, M. Nanocement/poly(vinyl alcohol) composites for endodontic applications. Mater. Chem. Phys. 2020, 254, 123337. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, J.; Feng, J.; Jiang, Y. Oxidation-Mediated Chitosan as Additives for Creation of Chitosan Aerogels with Diverse Three-Dimensional Interconnected Skeletons. Appl. Surf. Sci. 2017, 396, 1220–1225. [Google Scholar] [CrossRef]
- Chen, X.; Ware, H.O.T.; Baker, E.; Chu, W.; Hu, J.; Sun, C. The Development of an All-Polymer-Based Piezoelectric Photocurable Resin for Additive Manufacturing. Procedia CIRP 2017, 65, 157–162. [Google Scholar] [CrossRef]
- Guan, Q.F.; Han, Z.M.; Zhu, Y.B.; Xu, W.L.; Yang, H.B.; Ling, Z.C.; Yan, B.B.; Yang, K.P.; Yin, C.H.; Wu, H.A.; et al. Bio-Inspired Lotus-Fiber-Like Spiral Hydrogel Bacterial Cellulose Fibers. Nano Lett. 2021, 21, 952–958. [Google Scholar] [CrossRef]
- Wu, K.; Dong, W.; Pan, Y.; Cao, J.; Zhang, Y.; Long, D. Lightweight and Flexible Phenolic Aerogels with Three-Dimensional Foam Reinforcement for Acoustic and Thermal Insulation. Ind. Eng. Chem. Res. 2021, 60, 1241–1249. [Google Scholar] [CrossRef]
- Sakuma, W.; Yamasaki, S.; Fujisawa, S.; Kodama, T.; Shiomi, J.; Kanamori, K.; Saito, T. Mechanically Strong, Scalable, Mesoporous Xerogels of Nanocellulose Featuring Light Permeability, Thermal Insulation, and FlameSelf-Extinction. ACS Nano 2021, 15, 1436–1444. [Google Scholar] [CrossRef]
- Garemark, J.; Yang, X.; Sheng, X.; Cheung, O.; Sun, L.; Berglund, L.A.; Li, Y. Top-Down Approach Making Anisotropic Cellulose Aerogels as Universal Substrates for Multifunctionalization. ACS Nano 2020, 14, 7111–7120. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.; Chen, Z.; Peng, X.; Lai, H.; Liu, L.; Liu, Q.; Liu, C.; Zhong, L. Linking Renewable Cellulose Nanocrystal into Lightweight and Highly Elastic Carbon Aerogel. ACS Sustain. Chem. Eng. 2020, 8, 11921–11929. [Google Scholar] [CrossRef]
- Takeshita, S.; Yoda, S. Chitosan Aerogels: Transparent, Flexible Thermal Insulators. Chem. Mater. 2015, 27, 7569–7572. [Google Scholar] [CrossRef]
- Noroozi, M.; Panahi-Sarmad, M.; Abrisham, M.; Amirkiai, A.; Asghari, N.; Golbaten-Mofrad, H.; Karimpour-Motlagh, N.; Goodarzi, V.; Bahramian, A.R.; Zahiri, B. Nanostructure of Aerogels and Their Applications in Thermal Energy Insulation. ACS Appl. Energy Mater. 2019, 2, 5319–5349. [Google Scholar] [CrossRef]
- Gurikov, P.; Raman, S.P.; Griffin, J.S.; Steiner, S.A., III; Smirnova, I. 110th Anniversary: Solvent Exchange in the Processing of Biopolymer Aerogels: Current Status and Open Questions. Ind. Eng. Chem. Res. 2019, 58, 18590–18600. [Google Scholar] [CrossRef]
- Zhao, S.; Siqueira, G.; Drdova, S.; Norris, D.; Ubert, C.; Bonnin, A.; Galmarini, S.; Ganobjak, M.; Pan, Z.; Brunner, S.; et al. Additive Manufacturing of Silica Aerogels. Nature 2020, 584, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Hüsing, N.; Schubert, U. Aerogels-Airy Materials: Chemistry, Structure, and Properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Gu, W.; Wang, G.; Zhou, M.; Zhang, T.; Ji, G. Polyimide-Based Foams: Fabrication and Multifunctional Applications. ACS Appl. Mater. Interfaces 2020, 12, 48246–48258. [Google Scholar] [CrossRef]
- Zhao, T.; Tan, X.; Song, L.; Guo, L.; Liu, Y.; Kang, X.; Meng, X.; Wang, H.; Chu, W. Up-Scalable Conversion of White-Waste Polystyrene Foams to Sulfur, Phosphorus-Codoped Porous Carbon for High-Performance Lithium-Sulfur Batteries. ACS Appl. Energy Mater. 2020, 3, 9369–9378. [Google Scholar] [CrossRef]
- Zeng, Z.; Wu, T.; Han, D.; Ren, Q.; Siqueira, G.; Nyström, G. Ultralight, Flexible, and Biomimetic Nanocellulose/Silver Nanowire Aerogels for Electromagnetic Interference Shielding. ACS Nano 2020, 14, 2927–2938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, X.; Feng, J.; Qi, F.; Dianyu, E.; Jiang, Y.; Li, L.; Xiong, S.; Feng, J. Structure, compression and thermally insulating properties of cellulose diacetate-based aerogels. Mater. Des. 2020, 189, 108502. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Xiao, Q.; Xiao, Y.; Li, Z.; Xiong, S.; Ding, F.; He, J. Chitosan Based Aerogels with Low Shrinkage by Chemical Cross-Linking and Supramolecular Interaction. Gels 2022, 8, 131. https://doi.org/10.3390/gels8020131
Zhang S, Xiao Q, Xiao Y, Li Z, Xiong S, Ding F, He J. Chitosan Based Aerogels with Low Shrinkage by Chemical Cross-Linking and Supramolecular Interaction. Gels. 2022; 8(2):131. https://doi.org/10.3390/gels8020131
Chicago/Turabian StyleZhang, Sizhao, Qi Xiao, Yunyun Xiao, Zhengquan Li, Shixian Xiong, Feng Ding, and Junpeng He. 2022. "Chitosan Based Aerogels with Low Shrinkage by Chemical Cross-Linking and Supramolecular Interaction" Gels 8, no. 2: 131. https://doi.org/10.3390/gels8020131
APA StyleZhang, S., Xiao, Q., Xiao, Y., Li, Z., Xiong, S., Ding, F., & He, J. (2022). Chitosan Based Aerogels with Low Shrinkage by Chemical Cross-Linking and Supramolecular Interaction. Gels, 8(2), 131. https://doi.org/10.3390/gels8020131






