Maillard-Type Protein–Polysaccharide Conjugates and Electrostatic Protein–Polysaccharide Complexes as Delivery Vehicles for Food Bioactive Ingredients: Formation, Types, and Applications
Abstract
:1. Introduction
2. Formation and Characterization of Maillard-Type Protein–Polysaccharide Conjugates and Electrostatic Complexes
2.1. Maillard-Type Protein–Polysaccharide Conjugates
2.1.1. Formation of Covalent Protein–Polysaccharide Conjugates by Maillard Reaction
2.1.2. Characterization of Maillard-Type Protein–Polysaccharide Conjugates
2.1.3. Functional Properties of Maillard-Type Protein–Polysaccharide Conjugates
Solubility and Thermal Stability
Emulsifying and Stabilizing Properties
2.2. Electrostatic Protein–Polysaccharide Complexes
2.2.1. Formation of Electrostatic Protein–Polysaccharide Complexes
2.2.2. Rheological and Structural Characteristics of Electrostatic Protein–Polysaccharide Complexes
3. Different Types of Protein–Polysaccharide Complex- or Conjugate-Based Delivery Systems
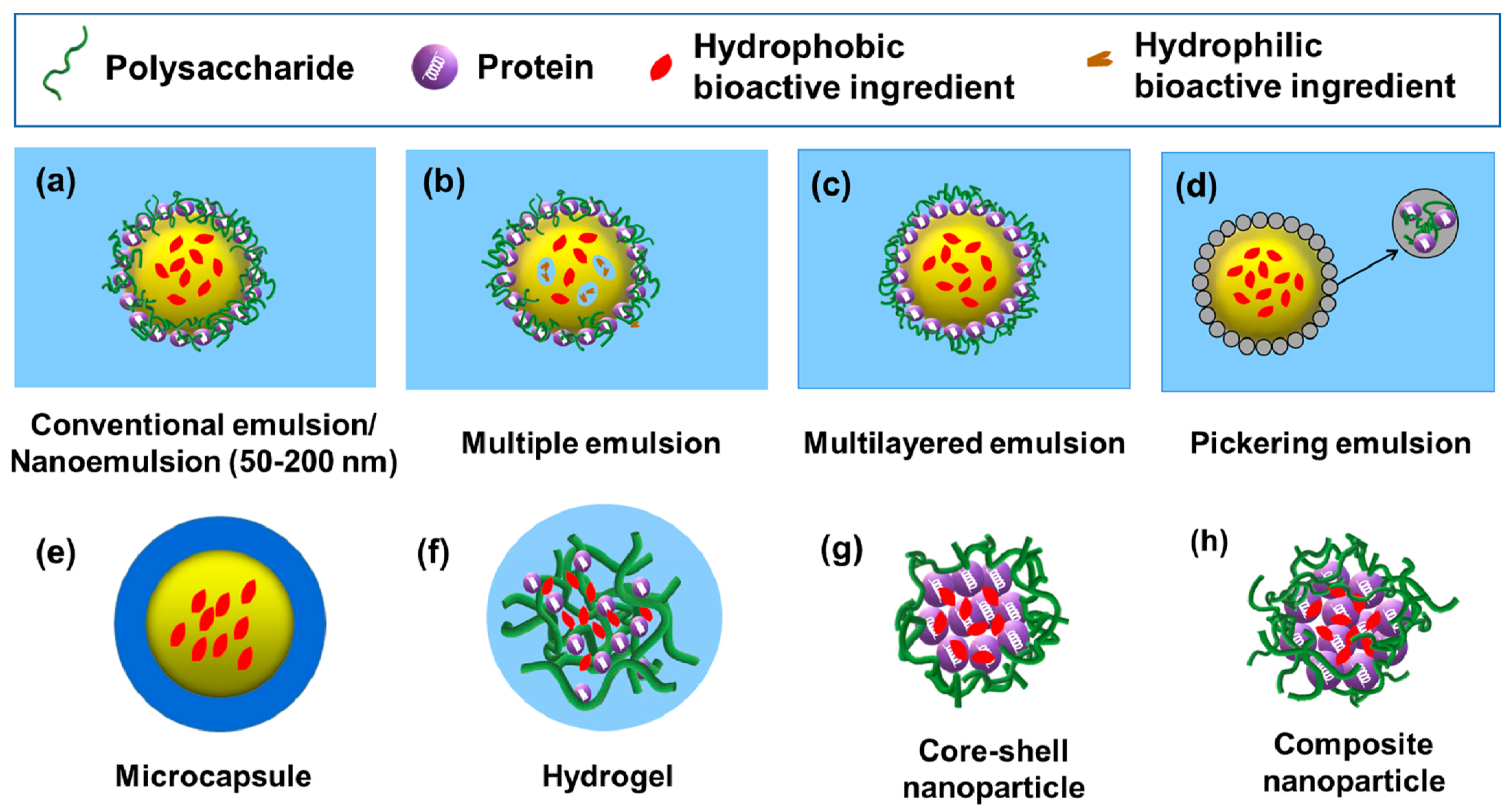
3.1. Emulsion-Based Delivery Systems
3.1.1. Conventional O/W Emulsions
3.1.2. Nanoemulsions (O/W)
3.1.3. Multiple Emulsions
3.1.4. Multilayered Emulsions
3.1.5. Pickering Emulsions
3.2. Microcapsule-Based Delivery Systems
3.3. Hydrogel-Based Delivery Systems
3.4. Nanoparticle-Based Delivery Systems
4. Applications of Protein–Polysaccharide Complexes/Conjugates as Delivery Systems for Food Bioactive Ingredients
4.1. Polyphenols
4.1.1. Curcumin
4.1.2. Resveratrol
4.2. Proteins and Bioactive Peptides
4.3. Carotenoids
4.3.1. Lutein
4.3.2. β-Carotene
4.3.3. Lycopene
4.4. Vitamins
4.5. Mineral (Iron)
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Z.; Huang, Q. Assembly of protein–polysaccharide complexes for delivery of bioactive ingredients: A perspective paper. J. Agric. Food Chem. 2019, 67, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Rajendran, S.R.; He, Q.S.; Bazinet, L.; Udenigwe, C.C. Encapsulation of food protein hydrolysates and peptides: A review. Rsc Adv. 2015, 5, 79270–79278. [Google Scholar] [CrossRef]
- Ru, Q.; Wang, Y.; Lee, J.; Ding, Y.; Huang, Q. Turbidity and rheological properties of bovine serum albumin/pectin coacervates: Effect of salt concentration and initial protein/polysaccharide ratio. Carbohyd. Polym. 2012, 88, 838–846. [Google Scholar] [CrossRef]
- de Oliveira, F.C.; Coimbra, J.S.d.R.; de Oliveira, E.B.; Zuñiga, A.D.G.; Rojas, E.E.G. Food protein-polysaccharide conjugates obtained via the Maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef]
- Naik, R.R.; Wang, Y.; Selomulya, C. Improvements of plant protein functionalities by Maillard conjugation and Maillard reaction products. Crit. Rev. Food Sci. Nutr. 2021, 1–26. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Y.; Yue, W.; Qin, W.; Dong, H.; Vasanthan, T. Nanostructures of protein-polysaccharide complexes or conjugates for encapsulation of bioactive compounds. Trends Food Sci. Technol. 2021, 109, 169–196. [Google Scholar] [CrossRef]
- Maillard, L. Action of amino acids on sugars. Formation of melanoidins in a methodical way. Compte-Rendu L’acad. Sci. 1912, 154, 66–68. [Google Scholar]
- Akhtar, M.; Ding, R. Covalently cross-linked proteins & polysaccharides: Formation, characterisation and potential applications. Curr. Opin. Colloid Interface Sci. 2017, 28, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Nooshkam, M.; Varidi, M. Maillard conjugate-based delivery systems for the encapsulation, protection, and controlled release of nutraceuticals and food bioactive ingredients: A review. Food Hydrocoll. 2020, 100, 105389. [Google Scholar] [CrossRef]
- Silván, J.M.; Assar, S.H.; Srey, C.; Del Castillo, M.D.; Ames, J.M. Control of the Maillard reaction by ferulic acid. Food Chem. 2011, 128, 208–213. [Google Scholar] [CrossRef]
- Zhu, D.; Damodaran, S.; Lucey, J.A. Physicochemical and emulsifying properties of whey protein isolate (WPI)—Dextran conjugates produced in aqueous solution. J. Agric. Food Chem. 2010, 58, 2988–2994. [Google Scholar] [CrossRef]
- He, W.; Tian, L.; Zhang, S.; Pan, S. A novel method to prepare protein-polysaccharide conjugates with high grafting and low browning: Application in encapsulating curcumin. LWT 2021, 145, 111349. [Google Scholar] [CrossRef]
- Usui, M.; Tamura, H.; Nakamura, K.; Ogawa, T.; Muroshita, M.; Azakami, H.; Kanuma, S.; Kato, A. Enhanced bactericidal action and masking of allergen structure of soy protein by attachment of chitosan through Maillard-type protein-polysaccharide conjugation. Nahrung 2004, 48, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ogawa, M.; Nakai, S.; Kato, A.; Kitts, D.D. Antioxidant activity of a Maillard-type Phosvitin—Galactomannan conjugate with emulsifying properties and heat stability. J. Agric. Food Chem. 1998, 46, 3958–3963. [Google Scholar] [CrossRef]
- Gentile, L. Protein-polysaccharide interactions and aggregates in food formulations. Curr. Opin. Colloid Interface Sci. 2020, 48, 18–27. [Google Scholar] [CrossRef]
- Kato, A.; Minaki, K.; Kobayashi, K. Improvement of emulsifying properties of egg white proteins by the attachment of polysaccharide through Maillard reaction in a dry state. J. Agric. Food Chem. 1993, 41, 540–543. [Google Scholar] [CrossRef]
- Bi, B.; Yang, H.; Fang, Y.; Nishinari, K.; Phillips, G.O. Characterization and emulsifying properties of β-lactoglobulin-gum Acacia Seyal conjugates prepared via the Maillard reaction. Food Chem. 2017, 214, 614–621. [Google Scholar] [CrossRef]
- Ledesma-Osuna, A.I.; Ramos-Clamont, G.; Guzman-Partida, A.M.; Vazquez-Moreno, L. Conjugates of bovine serum albumin with chitin oligosaccharides prepared through the Maillard reaction. J. Agric. Food Chem. 2010, 58, 12000–12005. [Google Scholar] [CrossRef]
- Sheng, L.; Su, P.; Han, K.; Chen, J.; Cao, A.; Zhang, Z.; Jin, Y.; Ma, M. Synthesis and structural characterization of lysozyme–pullulan conjugates obtained by the Maillard reaction. Food Hydrocoll. 2017, 71, 1–7. [Google Scholar] [CrossRef]
- Guan, Y.-G.; Lin, H.; Han, Z.; Wang, J.; Yu, S.-J.; Zeng, X.-A.; Liu, Y.-Y.; Xu, C.-H.; Sun, W.-W. Effects of pulsed electric field treatment on a bovine serum albumin–dextran model system, a means of promoting the Maillard reaction. Food Chem. 2010, 123, 275–280. [Google Scholar] [CrossRef]
- Wang, Q.; Ismail, B. Effect of Maillard-induced glycosylation on the nutritional quality, solubility, thermal stability and molecular configuration of whey proteinv. Int. Dairy J. 2012, 25, 112–122. [Google Scholar] [CrossRef]
- Doost, A.S.; Nasrabadi, M.N.; Goli, S.A.H.; van Troys, M.; Dubruel, P.; De Neve, N.; Van der Meeren, P. Maillard conjugation of whey protein isolate with water-soluble fraction of almond gum or flaxseed mucilage by dry heat treatment. Food Res. Int. 2020, 128, 108779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirestani, S.; Nasirpour, A.; Keramat, J.; Desobry, S.; Jasniewski, J. Structural properties of canola protein isolate-gum Arabic Maillard conjugate in an aqueous model system. Food Hydrocoll. 2018, 79, 228–234. [Google Scholar] [CrossRef]
- Xu, Z.-Z.; Huang, G.-Q.; Xu, T.-C.; Liu, L.-N.; Xiao, J.-X. Comparative study on the Maillard reaction of chitosan oligosaccharide and glucose with soybean protein isolate. Int. J. Biol. Macromol. 2019, 131, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, W.; Yan, T.; Wang, D.; Hou, F.; Miao, S.; Liu, D. Comparison of citrus pectin and apple pectin in conjugation with soy protein isolate (SPI) under controlled dry-heating conditions. Food Chem. 2020, 309, 125501. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Tang, G.; Wang, Q.; Zou, J.; Ma, M.; Huang, X. Molecular characteristics and foaming properties of ovalbumin-pullulan conjugates through the Maillard reaction. Food Hydrocoll. 2020, 100, 105384. [Google Scholar] [CrossRef]
- Jiménez-Castaño, L.; Villamiel, M.; López-Fandiño, R. Glycosylation of individual whey proteins by Maillard reaction using dextran of different molecular mass. Food Hydrocoll. 2007, 21, 433–443. [Google Scholar] [CrossRef]
- Al-Hakkak, J.; Al-Hakkak, F. Functional egg white-pectin conjugates prepared by controlled Maillard reaction. J. Food Eng. 2010, 100, 152–159. [Google Scholar] [CrossRef]
- Wang, L.; Wu, M.; Liu, H.-M. Emulsifying and physicochemical properties of soy hull hemicelluloses-soy protein isolate conjugates. Carbohyd. Polym. 2017, 163, 181–190. [Google Scholar] [CrossRef]
- Seo, C.W.; Yoo, B. Preparation of milk protein isolate/κ-carrageenan conjugates by maillard reaction in wet-heating system and their application to stabilization of oil-in-water emulsions. LWT 2021, 139, 110542. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Qin, W.; Gu, J.; Zhang, H.; Duan, Y.; Ma, H. Structure and functional properties of soy protein isolate-lentinan conjugates obtained in Maillard reaction by slit divergent ultrasonic assisted wet heating and the stability of oil-in-water emulsions. Food Chem. 2020, 331, 127374. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hou, F.; Zhao, H.; Wang, D.; Chen, W.; Miao, S.; Liu, D. Conjugation of soy protein isolate (SPI) with pectin by ultrasound treatment. Food Hydrocoll. 2020, 108, 106056. [Google Scholar] [CrossRef]
- Jian, W.; He, J.; Sun, Y.; Pang, J. Comparative studies on physicochemical properties of bovine serum albumin-glucose and bovine serum albumin-mannose conjugates formed via Maillard reaction. LWT Food Sci. Technol. 2016, 69, 358–364. [Google Scholar] [CrossRef]
- Zhou, Y.; Niu, H.; Luo, T.; Yun, Y.; Zhang, M.; Chen, W.; Zhong, Q.; Zhang, H.; Chen, H.; Chen, W. Effect of glycosylation with sugar beet pectin on the interfacial behaviour and emulsifying ability of coconut protein. Int. J. Biol. Macromol. 2021, 183, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, Y.; Sun, Q.; Wang, J.; Zheng, B.; Guo, Z. Structural characteristics and emulsifying properties of myofibrillar protein-dextran conjugates induced by ultrasound Maillard reaction. Ultrason. Sonochem. 2021, 72, 105458. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.N.; Nickerson, M.T. Review on plant protein-polysaccharide complex coacervation, and the functionality and applicability of formed complexes. J. Sci. Food Agric. 2018, 98, 5559–5571. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhao, Y.; Ding, J.; Lin, S. Investigation on complex coacervation between fish skin gelatin from cold-water fish and gum arabic: Phase behavior, thermodynamic, and structural properties. Food Res. Int. 2018, 107, 596–604. [Google Scholar] [CrossRef]
- Eghbal, N.; Yarmand, M.S.; Mousavi, M.; Degraeve, P.; Oulahal, N.; Gharsallaoui, A. Complex coacervation for the development of composite edible films based on LM pectin and sodium caseinate. Carbohyd. Polym. 2016, 151, 947–956. [Google Scholar] [CrossRef]
- Dong, D.; Hua, Y. Glycinin-gum arabic complex formation: Turbidity measurement and charge neutralization analysis. Food Res. Int. 2016, 89, 709–715. [Google Scholar] [CrossRef]
- Pillai, P.K.S.; Stone, A.K.; Guo, Q.; Guo, Q.; Wang, Q.; Nickerson, M.T. Effect of alkaline de-esterified pectin on the complex coacervation with pea protein isolate under different mixing conditions. Food Chem. 2019, 284, 227–235. [Google Scholar] [CrossRef]
- Zinoviadou, K.G.; Scholten, E.; Moschakis, T.; Biliaderis, C.G. Properties of emulsions stabilised by sodium caseinate-chitosan complexes. Int. Dairy J. 2012, 26, 94–101. [Google Scholar] [CrossRef]
- Souza, C.J.F.; Garcia-Rojas, E.E. Interpolymeric complexing between egg white proteins and xanthan gum: Effect of salt and protein/polysaccharide ratio. Food Hydrocoll. 2017, 66, 268–275. [Google Scholar] [CrossRef]
- Souza, C.J.F.; da Costa, A.R.; Souza, C.F.; Tosin, F.F.S.; Garcia-Rojas, E.E. Complex coacervation between lysozyme and pectin: Effect of pH, salt, and biopolymer ratio. Int. J. Biol. Macromol. 2018, 107, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lee, J.; Wang, Y.-W.; Huang, Q. Composition and rheological properties of β-lactoglobulin/pectin coacervates: Effects of salt concentration and initial protein/polysaccharide ratio. Biomacromolecules 2007, 8, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani Gorji, E.; Waheed, A.; Ludwig, R.; Toca-Herrera, J.L.; Schleining, G.; Ghorbani Gorji, S. Complex Coacervation of Milk Proteins with Sodium Alginate. J. Agr. Food Chem. 2018, 66, 3210–3220. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Cui, B. Comparison of rheological properties of different protein/gum arabic complex coacervates. J. Food Process Eng. 2019, 42, e13196. [Google Scholar] [CrossRef]
- Hasanvand, E.; Rafe, A. Rheological and structural properties of rice bran protein-flaxseed (Linum usitatissimum L.) gum complex coacervates. Food Hydrocoll. 2018, 83, 296–307. [Google Scholar] [CrossRef]
- Souza, C.J.F.; Souza, C.S.F.; Heckert Bastos, L.P.; Garcia-Rojas, E.E. Interpolymer complexation of egg white proteins and carrageenan: Phase behavior, thermodynamics and rheological properties. Int. J. Biol. Macromol. 2018, 109, 467–475. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, T.; Li, S.; Wang, Z.; Wen, C.; Wang, H.; Yu, C.; Ji, C. Stability, microstructure, and digestibility of whey protein isolate-Tremella fuciformis polysaccharide complexes. Food Hydrocoll. 2019, 89, 379–385. [Google Scholar] [CrossRef]
- Derkach, S.R.; Kuchina, Y.A.; Kolotova, D.S.; Voron’ko, N.G. Polyelectrolyte Polysaccharide—Gelatin Complexes: Rheology and Structure. Polymers 2020, 12, 266. [Google Scholar] [CrossRef] [Green Version]
- Xu, A.Y.; Melton, L.D.; Ryan, T.M.; Mata, J.P.; Rekas, A.; Williams, M.A.K.; McGillivray, D.J. Effects of polysaccharide charge pattern on the microstructures of β-lactoglobulin-pectin complex coacervates, studied by SAXS and SANS. Food Hydrocoll. 2018, 77, 952–963. [Google Scholar] [CrossRef]
- Jin, W.; Wang, Z.; Peng, D.; Shen, W.; Zhu, Z.; Cheng, S.; Li, B.; Huang, Q. Effect of pulsed electric field on assembly structure of α-amylase and pectin electrostatic complexes. Food Hydrocoll. 2020, 101, 105547. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, S.; Gong, J.; Miller, S.S.; Wang, Q.; Hua, Y. Stability of citral in oil-in-water emulsions protected by a soy protein–polysaccharide Maillard reaction product. Food Res. Int. 2015, 69, 357–363. [Google Scholar] [CrossRef]
- Araiza-Calahorra, A.; Akhtar, M.; Sarkar, A. Recent advances in emulsion-based delivery approaches for curcumin: From encapsulation to bioaccessibility. Trends Food Sci. Technol. 2018, 71, 155–169. [Google Scholar] [CrossRef]
- Zhang, F.; Cai, X.; Ding, L.; Wang, S. Effect of pH, ionic strength, chitosan deacetylation on the stability and rheological properties of O/W emulsions formulated with chitosan/casein complexes. Food Hydrocoll. 2021, 111, 106211. [Google Scholar] [CrossRef]
- Zha, F.; Dong, S.; Rao, J.; Chen, B. Pea protein isolate-gum Arabic Maillard conjugates improves physical and oxidative stability of oil-in-water emulsions. Food Chem. 2019, 285, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Dudhe, R.; Sharma, P. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Yin, B.; Deng, W.; Xu, K.; Huang, L.; Yao, P. Stable nano-sized emulsions produced from soy protein and soy polysaccharide complexes. J. Colloid Interf. Sci. 2012, 380, 51–59. [Google Scholar] [CrossRef]
- Liu, Q.-R.; Qi, J.-R.; Yin, S.-W.; Wang, J.-M.; Guo, J.; Feng, J.-L.; Cheng, M.; Cao, J.; Weng, J.-Y.; Yang, X.-Q. The influence of heat treatment on acid-tolerant emulsions prepared from acid soluble soy protein and soy soluble polysaccharide complexes. Food Res. Int. 2016, 89, 211–218. [Google Scholar] [CrossRef]
- Khan, A.Y.; Talegaonkar, S.; Iqbal, Z.; Ahmed, F.J.; Khar, R.K. Multiple emulsions: An overview. Curr. Drug Deliv. 2006, 3, 429–443. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Ochando-Pulido, J.M.; Segura-Carretero, A.; Martinez-Ferez, A. Stabilization of W/O/W multiple emulsion loaded with Hibiscus sabdariffa extract through protein-polysaccharide complexes. LWT 2018, 90, 389–395. [Google Scholar] [CrossRef]
- Li, B.; Jiang, Y.; Liu, F.; Chai, Z.; Li, Y.; Li, Y.; Leng, X. Synergistic effects of whey protein–polysaccharide complexes on the controlled release of lipid-soluble and water-soluble vitamins in W1/O/W2 double emulsion systems. Int. J. Food Sci. Technol. 2012, 47, 248–254. [Google Scholar] [CrossRef]
- O’Regan, J.; Mulvihill, D.M. Sodium caseinate-maltodextrin conjugate stabilized double emulsions: Encapsulation and stability. Food Res. Int. 2010, 43, 224–231. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M.; Assadpour, E. Preparation of a multiple emulsion based on pectin-whey protein complex for encapsulation of saffron extract nanodroplets. Food Chem. 2017, 221, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Gharehbeglou, P.; Jafari, S.M.; Hamishekar, H.; Homayouni, A.; Mirzaei, H. Pectin-whey protein complexes vs. small molecule surfactants for stabilization of double nano-emulsions as novel bioactive delivery systems. J. Food Eng. 2019, 245, 139–148. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Wandersleben, T.; Marqués, A.M.; Rubilar, M. Multilayer emulsions stabilized by vegetable proteins and polysaccharides. Curr. Opin. Colloid Interface Sci. 2016, 25, 51–57. [Google Scholar] [CrossRef]
- Shamsara, O.; Jafari, S.M.; Muhidinov, Z.K. Development of double layered emulsion droplets with pectin/β-lactoglobulin complex for bioactive delivery purposes. J. Mol. Liq. 2017, 243, 144–150. [Google Scholar] [CrossRef]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering emulsions: Preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J. Control Release 2019, 309, 302–332. [Google Scholar] [CrossRef]
- Santos, J.; Alcaide-González, M.A.; Trujillo-Cayado, L.A.; Carrillo, F.; Alfaro-Rodríguez, M.C. Development of food-grade Pickering emulsions stabilized by a biological macromolecule (xanthan gum) and zein. Int. J. Biol. Macromol. 2020, 153, 747–754. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Edible Pickering emulsions stabilized by ovotransferrin-gum arabic particles. Food Hydrocoll. 2019, 89, 590–601. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef] [Green Version]
- Timilsena, Y.P.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Digestion behaviour of chia seed oil encapsulated in chia seed protein-gum complex coacervates. Food Hydrocoll. 2017, 66, 71–81. [Google Scholar] [CrossRef]
- Jia, C.; Cao, D.; Ji, S.; Lin, W.; Zhang, X.; Muhoza, B. Whey protein isolate conjugated with xylo-oligosaccharides via maillard reaction: Characterization, antioxidant capacity, and application for lycopene microencapsulation. LWT 2020, 118, 108837. [Google Scholar] [CrossRef]
- Du, Y.-L.; Huang, G.-Q.; Wang, H.-O.; Xiao, J.-X. Effect of high coacervation temperature on the physicochemical properties of resultant microcapsules through induction of Maillard reaction between soybean protein isolate and chitosan. J. Food Eng. 2018, 234, 91–97. [Google Scholar] [CrossRef]
- Shaddel, R.; Hesari, J.; Azadmard-Damirchi, S.; Hamishehkar, H.; Fathi-Achachlouei, B.; Huang, Q. Use of gelatin and gum Arabic for encapsulation of black raspberry anthocyanins by complex coacervation. Int. J. Biol. Macromol. 2018, 107, 1800–1810. [Google Scholar] [CrossRef]
- Ozel, B.; Cikrikci, S.; Aydin, O.; Oztop, M.H. Polysaccharide blended whey protein isolate-(WPI) hydrogels: A physicochemical and controlled release study. Food Hydrocoll. 2017, 71, 35–46. [Google Scholar] [CrossRef]
- Le, X.T.; Rioux, L.-E.; Turgeon, S.L. Formation and functional properties of protein–polysaccharide electrostatic hydrogels in comparison to protein or polysaccharide hydrogels. Adv. Colloid Interface Sci. 2017, 239, 127–135. [Google Scholar] [CrossRef]
- Pei, Y.; Li, Z.; McClements, D.J.; Li, B. Comparison of structural and physicochemical properties of lysozyme/carboxymethylcellulose complexes and microgels. Food Res. Int. 2019, 122, 273–282. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, T.; Hu, Q.; Luo, Y. Low density lipoprotein/pectin complex nanogels as potential oral delivery vehicles for curcumin. Food Hydrocoll. 2016, 57, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Yao, P. Soy protein/soy polysaccharide complex nanogels: Folic acid loading, protection, and controlled delivery. Langmuir 2013, 29, 8636–8644. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Zhou, X.; Li, X.; Lin, W.; Chen, G.; Qiu, R. Self-assembled modified soy protein/dextran nanogel induced by ultrasonication as a delivery vehicle for riboflavin. Molecules 2016, 21, 282. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Huang, X.; Gong, Y.; Xiao, H.; McClements, D.J.; Hu, K. Enhancement of curcumin water dispersibility and antioxidant activity using core–shell protein–polysaccharide nanoparticles. Food Res. Int. 2016, 87, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Sun, C.; Dai, L.; Zhan, X.; Gao, Y. Structure, physicochemical stability and in vitro simulated gastrointestinal digestion properties of β-carotene loaded zein-propylene glycol alginate composite nanoparticles fabricated by emulsification-evaporation method. Food Hydrocoll. 2018, 81, 149–158. [Google Scholar] [CrossRef]
- Chen, G.; Dong, S.; Chen, Y.; Gao, Y.; Zhang, Z.; Li, S.; Chen, Y. Complex coacervation of zein-chitosan via atmospheric cold plasma treatment: Improvement of encapsulation efficiency and dispersion stability. Food Hydrocoll. 2020, 107, 105943. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Wang, X.; Zhang, X.; Li, Y.; Zhang, S. Microencapsulation of essential oils by complex coacervation method: Preparation, thermal stability, release properties and applications. Crit. Rev. Food Sci. Nutr. 2020, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, L.; Bao, X.; Yao, P. Curcumin, casein and soy polysaccharide ternary complex nanoparticles for enhanced dispersibility, stability and oral bioavailability of curcumin. Food Biosci. 2020, 35, 100569. [Google Scholar] [CrossRef]
- Ren, D.; Qi, J.; Xie, A.; Jia, M.; Yang, X.; Xiao, H. Encapsulation in lysozyme/A. Sphaerocephala Krasch polysaccharide nanoparticles increases stability and bioefficacy of curcumin. J. Funct. Foods 2017, 38, 100–109. [Google Scholar] [CrossRef]
- Wei, Y.; Cai, Z.; Wu, M.; Guo, Y.; Wang, P.; Li, R.; Ma, A.; Zhang, H. Core-shell pea protein-carboxymethylated corn fiber gum composite nanoparticles as delivery vehicles for curcumin. Carbohyd. Polym. 2020, 240, 116273. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Su, J.; Xie, W.; Tu, X.; Yuan, F.; Mao, L.; Gao, Y. Curcumin-loaded pea protein isolate-high methoxyl pectin complexes induced by calcium ions: Characterization, stability and in vitro digestibility. Food Hydrocoll. 2020, 98, 105284. [Google Scholar] [CrossRef]
- Okagu, O.D.; Verma, O.; McClements, D.J.; Udenigwe, C.C. Utilization of insect proteins to formulate nutraceutical delivery systems: Encapsulation and release of curcumin using mealworm protein-chitosan nano-complexes. Int. J. Biol. Macromol. 2020, 151, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Sarika, P.R.; James, N.R. Polyelectrolyte complex nanoparticles from cationised gelatin and sodium alginate for curcumin delivery. Carbohyd. Polym. 2016, 148, 354–361. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Alavi, F.; Momen, S.; Emam-Djomeh, Z.; Moosavi-Movahedi, A.A. Fabrication and Characterization of Curcumin-Loaded Complex Coacervates Made of Gum Arabic and Whey Protein Nanofibrils. Food Biophys. 2019, 14, 425–436. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Pei, Y.; Xiong, W.; Zhang, C.; Xu, W.; Liu, S.; Li, B. Curcumin encapsulated in the complex of lysozyme/carboxymethylcellulose and implications for the antioxidant activity of curcumin. Food Res. Int. 2015, 75, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Peng, G.; Zheng, S.; Wen, Z.; Gan, C.; Fan, Y. Fabrication of whey protein isolate-sodium alginate nanocomplex for curcumin solubilization and stabilization in a model fat-free beverage. Food Chem. 2021, 348, 129102. [Google Scholar] [CrossRef]
- Xie, H.; Xiang, C.; Li, Y.; Wang, L.; Zhang, Y.; Song, Z.; Ma, X.; Lu, X.; Lei, Q.; Fang, W. Fabrication of ovalbumin/κ-carrageenan complex nanoparticles as a novel carrier for curcumin delivery. Food Hydrocoll. 2019, 89, 111–121. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, L.; Tong, M.; Lu, Y.; Ouyang, X.-K.; Ling, J. Encapsulation of curcumin using fucoidan stabilized zein nanoparticles: Preparation, characterization, and in vitro release performance. J. Mol. Liq. 2021, 329, 115586. [Google Scholar] [CrossRef]
- Yan, J.-K.; Qiu, W.-Y.; Wang, Y.-Y.; Wu, J.-Y. Biocompatible Polyelectrolyte Complex Nanoparticles from Lactoferrin and Pectin as Potential Vehicles for Antioxidative Curcumin. J. Agr. Food Chem. 2017, 65, 5720–5730. [Google Scholar] [CrossRef]
- Xu, G.; Wang, C.; Yao, P. Stable emulsion produced from casein and soy polysaccharide compacted complex for protection and oral delivery of curcumin. Food Hydrocoll. 2017, 71, 108–117. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Xu, G.; Yin, B.; Yao, P. BSA-dextran emulsion for protection and oral delivery of curcumin. Food Hydrocoll. 2016, 61, 11–19. [Google Scholar] [CrossRef]
- Meena, S.; Prasad, W.; Khamrui, K.; Mandal, S.; Bhat, S. Preparation of spray-dried curcumin microcapsules using a blend of whey protein with maltodextrin and gum arabica and its in-vitro digestibility evaluation. Food Biosci. 2021, 41, 100990. [Google Scholar] [CrossRef]
- Su, J.; Cai, Y.; Zhi, Z.; Guo, Q.; Mao, L.; Gao, Y.; Yuan, F.; Van der Meeren, P. Assembly of propylene glycol alginate/β-lactoglobulin composite hydrogels induced by ethanol for co-delivery of probiotics and curcumin. Carbohyd. Polym. 2021, 254, 117446. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, L.; Yi, J.; Fan, Y.; Wu, X.; Zhang, Y. α-Lactalbumin and chitosan core-shell nanoparticles: Resveratrol loading, protection, and antioxidant activity. Food Funct. 2020, 11, 1525–1536. [Google Scholar] [CrossRef]
- Khan, M.A.; Yue, C.; Fang, Z.; Hu, S.; Cheng, H.; Bakry, A.M.; Liang, L. Alginate/chitosan-coated zein nanoparticles for the delivery of resveratrol. J. Food Eng. 2019, 258, 45–53. [Google Scholar] [CrossRef]
- Khan, M.A.; Chen, L.; Liang, L. Improvement in storage stability and resveratrol retention by fabrication of hollow zein-chitosan composite particles. Food Hydrocoll. 2021, 113, 106477. [Google Scholar] [CrossRef]
- Huang, X.; Dai, Y.; Cai, J.; Zhong, N.; Xiao, H.; McClements, D.J.; Hu, K. Resveratrol encapsulation in core-shell biopolymer nanoparticles: Impact on antioxidant and anticancer activities. Food Hydrocoll. 2017, 64, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Consoli, L.; Dias, R.A.O.; Rabelo, R.S.; Furtado, G.F.; Sussulini, A.; Cunha, R.L.; Hubinger, M.D. Sodium caseinate-corn starch hydrolysates conjugates obtained through the Maillard reaction as stabilizing agents in resveratrol-loaded emulsions. Food Hydrocoll. 2018, 84, 458–472. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Silva, H.D.; Soliva-Fortuny, R.; Martín-Belloso, O.; Vicente, A.A. Formation, stability and antioxidant activity of food-grade multilayer emulsions containing resveratrol. Food Hydrocoll. 2017, 71, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Raei, M.; Shahidi, F.; Farhoodi, M.; Jafari, S.M.; Rafe, A. Application of whey protein-pectin nano-complex carriers for loading of lactoferrin. Int. J. Biol. Macromol. 2017, 105, 281–291. [Google Scholar] [CrossRef]
- Mendanha, D.V.; Ortiz, S.E.M.; Favaro-Trindade, C.S.; Mauri, A.; Monterrey-Quintero, E.S.; Thomazini, M. Microencapsulation of casein hydrolysate by complex coacervation with SPI/pectin. Food Res. Int. 2009, 42, 1099–1104. [Google Scholar] [CrossRef]
- Jo, Y.-J.; van der Schaaf, U.S. Fabrication and characterization of double (W1/O/W2) emulsions loaded with bioactive peptide/polysaccharide complexes in the internal water (W1) phase for controllable release of bioactive peptide. Food Chem. 2021, 344, 128619. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ma, X.-Y.; Gong, W.; Li, X.; Huang, H.-B.; Zhu, X.-M. Nanoparticles based on carboxymethylcellulose-modified rice protein for efficient delivery of lutein. Food Funct. 2020, 11, 2380–2394. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Y.; Zhu, J.; Wang, T.; Wang, D.; Xu, Y. Zein/soluble soybean polysaccharide composite nanoparticles for encapsulation and oral delivery of lutein. Food Hydrocoll. 2020, 103, 105715. [Google Scholar] [CrossRef]
- Morales, E.A.C.; Doost, A.S.; Velazquez, G.; Van der Meeren, P. Comparison of low-and high-methoxyl pectin for the stabilization of whey protein isolate as carrier for lutein. Food Hydrocoll. 2021, 113, 106458. [Google Scholar] [CrossRef]
- Gumus, C.E.; Davidov-Pardo, G.; McClements, D.J. Lutein-enriched emulsion-based delivery systems: Impact of Maillard conjugation on physicochemical stability and gastrointestinal fate. Food Hydrocoll. 2016, 60, 38–49. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Wang, J.; Su, Y.; Chang, C.; Gu, L.; Yang, Y.; Li, J. Utilization of high internal phase emulsion stabilized by egg yolk-modified starch complex for the delivery of lutein. LWT 2021, 142, 111024. [Google Scholar] [CrossRef]
- Su, J.; Guo, Q.; Chen, Y.; Dong, W.; Mao, L.; Gao, Y.; Yuan, F. Characterization and formation mechanism of lutein pickering emulsion gels stabilized by β-lactoglobulin-gum arabic composite colloidal nanoparticles. Food Hydrocoll. 2020, 98, 105276. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, J.; Li, Y.; Nirasawa, S.; Cheng, Y. Conformation and emulsifying properties of deamidated wheat gluten-maltodextrin/citrus pectin conjugates and their abilities to stabilize β-carotene emulsions. Food Hydrocoll. 2019, 87, 129–141. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, Y.; Zhong, L.; Ma, N.; Zhao, L.; Ma, G.; Cheng, N.; Nakata, P.A.; Xu, J. In vitro digestion and cellular antioxidant activity of β-carotene-loaded emulsion stabilized by soy protein isolate-Pleurotus eryngii polysaccharide conjugates. Food Hydrocoll. 2021, 112, 106340. [Google Scholar] [CrossRef]
- Zhong, L.; Ma, N.; Wu, Y.; Zhao, L.; Ma, G.; Pei, F.; Hu, Q. Gastrointestinal fate and antioxidation of β-carotene emulsion prepared by oat protein isolate-Pleurotus ostreatus β-glucan conjugate. Carbohyd. Polym. 2019, 221, 10–20. [Google Scholar] [CrossRef]
- López-Monterrubio, D.; Lobato-Calleros, C.; Vernon-Carter, E.; Alvarez-Ramirez, J. Influence of β-carotene concentration on the physicochemical properties, degradation and antioxidant activity of nanoemulsions stabilized by whey protein hydrolyzate-pectin soluble complexes. LWT 2021, 111148. [Google Scholar] [CrossRef]
- Yi, J.; Gan, C.; Wen, Z.; Fan, Y.; Wu, X. Development of pea protein and high methoxyl pectin colloidal particles stabilized high internal phase pickering emulsions for β-carotene protection and delivery. Food Hydrocoll. 2021, 113, 106497. [Google Scholar] [CrossRef]
- Silva, D.; Favaro-Trindade, C.; Rocha, G.; Thomazini, M. Microencapsulation of lycopene by gelatin–pectin complex coacervation. J. Food Process. Pres. 2012, 36, 185–190. [Google Scholar] [CrossRef]
- Assadpour, E.; Maghsoudlou, Y.; Jafari, S.-M.; Ghorbani, M.; Aalami, M. Optimization of folic acid nano-emulsification and encapsulation by maltodextrin-whey protein double emulsions. Int. J. Biol. Macromol. 2016, 86, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Assadpour, E.; Jafari, S.-M.; Maghsoudlou, Y. Evaluation of folic acid release from spray dried powder particles of pectin-whey protein nano-capsules. Int. J. Biol. Macromol. 2017, 95, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Gao, J.; Ye, H.; Ren, G.; Ma, X.; Xie, H.; Fang, S.; Lei, Q.; Fang, W. Development of ovalbumin-pectin nanocomplexes for vitamin D3 encapsulation: Enhanced storage stability and sustained release in simulated gastrointestinal digestion. Food Hydrocoll. 2020, 106, 105926. [Google Scholar] [CrossRef]
- Kazemi-Taskooh, Z.; Varidi, M. Designation and characterization of cold-set whey protein-gellan gum hydrogel for iron entrapment. Food Hydrocoll. 2021, 111, 106205. [Google Scholar] [CrossRef]
- Yao, X.; Xu, K.; Shu, M.; Liu, N.; Li, N.; Chen, X.; Nishinari, K.; Phillips, G.O.; Jiang, F. Fabrication of iron loaded whey protein isolate/gum Arabic nanoparticles and its adsorption activity on oil-water interface. Food Hydrocoll. 2021, 115, 106610. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, Q.; Liu, Y.; Cai, Z. Enhancing the resistance of anthocyanins to environmental stress by constructing ovalbumin-propylene glycol alginate nanocarriers with novel configurations. Food Hydrocoll. 2021, 118, 106668. [Google Scholar] [CrossRef]
- Cuevas-Bernardino, J.C.; Leyva-Gutierrez, F.M.; Vernon-Carter, E.J.; Lobato-Calleros, C.; Román-Guerrero, A.; Davidov-Pardo, G. Formation of biopolymer complexes composed of pea protein and mesquite gum–Impact of quercetin addition on their physical and chemical stability. Food Hydrocoll. 2018, 77, 736–745. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Liu, C.; Zhu, J.; Fan, M.; Sun, X.; Wang, T.; Xu, Y.; Cao, Y. Fabrication of stable zein nanoparticles coated with soluble soybean polysaccharide for encapsulation of quercetin. Food Hydrocoll. 2019, 87, 342–351. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Li, X.; Zhang, Z.; Wei, Z.; Xing, Z.; Deng, S.; Duan, F. Okra polysaccharides/gelatin complex coacervate as pH-responsive and intestine-targeting delivery protects isoquercitin bioactivity. Int. J. Biol. Macromol. 2020, 159, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Han, Y.; Wang, Y.; Yang, X.; Sun, C.; Mao, L.; Gao, Y. Zein-hyaluronic acid binary complex as a delivery vehicle of quercetagetin: Fabrication, structural characterization, physicochemical stability and in vitro release property. Food Chem. 2019, 276, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lv, R.; Muhammad, A.I.; Guo, M.; Ding, T.; Ye, X.; Liu, D. Fabrication of (−)-epigallocatechin-3-gallate carrier based on glycosylated whey protein isolate obtained by ultrasound Maillard reaction. Ultrason. Sonochem. 2019, 58, 104678. [Google Scholar] [CrossRef]
- Fan, L.; Lu, Y.; Ouyang, X.-K.; Ling, J. Development and characterization of soybean protein isolate and fucoidan nanoparticles for curcumin encapsulation. Int. J. Biol. Macromol. 2021, 169, 194–205. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Okagu, O.D.; Jin, J.; Udenigwe, C.C. Impact of succinylation on pea protein-curcumin interaction, polyelectrolyte complexation with chitosan, and gastrointestinal release of curcumin in loaded-biopolymer nano-complexes. J. Mol. Liq. 2021, 325, 115248. [Google Scholar] [CrossRef]
- Yi, J.; Fan, Y.; Zhang, Y.; Wen, Z.; Zhao, L.; Lu, Y. Glycosylated α-lactalbumin-based nanocomplex for curcumin: Physicochemical stability and DPPH-scavenging activity. Food Hydrocoll. 2016, 61, 369–377. [Google Scholar] [CrossRef]
- Cho, H.; Lee, H.J.; Yu, K.S.; Choi, Y.M.; Hwang, K.T. Characterisation and food application of curcumin bound to sodium caseinate–polysaccharide electrostatic complexes. Int. J. Food Sci. Technol. 2017, 52, 1770–1776. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, H.; Li, D.; Duan, H.; Liang, L. Impact of oil type on the location, partition and chemical stability of resveratrol in oil-in-water emulsions stabilized by whey protein isolate plus gum Arabic. Food Hydrocoll. 2020, 109, 106119. [Google Scholar] [CrossRef]
- Ren, X.; Hou, T.; Liang, Q.; Zhang, X.; Hu, D.; Xu, B.; Chen, X.; Chalamaiah, M.; Ma, H. Effects of frequency ultrasound on the properties of zein-chitosan complex coacervation for resveratrol encapsulation. Food Chem. 2019, 279, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udenigwe, C.C. Bioinformatics approaches, prospects and challenges of food bioactive peptide research. Trends Food Sci. Technol. 2014, 36, 137–143. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Abioye, R.O.; Okagu, I.U.; Obeme-Nmom, J.I. Bioaccessibility of bioactive peptides: Recent advances and perspectives. Curr. Opin. Food Sci. 2021, 39, 182–189. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, T.; Mu, Y.; Jing, H.; Obadi, M.; Xu, B. Enhancing the stabilization of β-carotene emulsion using ovalbumin-dextran conjugates as emulsifier. Colloids Surfaces A 2021, 626, 126806. [Google Scholar] [CrossRef]
- Dhakal, S.P.; He, J. Microencapsulation of vitamins in food applications to prevent losses in processing and storage: A review. Food Res. Int. 2020, 137, 109326. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Gao, J.; Liu, C.; Shi, J.; Ni, F.; Shen, Q.; Xie, H.; Wang, K.; Lei, Q.; Fang, W.; Ren, G. The regulation of sodium alginate on the stability of ovalbumin-pectin complexes for VD3 encapsulation and in vitro simulated gastrointestinal digestion study. Food Res. Int. 2021, 140, 110011. [Google Scholar] [CrossRef]
- Sun, X.; Sarteshnizi, R.A.; Boachie, R.T.; Okagu, O.D.; Abioye, R.O.; Pfeilsticker Neves, R.; Ohanenye, I.C.; Udenigwe, C.C. Peptide-Mineral Complexes: Understanding Their Chemical Interactions, Bioavailability, and Potential Application in Mitigating Micronutrient Deficiency. Foods 2020, 9, 1402. [Google Scholar] [CrossRef] [PubMed]
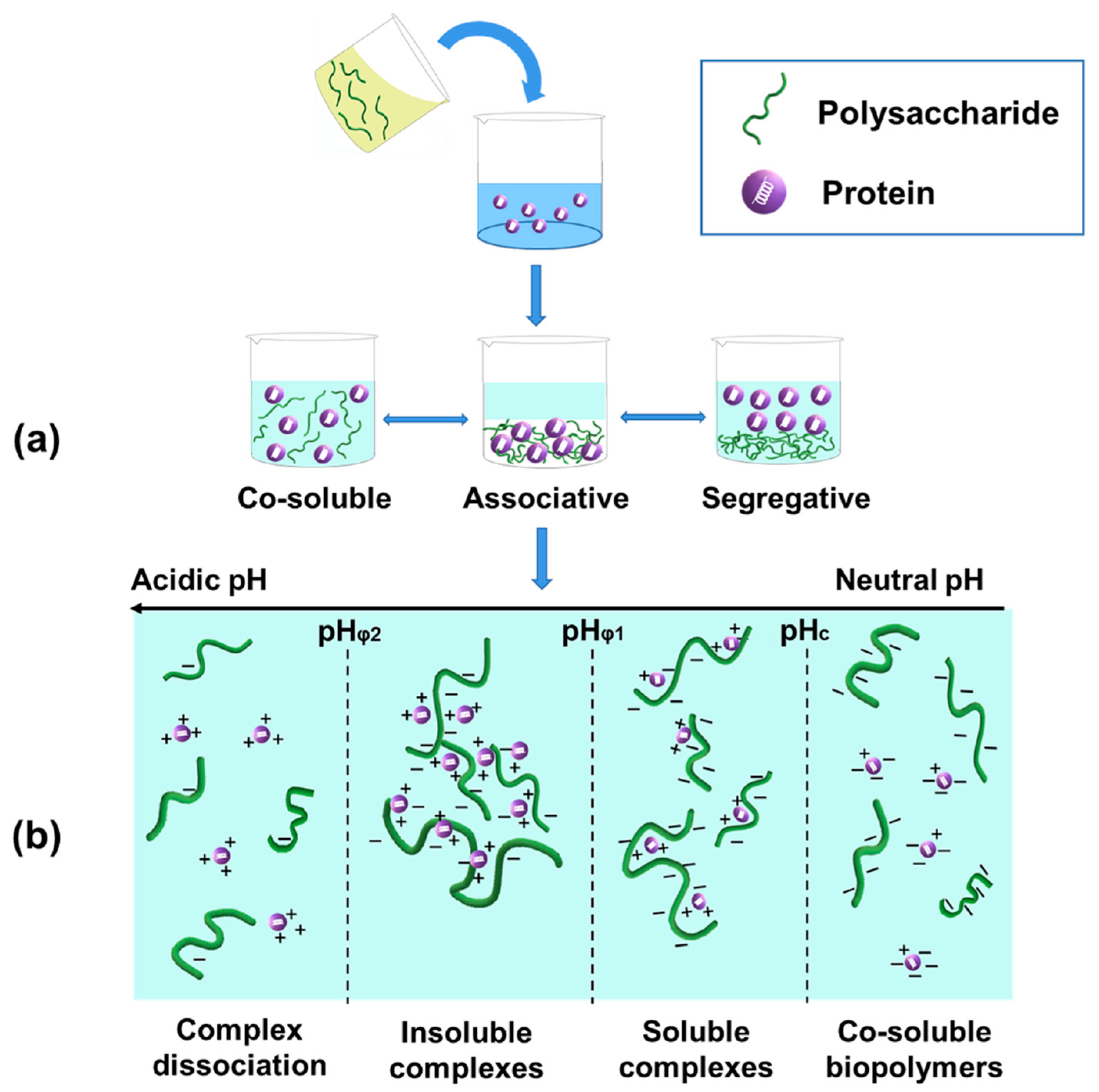

| Maillard-Type Protein–Polysaccharide Conjugates | Electrostatic Protein–Polysaccharide Complexes | |
|---|---|---|
| Formation | Covalent bonding between reducing ends of carbohydrates and amino groups of proteins [4,8,9,10] | Different phase systems between proteins and polysaccharides, including co-soluble biopolymers, complex coacervation, and thermodynamic incompatibility [36] |
| Structural characteristics | Secondary structures analyzed by CD [19,22] and FTIR [23,24] | Secondary structures analyzed by CD [49] and FTIR [50]; Microstructures analyzed by Cryo-SEM [49], SAXS and SANS [51] |
| Functional properties | Enhanced functional properties compared to native proteins: water solubility [25,27]; thermal stability [29]; emulsifying property [25,31,32]; emulsifying stability [25,29,34] | Rheological properties [46,47,48] |
| Types | Common Preparation Methods | Features | Nature of Commonly Encapsulated Compounds | References |
|---|---|---|---|---|
| Conventional O/W emulsions | High-energy methods (high-shear mixers or high-pressure homogenizers) | Mean droplet radii (0.2–100 μm); thermodynamically unstable systems | Lipophilic | [53,54,55,56] |
| Nanoemulsions (O/W) | High-energy methods (high-speed blenders, high-pressure homogenizers, microfluidizers or ultrasonic probes); Low-energy methods (phase inversion and solvent mixing approaches) | Mean droplet radii (50–200 nm); thermodynamically stable isotropic systems | Lipophilic | [54,57,58,59] |
| Multiple emulsions (W1/O/W2) | Producing primary W/O emulsions before generating W1/O/W2 emulsions | Presence of both water and oil compartments | Hydrophilic and lipophilic | [60,61,62,63,64,65] |
| Multilayered emulsions (O/W) | Layer-by-layer (LbL) electrostatic deposition technique | Stabilized by a multilayered interfacial membrane; good physical stability to environmental stresses | Lipophilic | [54,66,67] |
| Pickering emulsions (O/W) | High-energy methods (Rotor-stator homogenization, high-pressure homogenization, sonication) | Stabilized by solid particles; long-term physical stability | Lipophilic | [54,68,69,70] |
| Microcapsules | Emulsion-spray drying; double emulsion–complex coacervation method | Containing a membrane shell | Lipophilic | [71,72,73,74,75] |
| Hydrogels | Complex coacervation and thermal treatment to induce gelation | Three-dimensional networks; polymer crosslinking through physical, ionic or covalent interactions; including microgels (d. μm 1–350) and nanogels (d. nm 20–250) | Hydrophilic | [76,77,78,79,80,81,82] |
| Core-shell nanoparticles | Coating protein nanoparticles with polysaccharides | Including protein inner core and polysaccharide shell layer | Lipophilic | [1,83] |
| Composite nanoparticles | Anti-solvent precipitation; emulsification–evaporation method | Formation of the protein–polysaccharide complexes prior to loading of bioactive compounds | Lipophilic | [1,84,85] |
| Bioactive Ingredient | Composition of Delivery System 1 | Type of Delivery System | Improved Properties of Encapsulated Bioactive Ingredient | References |
|---|---|---|---|---|
| Polyphenols | ||||
| Curcumin | Casein-soy polysaccharide | Core-shell nanoparticle | Long-term dispersion stability; oral bioavailability | [87] |
| Curcumin | Lysozyme-A. Sphaerocephala Krasch polysaccharide; pea protein–carboxymethylated corn fiber gum; pea protein isolate–high methoxyl pectin | Core-shell nanoparticle | Chemical, thermal, and photo stabilities | [88,89,90] |
| Curcumin | Insect protein–chitosan | Core-shell nanoparticle | Release profile | [91] |
| Curcumin | Cationised gelatin–sodium alginate; whey protein nanofibril–gum arabic | Core-shell nanoparticle | In vitro antioxidant and anticancer activities | [92,93] |
| Curcumin | Whey protein isolate–sodium alginate; ovalbumin–κ-carrageenan | Composite nanoparticle | Dispersion, light and chemical stabilities | [94,95,96] |
| Curcumin | Zein–fucoidan | Composite nanoparticle | Sustained release | [97] |
| Curcumin | Lactoferrin–pectin | Composite nanoparticle | In vitro antioxidant activities | [98] |
| Curcumin | Casein–soy soluble polysaccharide | Nanoemulsion | Storage stability; controlled release; oral bioavailability | [99] |
| Curcumin | Bovine serum albumin–dextran conjugate | Nanoemulsion | Chemical stability; oral bioavailability | [100] |
| Curcumin | Whey protein–maltodextrin and gum arabic | Microcapsule | Sustained release | [101] |
| Curcumin | β-lactoglobulin–propylene glycol alginate | Hydrogel | Sustained release; light and storage stabilities | [102] |
| Resveratrol | α-lactalbumin–chitosan; | Core-shell nanoparticle | Light, heat and storage stabilities | [103] |
| Resveratrol | Zein–alginate/chitosan; zein–chitosan | Core-shell nanoparticle | Sustained release; bioaccessibility; storage stability | [104,105] |
| Resveratrol | Zein–pectin; α-lactalbumin–chitosan | Core-shell nanoparticle | In vitro antioxidant and anticancer activities | [103,106] |
| Resveratrol | Sodium caseinate–corn starch hydrolysate conjugate | O/W emulsion | In vitro antioxidant activities | [107] |
| Resveratrol | Lactoferrin–alginate | Multilayered emulsion | In vitro antioxidant activity | [108] |
| Proteins and bioactive peptides | ||||
| Lactoferrin | Whey protein isolate–high methoxyl pectin | Nanoparticle | Not determined | [109] |
| Casein hydrolysate | Soybean protein isolate–pectin | Microcapsule | Attenuated bitter taste; decreased hygroscopicity | [110] |
| Bioactive peptide | Bioactive peptide–pectin/chitosan | Double emulsion | Controlled release | [111] |
| Carotenoids | ||||
| Lutein | Modified rice protein–carboxymethylcellulose | Core-shell nanoparticle | Controlled release; inhibited the proliferation of breast cancer cells; increased the lutein uptake rate and absorption | [112] |
| Lutein | Zein–soluble soybean polysaccharide | Core-shell nanoparticle | Bioaccessibility | [113] |
| Lutein | Whey protein isolate–pectin | Core-shell nanoparticle | Storage stability | [114] |
| Lutein | Casein–dextrin conjugate | O/W emulsion | Dispersion stability | [115] |
| Lutein | Egg yolk–modified starch | O/W emulsion | Physical and storage stabilities; low lipid oxidation | [116] |
| Lutein | β-lactoglobulin-gum arabic | Pickering emulsion | Storage stability | [117] |
| β-Carotene | Soy protein isolate–Pleurotus eryngii polysaccharide conjugate; wheat gluten–maltodextrin/citrus pectin conjugate; oat protein isolate–Pleurotus ostreatus β-glucan conjugate | O/W emulsion | Bioaccessibiliy; in vitro antioxidant activity | [118,119,120] |
| β-Carotene | Whey protein hydrolysate–pectin | Nanoemulsion | Storage stability; in vitro antioxidant activity | [121] |
| β-Carotene | Pea protein–high methoxyl pectin | Pickering emulsion | pH stability | [122] |
| Lycopene | Gelatin–pectin | Microcapsule | No desirable storage stability | [123] |
| Lycopene | Whey protein isolate–xylo-oilgosaccharide conjugate | Microcapsule | Storage stability; bioaccessibility | [73] |
| Vitamins | ||||
| Folic acid | Soy protein–soy polysaccharide | Nanogel | Water dispersibility at acidic conditions; chemical, light and heat stabilities | [81] |
| Folic acid | Whey protein–maltodextrin | Double emulsion | Not determined | [124] |
| Folic acid | Whey protein–pectin | Double emulsion | Sustained release | [125] |
| Vitamin D3 | Ovalbumin–pectin | Microcapsule | Sustained release | [126] |
| Mineral | ||||
| Iron | Whey protein isolate–gellan gum | Hydrogel | Burst release in simulated gastric digestion | [127] |
| Iron | Whey protein isolate–gum arabic | Nanoparticle | Sustained release | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Wang, H.; Li, S.; Song, C.; Zhang, S.; Ren, J.; Udenigwe, C.C. Maillard-Type Protein–Polysaccharide Conjugates and Electrostatic Protein–Polysaccharide Complexes as Delivery Vehicles for Food Bioactive Ingredients: Formation, Types, and Applications. Gels 2022, 8, 135. https://doi.org/10.3390/gels8020135
Sun X, Wang H, Li S, Song C, Zhang S, Ren J, Udenigwe CC. Maillard-Type Protein–Polysaccharide Conjugates and Electrostatic Protein–Polysaccharide Complexes as Delivery Vehicles for Food Bioactive Ingredients: Formation, Types, and Applications. Gels. 2022; 8(2):135. https://doi.org/10.3390/gels8020135
Chicago/Turabian StyleSun, Xiaohong, Hao Wang, Shengnan Li, Chunli Song, Songyuan Zhang, Jian Ren, and Chibuike C. Udenigwe. 2022. "Maillard-Type Protein–Polysaccharide Conjugates and Electrostatic Protein–Polysaccharide Complexes as Delivery Vehicles for Food Bioactive Ingredients: Formation, Types, and Applications" Gels 8, no. 2: 135. https://doi.org/10.3390/gels8020135
APA StyleSun, X., Wang, H., Li, S., Song, C., Zhang, S., Ren, J., & Udenigwe, C. C. (2022). Maillard-Type Protein–Polysaccharide Conjugates and Electrostatic Protein–Polysaccharide Complexes as Delivery Vehicles for Food Bioactive Ingredients: Formation, Types, and Applications. Gels, 8(2), 135. https://doi.org/10.3390/gels8020135








