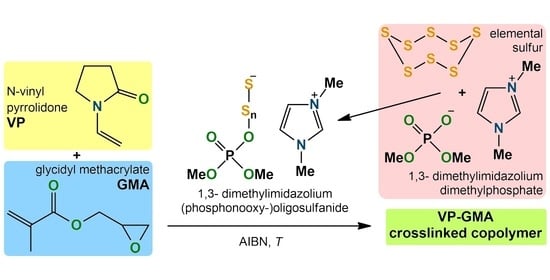
Formation of Hydrogels Based on a Copolymer of N-Vinyl-2-pyrrolidone and Glycidyl Methacrylate in the Presence of the Reaction Product of 1,3-Dimethylmidazolium Dimethylphosphate and Elemental Sulfur
Abstract
:1. Introduction
2. Results and Discussion
2.1. Interaction between GMA and POOS
2.2. Synthesis and Characterization of VP and GMA Copolymers Obtained in the Presence of POOS
2.3. Swelling Kinetics and Crosslink Density
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Structure Characterization Methods
4.3. Reaction of GMA and POOS
4.4. Synthesis of the VP-GMA-POOS Copolymers
4.5. Testing the Swelling Kinetics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, G.; Zhang, T.; Ahmad, S.; Cheng, J.; Guo, M. Physicochemical and adhesive properties, microstructure and storage stability of whey protein-based paper glue. Int. J. Adhes. Adhes. 2013, 41, 198–205. [Google Scholar] [CrossRef]
- Morozov, V.N.; Mikheev, A.Y. Water-soluble polyvinylpyrrolidone nanofilters manufactured by electrospray-neutralization technique. J. Membr. Sci. 2012, 403–404, 110–120. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Khafagy, R.M.; Bishay, S.T.; Saleh, N.M. Effective dye removal and water purification using the electric and magnetic Zn0.5Co0.5Al0.5Fe1.46La0.04O4/polymer core–shell nanocomposites. J. Alloys Compd. 2013, 578, 121–131. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. The Use of Poly(N-vinyl pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M. Poly(vinylpyrrolidone)—A Versatile Polymer for Biomedical and Beyond Medical Applications. Polym. Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Papagiorgis, P.G.; Manoli, A.; Alexiou, A.; Karacosta, P.; Karagiorgis, X.; Papaparaskeva, G.; Bernasconi, C.; Bodnarchuk, M.I.; Kovalenko, M.V.; Krasia-Christoforou, T.; et al. Robust Hydrophobic and Hydrophilic Polymer Fibers Sensitized by Inorganic and Hybrid Lead Halide Perovskite Nanocrystal Emitters. Front. Chem. 2019, 7, 87. [Google Scholar] [CrossRef]
- Grünig, L.; Handge, U.A.; Koll, J.; Gronwald, O.; Weber, M.; Hankiewicz, B.; Scharnagl, N.; Abetz, V. Hydrophilic Dual Layer Hollow Fiber Membranes for Ultrafiltration. Membranes 2020, 10, 143. [Google Scholar] [CrossRef]
- Alpatova, A.; Kim, E.-S.; Sun, X.; Hwang, G.; Liu, Y.; El-Din, M.G. Fabrication of porous polymeric nanocomposite membranes with enhanced anti-fouling properties: Effect of casting composition. J. Membr. Sci. 2013, 444, 449–460. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, K.; Yao, J.; Webley, P.A.; Smart, S.; Wang, H. Effect of the addition of polyvinylpyrrolidone as a pore-former on microstructure and mechanical strength of porous alumina ceramics. Ceram. Int. 2013, 39, 7551–7556. [Google Scholar] [CrossRef]
- Wardani, A.K.; Ariono, D.; Subagjo, S.; Wenten, I.G. Fouling tendency of PDA/PVP surface modified PP membrane. Surf. Interfaces 2020, 19, 100464. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, T.; Kolli, V.; Karmakar, S.; Sarkar, N. Functionalisation of Polyvinylpyrrolidone on Gold Nanoparticles Enhances Its Anti-Amyloidogenic Propensity towards Hen Egg White Lysozyme. Biomedicines 2017, 5, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demeter, M.; Călina, I.; Scărișoreanu, A.; Micutz, M. E-Beam Cross-Linking of Complex Hydrogels Formulation: The Influence of Poly(Ethylene Oxide) Concentration on the Hydrogel Properties. Gels 2022, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Burkert, S.; Schmidt, T.; Gohs, U.; Dorschner, H.; Arndt, K. Cross-linking of poly(N-vinyl pyrrolidone) films by electron beam irradiation. Radiat. Phys. Chem. 2007, 76, 1324–1328. [Google Scholar] [CrossRef]
- Lopérgolo, L.; Lugão, A.; Catalani, L. Direct UV photocrosslinking of poly(N-vinyl-2-pyrrolidone) (PVP) to produce hydrogels. Polymer 2003, 44, 6217–6222. [Google Scholar] [CrossRef] [Green Version]
- Maciejewska, B.M.; Wychowaniec, J.K.; Woźniak-Budych, M.; Popenda, L.; Warowicka, A.; Golba, K.; Litowczenko, J.; Fojud, Z.; Wereszczyńska, B.; Jurga, S. UV cross-linked polyvinylpyrrolidone electrospun fibres as antibacterial surfaces. Sci. Technol. Adv. Mater. 2019, 20, 979–991. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.C.; Rodriguez, F.; Thurston, D.A. Crosslinking aqueous poly(vinyl pyrrolidone) solutions by persulfate. J. Appl. Polym. Sci. 1979, 23, 2453–2462. [Google Scholar] [CrossRef]
- Tapdiqov, S.; Zeynalov, N.; Babayeva, D.; Nasiyyati, E.; Humbatova, S. Copolymerization of N-vinylpyrrolidone with N,N'-methylen-bis-acrylamide: Properties and Structure. Am. J. Polym. Sci. 2015, 5, 18–23. [Google Scholar] [CrossRef]
- Rueda, J.C.; Komber, H.; Cedrón, J.C.; Voit, B.; Shevtsova, G. Synthesis of New Hydrogels by Copolymerization of Poly(2-methyl-2-oxazoline) Bis(macromonomers) and N-Vinylpyrrolidone. Macromol. Chem. Phys. 2003, 204, 947–953. [Google Scholar] [CrossRef]
- Benamer, S.; Mahlous, M.; Boukrif, A.; Mansouri, B.; Larbi Youcef, S. Synthesis and characterisation of hydrogels based on poly(vinyl pyrrolidone). Nucl. Instrum. Methods Phys. Res. B 2006, 248, 284–290. [Google Scholar] [CrossRef]
- Bonelli, N.; Poggi, G.; Chelazzi, D.; Giorgi, R.; Baglioni, P. Poly(vinyl alcohol)/poly(vinyl pyrrolidone) hydrogels for the cleaning of art. J. Colloid Interface Sci. 2019, 536, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Mezhuev, Y.O.; Varankin, A.V.; Luss, A.L.; Dyatlov, V.A.; Tsatsakis, A.M.; Shtilman, M.I.; Korshak, Y.V. Immobilization of dopamine on the copolymer of N-vinyl-2-pyrrolidone and allyl glycidyl ether and synthesis of new hydrogels. Polym. Int. 2020, 69, 1275–1282. [Google Scholar] [CrossRef]
- Parsons, P.J.; Penkett, C.S.; Shell, A.J. Tandem Reactions in Organic Synthesis: Novel Strategies for Natural Product Elaboration and the Development of New Synthetic Methodology. Chem. Rev. 1996, 96, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T.; Price, G. Glycidyl methacrylate and N-vinylpyrrolidinone copolymers: Synthesis and nuclear magnetic resonance characterization. Polymer 1994, 35, 3530–3534. [Google Scholar] [CrossRef]
- Sánchez-Chaves, M.; Martínez, G.; López Madruga, E.; Fernández-Monreal, C. Synthesis of statistical glycidyl methacrylate-N-vinyl pyrrolidone copolymers and their reaction with naproxen. J. Polym. Sci. A Polym. Chem. 2002, 40, 1192–1199. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, D.; Huang, X.; Zhao, W.; Zhao, C. Hexanediamine functionalized poly (glycidyl methacrylate-co-N-vinylpyrrolidone) particles for bilirubin removal. J. Colloid Interface Sci. 2017, 504, 214–222. [Google Scholar] [CrossRef]
- Tarasova, N.; Krivoborodov, E.; Egorova, A.; Zanin, A.; Glukhov, L.; Toropygin, I.; Mezhuev, Y. Reaction of 1,3-dimethylimidazolium dimethylphosphate with elemental sulfur. Pure Appl. Chem. 2020, 92, 1297–1304. [Google Scholar] [CrossRef]
- Tarasova, N.; Krivoborodov, E.; Zanin, A.; Mezhuev, Y. Ionic liquids: Green solvents and reactive compounds? Reaction of tri-n-butylmethylphosphonium dimethylphosphate with elemental sulfur. Pure Appl. Chem. 2021, 93, 29–37. [Google Scholar] [CrossRef]
- Tarasova, N.; Zanin, A.; Krivoborodov, E.; Motyakin, M.; Levina, I.; Dyatlov, V.; Toropygin, I.; Dyakonov, V.; Mezhuev, Y. The product of interaction of elemental sulfur and dimethylphosphate 1,3-dimethylimidazolium is a new green initiator of formaldehyde polymerization. Green Chem. Lett. Rev. 2021, 14, 435–441. [Google Scholar] [CrossRef]
- Tarasova, N.; Krivoborodov, E.; Zanin, A.; Toropygin, I.; Pascal, E.; Dyatlov, V.; Mezhuev, Y. Anionic Polymerization of Ethyl 2-Cyanoacrylate Initiated by 1,3-Dimethylimidazolium (phosphonooxy-) oligosulfanide. Macromol. Res. 2021, 29, 847. [Google Scholar] [CrossRef]
- Brar, A.; Kumar, R. Sequencing of N-vinyl-2-pyrrolidone/glycidyl methacrylate copolymers by one-dimensional and two-dimensional nuclear magnetic resonance spectroscopy. J. Appl. Polym. Sci. 2002, 84, 50–60. [Google Scholar] [CrossRef]
- Soundararajan, S.; Reddy, B.S.R. Glycidyl methacrylate and N-vinyl-2-pyrrolidone copolymers: Synthesis, characterization, and reactivity ratios. J. Appl. Polym. Sci. 1991, 43, 251–258. [Google Scholar] [CrossRef]
- Tarasova, N.; Zanin, A.; Krivoborodov, E.; Toropygin, I.; Pascal, E.; Mezhuev, Y. The New Approach to the Preparation of Polyacrylamide-Based Hydrogels: Initiation of Polymerization of Acrylamide with 1,3-Dimethylimidazolium (Phosphonooxy-)Oligosulphanide under Drying Aqueous Solutions. Polymers 2021, 13, 1806. [Google Scholar] [CrossRef]
- Zang, Y.; Muller, R.; Froelich, D. Determination of crosslinking density of polymer networks by mechanical data in simple extension and by swelling degree at equilibrium. Polymer 1989, 30, 2060–2062. [Google Scholar] [CrossRef]









| Number of Item/ Components | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| GMA-POOS, vol% | 80 | 70 | 60 | 50 | 40 | 30 |
| VP (ml), vol% | 20 | 30 | 40 | 50 | 60 | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasova, N.; Krivoborodov, E.; Zanin, A.; Pascal, E.; Toropygin, I.; Artyukhov, A.; Muradyan, S.; Mezhuev, Y. Formation of Hydrogels Based on a Copolymer of N-Vinyl-2-pyrrolidone and Glycidyl Methacrylate in the Presence of the Reaction Product of 1,3-Dimethylmidazolium Dimethylphosphate and Elemental Sulfur. Gels 2022, 8, 136. https://doi.org/10.3390/gels8020136
Tarasova N, Krivoborodov E, Zanin A, Pascal E, Toropygin I, Artyukhov A, Muradyan S, Mezhuev Y. Formation of Hydrogels Based on a Copolymer of N-Vinyl-2-pyrrolidone and Glycidyl Methacrylate in the Presence of the Reaction Product of 1,3-Dimethylmidazolium Dimethylphosphate and Elemental Sulfur. Gels. 2022; 8(2):136. https://doi.org/10.3390/gels8020136
Chicago/Turabian StyleTarasova, Natalia, Efrem Krivoborodov, Alexey Zanin, Ekaterina Pascal, Ilya Toropygin, Alexander Artyukhov, Samson Muradyan, and Yaroslav Mezhuev. 2022. "Formation of Hydrogels Based on a Copolymer of N-Vinyl-2-pyrrolidone and Glycidyl Methacrylate in the Presence of the Reaction Product of 1,3-Dimethylmidazolium Dimethylphosphate and Elemental Sulfur" Gels 8, no. 2: 136. https://doi.org/10.3390/gels8020136
APA StyleTarasova, N., Krivoborodov, E., Zanin, A., Pascal, E., Toropygin, I., Artyukhov, A., Muradyan, S., & Mezhuev, Y. (2022). Formation of Hydrogels Based on a Copolymer of N-Vinyl-2-pyrrolidone and Glycidyl Methacrylate in the Presence of the Reaction Product of 1,3-Dimethylmidazolium Dimethylphosphate and Elemental Sulfur. Gels, 8(2), 136. https://doi.org/10.3390/gels8020136