Advanced Nanocomposite Hydrogels for Cartilage Tissue Engineering
Abstract
:1. Introduction
2. Nanocomposite Hydrogels
2.1. Carbon Nanomaterial Based Hydrogels
2.2. Nanocomposite Hydrogels from Polymer Nanoparticles
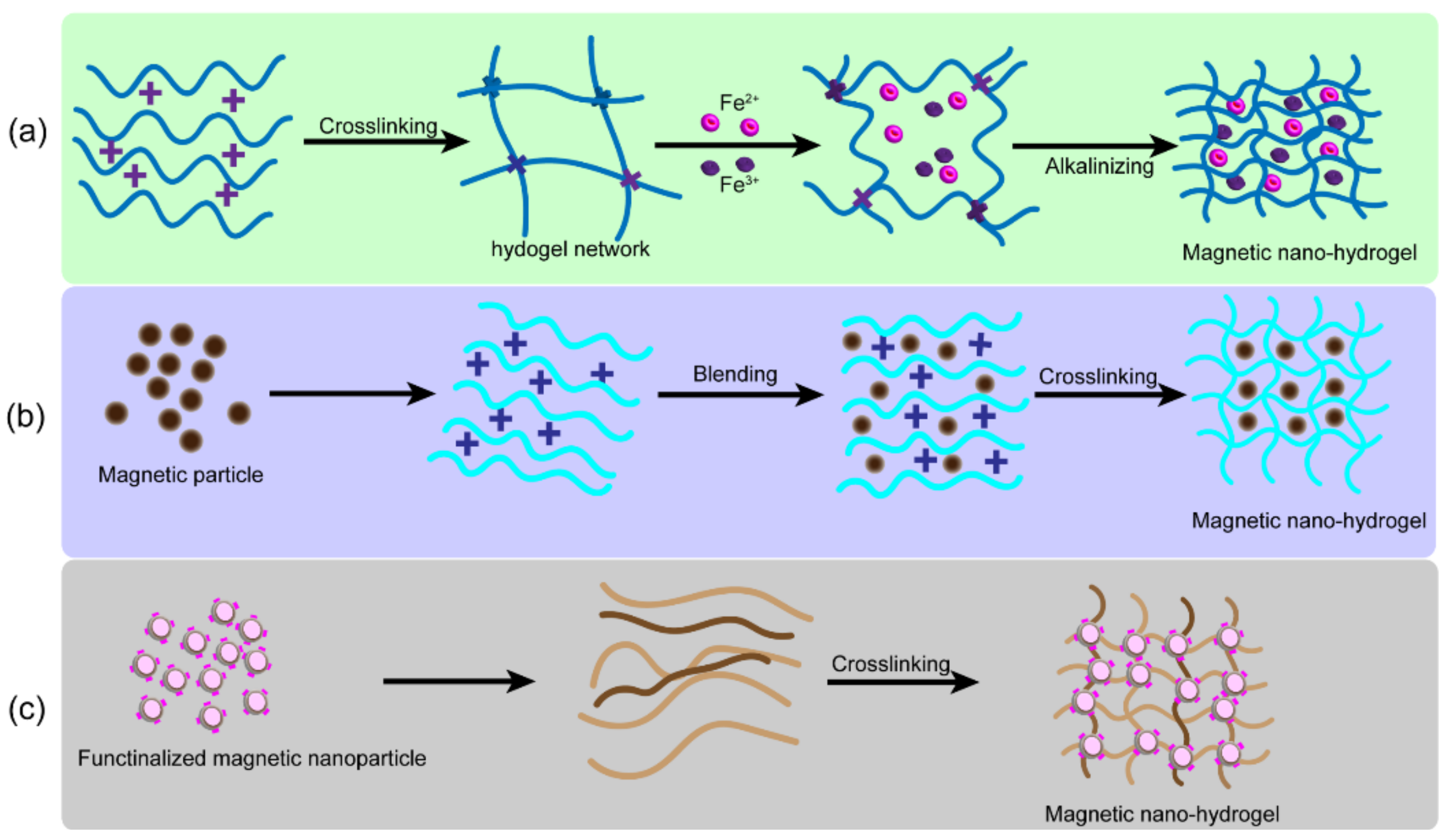
2.3. Nanocomposite Hydrogels from Magnetic Nanoparticles
2.4. Nanocomposite Hydrogels from Other Metal and Metal-Oxide Nanocomposites
2.5. Nanocomposite Hydrogels from Inorganic Nanoparticles of Non-Metallic Origin
2.6. Exosome-Loaded Hydrogel
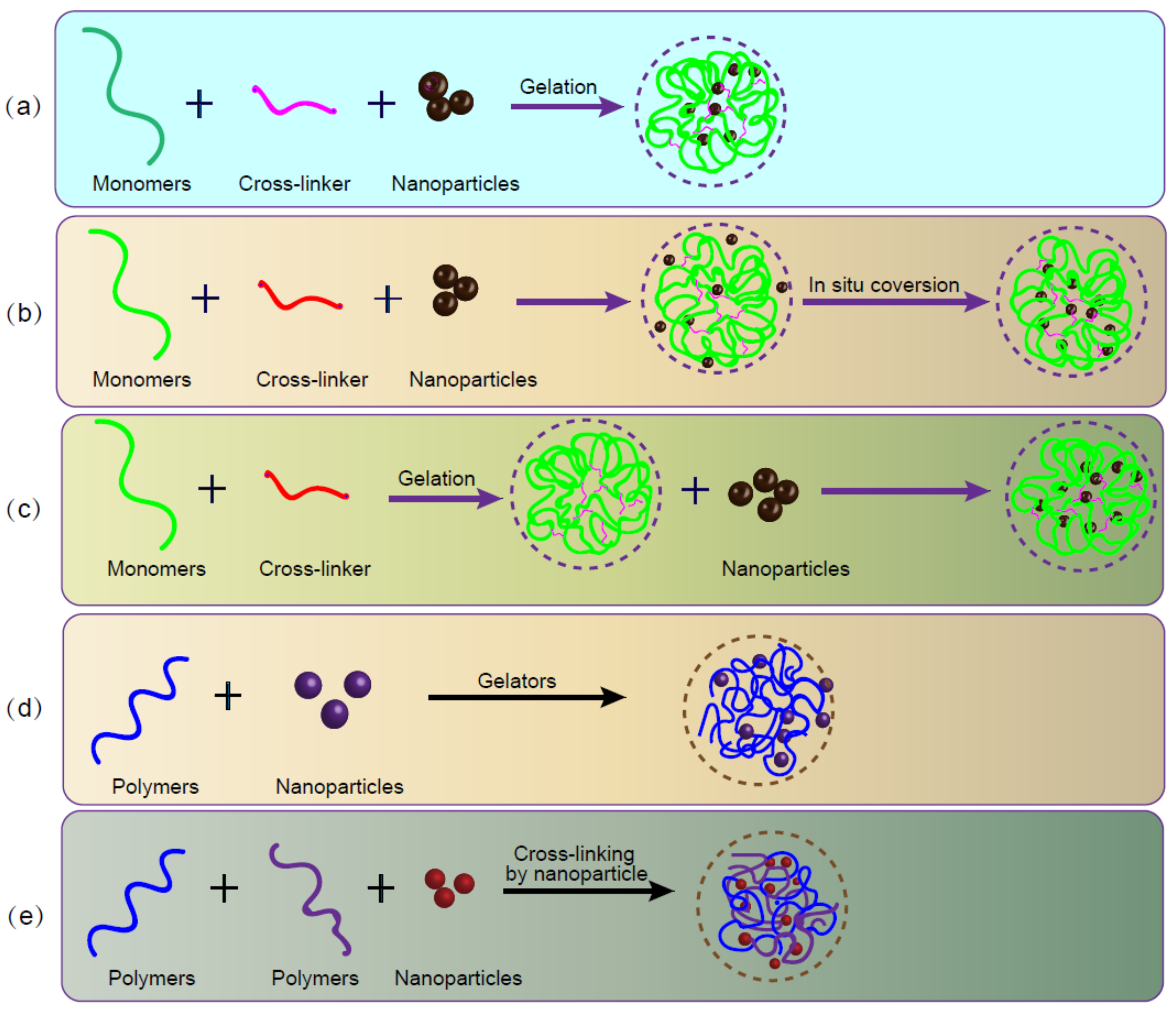
3. Crosslinking of Nanocomposite Hydrogels
4. Fabrication of Nanocomposite Hydrogels
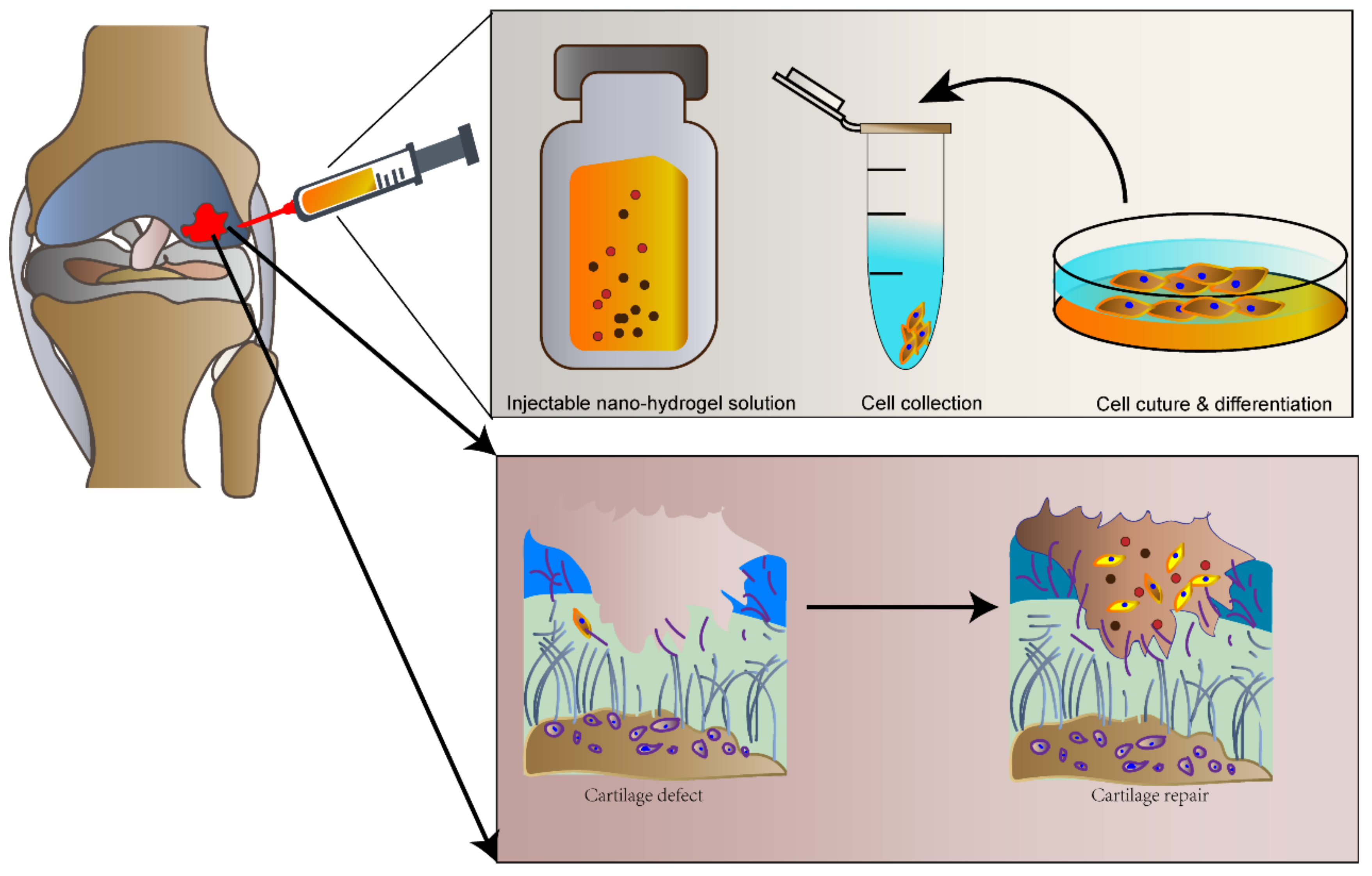
4.1. Injectable Nanohydrogel
4.2. 3D Bioprinting Nanohydrogel
4.3. Electrospinning of NC Hydrogels
5. Conclusions and Prospects
Authors Contribution
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, X.; Wang, S.; Zhan, S.; Niu, J.; Tao, K.; Zhang, Y.; Lin, J. The prevalence of symptomatic knee osteoarthritis in China: Results from the China health and retirement longitudinal study. Arthritis Rheumatol. 2016, 68, 648–653. [Google Scholar] [CrossRef]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef]
- Asadi, N.; Alizadeh, E.; Salehi, R.; Khalandi, B.; Davaran, S.; Akbarzadeh, A. Nanocomposite hydrogels for cartilage tissue engineering: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products—Sciencedirect. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Yahya, E.B.; Amirul, A.A.; Abdul Khalil, H.P.S.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; Atty Sofea, A.K.; Adnan, A.S. Insights into the role of biopolymer aerogel scaffolds in tissue engineering and regenerative medicine. Polymers 2021, 13, 1612. [Google Scholar] [CrossRef]
- Eslahi, N.; Abdorahim, M.; Simchi, A. Smart polymeric hydrogels for cartilage tissue engineering: A review on the chemistry and biological functions. Biomacromolecules 2016, 17, 3441–3463. [Google Scholar] [CrossRef]
- Gutierrez, A.M.; Frazar, E.M.; X Klaus, M.V.; Paul, P.; Hilt, J.Z. Hydrogels and Hydrogel Nanocomposites: Enhancing Healthcare Through Human and Environmental Treatment. Adv. Healthc. Mater. 2021, 10, e2101820. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, M.; Zhang, Y.; Yin, J.; Pei, R. Nanocomposite hydrogels for tissue engineering applications. Nanoscale 2020, 12, 14976–14995. [Google Scholar] [CrossRef]
- Sui, B.; Li, Y.; Yang, B. Nanocomposite hydrogels based on carbon dots and polymers. Chin. Chem. Lett. 2020, 31, 1443–1447. [Google Scholar] [CrossRef]
- He, C.; Yan, H.; Li, X.; Wang, X. In situ fabrication of carbon dots-based lubricants using a facile ultrasonic approach. Green Chem. 2019, 21, 2279–2285. [Google Scholar] [CrossRef]
- He, C.; Shuang, E.; Yan, H.; Li, X. Structural engineering design of carbon dots for lubrication. Chin. Chem. Lett. 2021, 32, 2693–2714. [Google Scholar] [CrossRef]
- De Mori, A.; Peña Fernández, M.; Blunn, G.; Tozzi, G.; Roldo, M. 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering. Polymmers 2018, 10, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Z.; Landish, B.; Chi, Z.; Nannan, C.; Jingyu, D.; Sen, L.; Xiangjin, L. 3D printing hydrogel with graphene oxide is functional in cartilage protection by influencing the signal pathway of rank/rankl/opg. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 244–252. [Google Scholar] [CrossRef]
- Chen, J.; Shi, X.; Ren, L.; Wang, Y. Graphene oxide/PVA inorganic/organic interpenetrating hydrogels with excellent mechanical properties and biocompatibility. Carbon 2017, 111, 18–27. [Google Scholar] [CrossRef]
- Cao, J.; Meng, Y.; Zhao, X.; Ye, L. Dual-anchoring intercalation structure and enhanced bioactivity of poly(vinyl alcohol)/graphene oxide–hydroxyapatite nanocomposite hydrogels as artificial cartilage replacement. Ind. Eng. Chem. Res. 2020, 59, 20359–20370. [Google Scholar] [CrossRef]
- Ghobril, C.; Rodriguez, E.K.; Nazarian, A.; Grinstaff, M.W. Recent advances in dendritic macromonomers for hydrogel formation and their medical applications. Biomacromolecules 2016, 17, 1235–1252. [Google Scholar] [CrossRef]
- Geiger, B.C.; Wang, S.; Padera, R.F., Jr.; Grodzinsky, A.J.; Hammond, P.T. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci. Transl. Med. 2018, 10, eaat8800. [Google Scholar] [CrossRef] [Green Version]
- Degoricija, L.; Bansal, P.N.; Söntjens, S.; Joshi, N.; Takahashi, M.; Snyder, B.; Grinstaff, M. Hydrogels for osteochondral repair based on photocrosslinkable carbamate dendrimers. Biomacromolecules 2008, 9, 2863–2872. [Google Scholar] [CrossRef] [Green Version]
- Seo, B.B.; Choi, H.; Koh, J.T.; Song, S.C. Sustained bmp-2 delivery and injectable bone regeneration using thermosensitive polymeric nanoparticle hydrogel bearing dual interactions with bmp-2. J. Control. Release 2015, 209, 67–76. [Google Scholar] [CrossRef]
- Asadi, N.; Alizadeh, E.; Azizeh, R.D.B.; Mostafavi, E.; Akbarzadeh, A.; Davaran, S. Fabrication and in vitro evaluation of nanocomposite hydrogel scaffolds based on gelatin/PCL–PEG–PCL for cartilage tissue engineering. Acs. Omega 2019, 4, 449–457. [Google Scholar] [CrossRef]
- Abdeen, M.; Albert, A.; Maxhera, B.; Hoffmann, T.; Petrov, G.; Sixt, S.; Roussel, E.; Westenfeld, R.; Lichtenberg, A.; Saeed, D. Implanting permanent left ventricular assist devices in patients on veno-arterial extracorporeal membrane oxygenation support: Do we really need a cardiopulmonary bypass machine? Eur. J. Cardiothorac. Surg. 2016, 50, 542–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antman-Passig, M.; Shefi, O. Remote magnetic orientation of 3D collagen hydrogels for directed neuronal regeneration. Nano. Lett. 2016, 16, 2567–2573. [Google Scholar] [CrossRef] [PubMed]
- Rodkate, N.; Rutnakornpituk, M. Multi-responsive magnetic microsphere of poly(n-isopropylacrylamide)/carboxymethylchitosan hydrogel for drug controlled release. Carbohydr. Polym. 2016, 151, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Omidinia-Anarkoli, A.; Boesveld, S.; Tuvshindorj, U.; Rose, J.C.; Haraszti, T.; De Laporte, L. An injectable hybrid hydrogel with oriented short fibers induces unidirectional growth of functional nerve cells. Small 2017, 13, 1702207. [Google Scholar] [CrossRef]
- Bowser, D.A.; Moore, M.J. Biofabrication of neural microphysiological systems using magnetic spheroid bioprinting. Biofabrication 2020, 12, 015002. [Google Scholar] [CrossRef]
- Gao, F.; Xie, W.; Miao, Y.; Wang, D.; Guo, Z.; Ghosal, A.; Li, Y.; Wei, Y.; Feng, S.S.; Zhao, L.; et al. Magnetic Hydrogel with Optimally Adaptive Functions for Breast Cancer Recurrence Prevention. Adv. Healthc. Mater. 2019, 8, e1900203. [Google Scholar] [CrossRef]
- Manjua, A.C.; Alves, V.D.; Crespo, J.G.; Portugal, C.A.M. Magnetic responsive PVA hydrogels for remote modulation of protein sorption. ACS Appl. Mater. Interfaces 2019, 11, 21239–21249. [Google Scholar] [CrossRef]
- Zou, J.; Shi, M.; Liu, X.; Jin, C.; Xing, X.; Qiu, L.; Tan, W. Aptamer-functionalized exosomes: Elucidating the cellular uptake mechanism and the potential for cancer-targeted chemotherapy. Anal. Chem. 2019, 91, 2425–2430. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Song, S.C. Thermosensitive/superparamagnetic iron oxide nanoparticle-loaded nanocapsule hydrogels for multiple cancer hyperthermia. Biomaterials 2016, 106, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.C.; Cámara-Torres, M.; Rahimi, K.; Köhler, J.; Möller, M.; De Laporte, L. Nerve cells decide to orient inside an injectable hydrogel with minimal structural guidance. Nano. Lett. 2017, 17, 3782–3791. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, H.; Yasa, I.C.; Yasa, O.; Tabak, A.F.; Giltinan, J.; Sitti, M. 3D-printed biodegradable microswimmer for theranostic cargo delivery and release. ACS Nano. 2019, 13, 3353–3362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrow, M.; Taylor, A.; Fuentes-Caparrós, A.M.; Sharkey, J.; Daniels, L.M.; Mandal, P.; Park, B.K.; Murray, P.; Rosseinsky, M.J.; Adams, D.J. Spions for cell labelling and tracking using MRI: Magnetite or maghemite? Biomater. Sci. 2017, 6, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Hai, H.T.; Kura, H.; Takahashi, M.; Ogawa, T. Facile synthesis of fe3o4 nanoparticles by reduction phase transformation from gamma-Fe2O3 nanoparticles in organic solvent. J. Colloid. Interface. Sci. 2010, 341, 194–199. [Google Scholar] [CrossRef]
- Orr, A.L.; Vargas, L.; Turk, C.N.; Baaten, J.E.; Matzen, J.T.; Dardov, V.J.; Attle, S.J.; Li, J.; Quackenbush, D.C.; Goncalves, R.L.; et al. Suppressors of superoxide production from mitochondrial complex III. Nat. Chem. Biol. 2015, 11, 834–836. [Google Scholar] [CrossRef] [Green Version]
- Brito-Pereira, R.; Correia, D.; Ribeiro, C.; Francesko, A.; Etxebarria, I.; Álvarez, L.P.; Vilas, J.L.; Martins, P.; Lanceros-Mendez, S. Silk fibroin-magnetic hybrid composite electrospun fibers for tissue engineering applications. Compos. Part B Eng. 2018, 141, 70–75. [Google Scholar] [CrossRef]
- Hermenegildo, B.; Ribeiro, C.; Pérez-Lvarez, L.; Vilas, J.L.; Lanceros-Méndez, S. Hydrogel-based magnetoelectric microenvironments for tissue stimulation. Colloids Surf. B Biointerfaces 2019, 181, 1041–1047. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, C.; Yan, S.; Liu, Q.; Hou, M.; Xu, Y.; Guo, R. Non-invasive monitoring of in vivo hydrogel degradation and cartilage regeneration by multiparametric mr imaging. Theranostics 2018, 8, 1146–1158. [Google Scholar] [CrossRef]
- Huang, J.; Jia, Z.; Liang, Y.; Huang, Z.; Rong, Z.; Xiong, J.; Wang, D. Pulse electromagnetic fields enhance the repair of rabbit articular cartilage defects with magnetic nano-hydrogel. RSC Adv. 2020, 10, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Brady, M.A.; Talvard, L.; Vella, A.; Ethier, C.R. Bio-inspired design of a magnetically active trilayered scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 1298–1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betsch, M.; Cristian, C.; Lin, Y.Y.; Blaeser, A.; Schöneberg, J.; Vogt, M.; Buhl, E.M.; Fischer, H.; Duarte Campos, D.F. Incorporating 4d into bioprinting: Real-time magnetically directed collagen fiber alignment for generating complex multilayered tissues. Adv. Healthc. Mater. 2018, 7, e1800894. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Foldager, C.B.; Pei, M.; Hui, J.H. Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration. Stem. Cell. Rev. Rep. 2014, 10, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ding, B.; Yan, X.; Peng, L.; Duan, J.; Yang, S.; Cheng, L.; Chen, D. Polyethylene glycol modified pamam dendrimer delivery of kartogenin to induce chondrogenic differentiation of mesenchymal stem cells. Nanomedicine 2017, 13, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- Stowers, R.S.; Drinnan, C.T.; Chung, E.; Suggs, L.J. Mesenchymal stem cell response to TGF-β1 in both 2D and 3D environments. Biomater. Sci. 2013, 1, 860–869. [Google Scholar] [CrossRef]
- Ren, X.; Weisgerber, D.W.; Bischoff, D.; Lewis, M.S.; Reid, R.R.; He, T.C.; Yamaguchi, D.T.; Miller, T.A.; Harley, B.A.; Lee, J.C. Nanoparticulate mineralized collagen scaffolds and bmp-9 induce a long-term bone cartilage construct in human mesenchymal stem cells. Adv. Healthc. Mater. 2016, 5, 1821–1830. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Shi, D.; Shen, Y.; Xu, Z.; Dai, J.; Chen, D.; Teng, H.; Jiang, Q. Full-thickness cartilage defects are repaired via a microfracture technique and intraarticular injection of the small-molecule compound kartogenin. Arthritis Res. Ther. 2015, 17, 20. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Zhu, P.; Huang, H.; Zheng, Y.; Liu, J.; Feng, L.; Guo, H.; Tang, S.; Guo, R. Functionalization of novel theranostic hydrogels with kartogenin-grafted uspio nanoparticles to enhance cartilage regeneration. ACS Appl. Mater. Interfaces 2019, 11, 34744–34754. [Google Scholar] [CrossRef]
- Reddi, A.H.; Becerra, J.; Andrades, J.A. Nanomaterials and hydrogel scaffolds for articular cartilage regeneration. Tissue Eng. Part B Rev. 2011, 17, 301–305. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, J.; Liu, J.; Luo, Y.; Wan, Y. Fabrication and characterization of layered chitosan/silk fibroin/nano-hydroxyapatite scaffolds with designed composition and mechanical properties. Biomed. Mater. 2015, 10, 045013. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, D.; Peng, L.; Chen, Y.F.; Cui, J.; Xiong, J.; Lu, W.; Duan, L.; Chen, K.; Zeng, Y.; et al. Repair of rabbit cartilage defect based on the fusion of rabbit bone marrow stromal cells and Nano-HA/PLLA composite material. Artif. Cells Nanomed. Biotechnol. 2017, 45, 115–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théry, C.A.-O.; Witwer, K.A.-O.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (misev2018): A position statement of the international society for extracellular vesicles and update of the misev2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Zhang, G.; Feng, X.; Liu, H.; Li, F.; Wang, M.; Li, H. Room-temperature self-healing tough nanocomposite hydrogel crosslinked by zirconium hydroxide nanoparticles. Compos. Sci. Technol. 2017, 140, 54–62. [Google Scholar] [CrossRef]
- Teow, S.Y.; Wong, M.M.; Yap, H.Y.; Peh, S.C.; Shameli, K. Bactericidal properties of plants-derived metal and metal oxide nanoparticles (NPS). Molecules 2018, 23, 1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P. Nano-TiO2 doped chitosan scaffold for the bone tissue engineering applications. Int. J. Biomater. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Grant, S.A.; Zhu, J.; Gootee, J.; Snider, C.L.; Bellrichard, M.; Grant, D.A. Gold nanoparticle-collagen gels for soft tissue augmentation. Tissue Eng. Part A 2018, 24, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Teow, S.Y.; Pushpamalar, J. Application of metal nanoparticle–hydrogel composites in tissue regeneration. Bioengineering 2019, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Motealleh, A.; Kehr, N.S. Nanocomposite hydrogels and their applications in tissue engineering. Adv. Healthc. Mater. 2017, 6, 1600938. [Google Scholar] [CrossRef]
- Xia, S.; Song, S.; Ren, X.; Gao, G. Highly tough, anti-fatigue and rapidly self-recoverable hydrogels reinforced with core-shell inorganic-organic hybrid latex particles. Soft Matter. 2017, 13, 6059–6067. [Google Scholar] [CrossRef]
- Bonifacio, M.A.; Cochis, A.; Cometa, S.; Scalzone, A.; Gentile, P.; Procino, G.; Milano, S.; Scalia, A.C.; Rimondini, L.; De Giglio, E. Advances in cartilage repair: The influence of inorganic clays to improve mechanical and healing properties of antibacterial gellan gum-manuka honey hydrogels. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110444. [Google Scholar] [CrossRef]
- Duan, L.; Xu, X.; Xu, L.; Chen, H.; Li, X.; Alahdal, M.; Xiao, Y.; Liang, Y.; Xia, J. Exosome-mediated drug delivery for cell-free therapy of osteoarthritis. Curr. Med. Chem. 2021, 28, 6458–6483. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, J.; Xiong, J.; Sun, S.; Xia, J.; Yang, L.; Liang, Y. Stem cell-derived nanovesicles: A novel cell-free therapy for wound healing. Stem. Cells Int. 2021, 2021, 1285087. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.; Xu, L.; Prasadam, I.; Duan, L.; Xiao, Y.; Xia, J. Non-surgical osteoarthritis therapy, intra-articular drug delivery towards clinical applications. J. Drug Target. 2021, 29, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liang, Y.; Li, X.; Ouyang, K.; Wang, M.; Cao, T.; Li, W.; Liu, J.; Xiong, J.; Li, B.; et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials 2021, 269, 120539. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, X.; Li, X.; Xiong, J.; Li, B.; Duan, L.; Wang, D.; Xia, J. Chondrocyte-targeted microrna delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl. Mater. Interfaces 2020, 12, 36938–36947. [Google Scholar] [CrossRef]
- Duan, L.; Xu, L.; Xu, X.; Qin, Z.; Zhou, X.; Xiao, Y.; Liang, Y.; Xia, J. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale 2021, 13, 1387–1397. [Google Scholar] [CrossRef]
- Chen, X.; Shi, Y.; Xue, P.; Ma, X.; Li, J.; Zhang, J. Mesenchymal stem cell-derived exosomal microRNA-136–5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res. Ther. 2020, 22, 256. [Google Scholar] [CrossRef]
- Huang, J.; Xiong, J.; Yang, L.; Zhang, J.; Sun, S.; Liang, Y. Cell-free exosome-laden scaffolds for tissue repair. Nanoscale 2021, 13, 8740–8750. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Li, Y.; Niu, X.; Zhao, B.; Wang, Y. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 2017, 9, 4430–4438. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Xu, X.; Chen, C.; Akiyama, K.; Snead, M.L.; Shi, S. Dental mesenchymal stem cells encapsulated in an alginate hydrogel co-delivery microencapsulation system for cartilage regeneration. Acta Biomater. 2013, 9, 9343–9350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Wang, X.; Li, Y.; Ren, M.; He, P.; Wang, L.; Xu, J.; Yang, S.; Ji, P. Dendrimer-modified gelatin methacrylate hydrogels carrying adipose-derived stromal/stem cells promote cartilage regeneration. Stem. Cell. Res. 2022, 13, 26. [Google Scholar] [CrossRef]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano. Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Zhang, N.; Lock, J.; Sallee, A.; Liu, H. Magnetic nanocomposite hydrogel for potential cartilage tissue engineering: Synthesis, characterization, and cytocompatibility with bone marrow derived mesenchymal stem cells. ACS Appl. Mater. Interfaces 2015, 7, 20987–20998. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Rey-Rico, A.; Venkatesan, J.K.; Johnstone, B.; Cucchiarini, M. Transforming growth factor beta-releasing scaffolds for cartilage tissue engineering. Tissue Eng. Part B Rev. 2014, 20, 106–125. [Google Scholar] [CrossRef]
- Biondi, M.; Borzacchiello, A.; Mayol, L. Nanoparticle-integrated hydrogels as multifunctional composite materials for biomedical applications. Gels 2015, 1, 162–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, F.; Li, X.; Wang, Q.; Liao, L.; Zhang, C. Nanocomposite hydrogels and their applications in drug delivery and tissue engineering. Nanocomposite Hydrogels Appl. Drug Deliv. Tissue Eng. 2015, 11, 40–52. [Google Scholar] [CrossRef]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef]
- Jayaraman, P.; Gandhimathi, C.; Venugopal, J.R.; Becker, D.L.; Ramakrishna, S.; Srinivasan, D.K. Controlled release of drugs in electrosprayed nanoparticles for bone tissue engineering. Adv. Drug Deliv. Rev. 2015, 94, 77–95. [Google Scholar] [CrossRef]
- Eftekhari, H.; Jahandideh, A.; Asghari, A.; Akbarzadeh, A.; Hesaraki, S. Assessment of polycaprolacton (PCL) nanocomposite scaffold compared with hydroxyapatite (ha) on healing of segmental femur bone defect in rabbits. Artif. Cells Nanomed. Biotechnol. 2017, 45, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Seo, S.K.; Cho, S.; Kim, H.S.; Lee, C.H. Characteristics of sodium polyacrylate/nano-sized carbon hydrogel for biomedical patch. J. Nanosci. Nanotechnol. 2018, 18, 1611–1614. [Google Scholar] [CrossRef] [PubMed]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle-hydrogel composites: Concept, design, and applications of these promising, multi-functional materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef] [PubMed]
- Chahine, N.O.; Collette, N.M.; Thomas, C.B.; Genetos, D.C.; Loots, G.G. Nanocomposite scaffold for chondrocyte growth and cartilage tissue engineering: Effects of carbon nanotube surface functionalization. Tissue Eng. Part A. 2014, 20, 2305–2315. [Google Scholar] [CrossRef]
- Ren, P.; Zhang, H.; Dai, Z.; Ren, F.; Wu, Y.; Hou, R.; Zhu, Y.; Fu, J. Stiff micelle-crosslinked hyaluronate hydrogels with low swelling for potential cartilage repair. J. Mater. Chem. B. 2019, 7, 5490–5501. [Google Scholar] [CrossRef]
- Zhu, M.; Wei, K.; Lin, S.; Chen, X.; Wu, C.-C.; Li, G.; Bian, L. Bioadhesive polymersome for localized and sustained drug delivery at pathological sites with harsh enzymatic and fluidic environment via supramolecular host–guest complexation. Small 2018, 14, 1702288. [Google Scholar] [CrossRef]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef]
- Lu, M.; Huang, Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials 2020, 242, 119925. [Google Scholar] [CrossRef]
- Awasthi, S.; Gaur, J.K.; Pandey, S.K.; Bobji, M.S.; Srivastava, C. High-strength, strongly bonded nanocomposite hydrogels for cartilage repair. ACS Appl. Mater. Interfaces 2021, 13, 24505–24523. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Guo, Q. Silk fibroin hydrogel scaffolds incorporated with chitosan nanoparticles repair articular cartilage defects by regulating tgf-β1 and bmp-2. Arthritis Res. Ther. 2021, 23, 50. [Google Scholar] [CrossRef]
- Lourenço, A.H.; Torres, A.L.; Vasconcelos, D.P.; Ribeiro-Machado, C.; Barbosa, J.N.; Barbosa, M.A.; Barrias, C.C.; Ribeiro, C.C. Osteogenic, anti-osteoclastogenic and immunomodulatory properties of a strontium-releasing hybrid scaffold for bone repair. Mater. Sci. Eng. C Mater Biol. Appl. 2019, 99, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Hu, Y.; Zeng, M.; Li, M.; Lin, S.; Zhou, Y.; Xie, J. Design and evaluation of nano-hydroxyapatite/poly(vinyl alcohol) hydrogels coated with poly(lactic-co-glycolic acid)/nano-hydroxyapatite/poly(vinyl alcohol) scaffolds for cartilage repair. J. Orthop. Surg. Res. 2019, 14, 446. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Liang, Y.; Huang, Z.; Zhao, P.; Liang, Q.; Liu, Y.; Duan, L.; Liu, W.; Zhu, F.; Bian, L.; et al. Magnetic enhancement of chondrogenic differentiation of mesenchymal stem cells. ACS Biomater. Sci. Eng. 2019, 5, 2200–2207. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, W.; Liang, Y.; Li, L.; Duan, L.; Chen, J.; Zhu, F.; Lai, Y.; Zhu, W.; You, W.; et al. Preparation and biocompatibility of diphasic magnetic nanocomposite scaffold. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 87, 70–77. [Google Scholar] [CrossRef]
- Khader, A.; Arinzeh, T.L. Biodegradable zinc oxide composite scaffolds promote osteochondral differentiation of mesenchymal stem cells. Biotechnol. Bioeng. 2020, 117, 194–209. [Google Scholar] [CrossRef]
- Söntjens, S.H.M.; Nettles, D.L.; Carnahan, M.A.; Setton, L.A.; Grinstaff, M.W. Biodendrimer-based hydrogel scaffolds for cartilage tissue repair. Biomacromolecules 2006, 7, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, F.; Bagheri-Khoulenjani, S.; Olov, N.; Zeini, D.; Solouk, A.; Mirzadeh, H. Fabrication of nanocomposite/nanofibrous functionally graded biomimetic scaffolds for osteochondral tissue regeneration. J. Biomed. Mater. Res. A 2021, 109, 1657–1669. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Manga, Y.B.; Ostrikov, K.K.; Chiang, W.H.; Pandey, A.; Nyambat, B.; Chuang, E.Y.; Chen, C.H. Microplasma cross-linked graphene oxide-gelatin hydrogel for cartilage reconstructive surgery. ACS Appl. Mater. Interfaces 2020, 12, 86–95. [Google Scholar] [CrossRef]
- Meng, Y.; Cao, J.; Chen, Y.; Yu, Y.; Ye, L. 3D printing of a poly(vinyl alcohol)-based nano-composite hydrogel as an artificial cartilage replacement and the improvement mechanism of printing accuracy. J. Mater. Chem. B 2020, 8, 677–690. [Google Scholar] [CrossRef]
- Olate-Moya, F.; Arens, L.; Wilhelm, M.; Mateos-Timoneda, M.A.; Engel, E.; Palza, H. Chondroinductive alginate-based hydrogels having graphene oxide for 3D printed scaffold fabrication. ACS Appl. Mater. Interfaces 2020, 12, 4343–4357. [Google Scholar] [CrossRef]
- Cai, H.; Wang, P.; Xu, Y.; Yao, Y.; Liu, J.; Li, T.; Sun, Y. BMSCS-assisted injectable Col I hydrogel-regenerated cartilage defect by reconstructing superficial and calcified cartilage. Regen. Biomater. 2020, 7, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, Y.P.; Moses, J.C.; Bhardwaj, N.; Mandal, B.B. Injectable hydrogels: A new paradigm for osteochondral tissue engineering. J. Mater. Chem. B 2018, 6, 5499–5529. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugam, A.; Kumar, R.A.; Priya, M.V.; Nair, S.V.; Jayakumar, R. An overview of injectable polymeric hydrogels for tissue engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Lee, S.S.; Choi, G.E.; Lee, H.J.; Kim, Y.; Choy, J.H.; Jeong, B. Layered double hydroxide and polypeptide thermogel nanocomposite system for chondrogenic differentiation of stem cells. ACS Appl. Mater. Interfaces 2017, 9, 42668–42675. [Google Scholar] [CrossRef] [PubMed]
- Kuang, L.; Ma, X.; Ma, Y.; Yao, Y.; Tariq, M. Self-assembled injectable nanocomposite hydrogels coordinated by in situ generated cap nanoparticles for bone regeneration. ACS Appl. Mater. Interfaces 2019, 11, 17234–17246. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Kaunas, R.; Gaharwar, A.K. Hydrogel bioink reinforcement for additive manufacturing: A focused review of emerging strategies. Adv. Mater. 2020, 32, e1902026. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Xiong, J.; Wang, D.; Zhang, J.; Yang, L.; Sun, S.; Liang, Y. 3D bioprinting of hydrogels for cartilage tissue engineering. Gels 2021, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Feng, Q.; Xu, J.; Xu, X.; Tian, F.; Yeung, K.W.K.; Bian, L. Self-assembled injectable nanocomposite hydrogels stabilized by bisphosphonate-magnesium (Mg2+) coordination regulates the differentiation of encapsulated stem cells via dual crosslinking. Adv. Funct. Mater. 2017, 27, 1701642. [Google Scholar] [CrossRef]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.I.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef]
- Xia, H.; Zhao, D.; Zhu, H.; Hua, Y.; Xiao, K.; Xu, Y.; Liu, Y.; Chen, W.; Liu, Y.; Zhang, W.; et al. Lyophilized scaffolds fabricated from 3D-printed photocurable natural hydrogel for cartilage regeneration. ACS Appl. Mater. Interfaces 2018, 10, 31704–31715. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.J.; O’Brien, J.; Zhang, L.G. Integrating biologically inspired nanomaterials and table-top stereolithography for 3D printed biomimetic osteochondral scaffolds. Nanoscale 2015, 7, 14010–14022. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ding, X.; Yu, X.; Chen, X.; Zhang, X.; Cui, S.; Shi, J.; Chen, J.; Yu, L.; Chen, S.; et al. Cell-free bilayered porous scaffolds for osteochondral regeneration fabricated by continuous 3D-printing using nascent physical hydrogel as ink. Adv. Healthc. Mater. 2021, 10, e2001404. [Google Scholar] [CrossRef] [PubMed]
- Shalom, H.; Kapishnikov, S.; Brumfeld, V.; Naveh, N.; Tenne, R.; Lachman, N. Strong, tough and bio-degradable polymer-based 3D-ink for fused filament fabrication (FFF) using WS2 nanotubes. Sci. Rep. 2020, 10, 8892. [Google Scholar] [CrossRef] [PubMed]
- Seiyama, T.; Kagawa, S. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1966, 38, 1502–1503. [Google Scholar] [CrossRef]
- Lang, J.H.; Xue, L.I.; Liu, X.Y.; Yang, J.H. Preparation and characterization of zno nanorods. Jilin. Norm. Univ. J. (Nat. Sci. Ed.) 2009, 2, 35–37. [Google Scholar]
- Gao, Y.J.; Zhang, W.C.; Wu, X.L.; Xia, Y.; Huang, G.S.; Xu, L.L.; Shen, J.C.; Siu, G.G.; Chu, P.K. Hydrothermal self-assembling of zno nanorods into sphere-like superstructures and their optical characteristics. Appl. Surf. Sci. 2008, 255, 1982–1987. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Jamshidi, A. A new strategy for fabrication of bone scaffolds using electrospun nano-HAp/PHB fibers and protein hydrogels. Chem. Eng. J. 2016, 289, 48–58. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Maio, A.; Botta, L.; Rigogliuso, S.; Ghersi, G. Electrospun PCL/GO-g-PEG structures: Processing morphology properties relationships. Compos. Part A Appl. Sci. Manuf. 2017, 92, 97–107. [Google Scholar] [CrossRef]
- Chen, W.; Wang, C.; Gao, Y.; Wu, Y.; Wu, G.; Shi, X.; Du, Y.; Deng, H. Incorporating chitin derived glucosamine sulfate into nanofibers via coaxial electrospinning for cartilage regeneration. Carbohydr. Polym. 2020, 229, 115544. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Udangawa, R.N.; Chen, J.; Mancinelli, C.D.; Garrudo, F.F.F.; Mikael, P.E.; Cabral, J.M.S.; Ferreira, F.C.; Linhardt, R.J. Kartogenin-loaded coaxial PGS/PCL aligned nanofibers for cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110291. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.M.; Oyen, M.L. Hydrogel composite materials for tissue engineering scaffolds. JOM 2013, 65, 505–516. [Google Scholar] [CrossRef]
- Naghieh, S.; Foroozmehr, E.; Badrossamay, M.; Kharaziha, M. Combinational processing of 3D printing and electrospinning of hierarchical poly(lactic acid)/gelatin-forsterite scaffolds as a biocomposite: Mechanical and biological assessment. Mater. Design. 2017, 133, 128–135. [Google Scholar] [CrossRef]
- Bas, O.; De-Juan-Pardo, E.M.; Meinert, C.; D’Angella, D.; Baldwin, J.G.; Bray, L.J.; Wellard, R.M.; Kollmannsberger, S.; Rank, E.; Werner, C.; et al. Biofabricated soft network composites for cartilage tissue engineering. Biofabrication 2017, 9, 025014. [Google Scholar] [CrossRef]
- Ran, N.; Gao, X.; Dong, X.; Li, J.; Lin, C.; Geng, M.; Yin, H. Effects of exosome-mediated delivery of myostatin propeptide on functional recovery of mdx mice. Biomaterials 2020, 236, 119826. [Google Scholar] [CrossRef] [PubMed]
- Kisiday, J.; Jin, M.; Kurz, B.; Hung, H.; Semino, C.; Zhang, S.; Grodzinsky, A.J. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair. Proc. Natl. Acad. Sci. USA 2002, 99, 9996–10001. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Xu, Y.; Li, Y.; Jia, L.; Zhou, G. 3D printing electrospinning fiber-reinforced decellularized extracellular matrix for cartilage regeneration. Chem. Eng. J. 2020, 382, 122986. [Google Scholar] [CrossRef]
- Feng, B.; Ji, T.; Wang, X.; Fu, W.; Li, F. Engineering cartilage tissue based on cartilage-derived extracellular matrix CECM/PCL hybrid nanofibrous scaffold. Mater. Design. 2020, 193, 108773. [Google Scholar] [CrossRef]

| Nanoparticles | Hydrogel | Preparation Method | In Vitro/Vivo Model | Function or Potential Applications | Ref |
|---|---|---|---|---|---|
| Carbon nanotube | Molten agarose | Two-dimensional culture | Three-dimensional (3D) pellet cultures | Promote chondrogenic ECM | [84] |
| Micelle | Methacrylated hyaluronic acid (MeHA) hydrogels | Photo-crosslink | Rabbit cartilage defects | Improve the mechanical properties: low swelling, effective self-recovery, and efficient energy dissipation | [85] |
| Polymersome | β-CD-grafted hyaluronic acid macromer | Crosslinking | Rat model of knee osteoarthritis | Localized and sustained drug release | [86] |
| Exosomes | Porcine cartilage/GelMA | 3D desktop-stereolithography technology | Rabbit model of osteochondral defect | Promote chondrocyte migration and cartilage regeneration | [87] |
| Human umbilical cord mesenchymal stem cell-derived small extracellular vesicles (hUC-MSCs-sEVs) | Gelatin methacrylate (Gelma)/nanoclay hydrogel (Gel-nano) | Chemical crosslinking by ultraviolet radiation | Full thickness cylindrical cartilage defects in rats | Promote cartilage regeneration | [88] |
| Titanium oxide (TiO2), carbon nanotubes (CNTs) | polyacrylamide (PAM) | Free radical polymerization reaction | In vitro evaluation | Enhance mechanical behavior and puncture resistance | [89] |
| Chitosan (CS) nanoparticles | Silk fibroin (SF) hydrogel | Ultrasound-induced crosslinking | Rabbit model of knee cartilage defects | Stimulate chondrogenic of BMSC and repair articular cartilage defects | [90] |
| Sr-doped hydroxyapatite (HAP) microspheres | RGD-alginate | Crosslinking | In vitro model | Bone repair | [91,92] |
| Nano-hydroxyapatite/poly(vinyl alcohol) hydrogels | Poly(lactic-co-glycolic acid)/nano-hydroxyapatite/poly(vinyl alcohol) | Crosslinking by freeze–thaw | In vitro culture | Promote chondrogenesis | |
| Magnetic nanoparticles | poly(vinyl alcohol) and nano-hydroxyapatite (n-HAP), | Ultrasonic dispersion method and freeze–thaw crosslinking | In vitro model | Promote proliferation and differentiation of the BMSCs | [52,93] |
| Magnetic nanoparticles | PLGA/Col-I-PLGA/n-HAP | Low-temperature deposition manufacturing | In vitro model | Cell compatibility | [94] |
| Zinc oxide | Polycaprolactone (PCL) | Electrospinning technique | In vitro model | Enhance osteochondral differentiation | [95] |
| Poly(amidoamine) (PAMAM) dendrimers | gelatin methacrylate (GelMA) hydrogel | Photo-crosslink | Rat knee cartilage defect | Promote cartilage tissue regeneration | [73] |
| Biodendrimer | PEG3400-(PGLSA-MA4)2 macromer | UV–photo-crosslink | In vitro model | Promote cartilage regeneration | [96] |
| n-HAP | PCL/gelatin | 3D printing | In vitro cytotoxicity evaluation | Promote MSC proliferation | [97] |
| Graphene oxide (GO) | Gelatin hydrogel | Microplasma-assisted crosslinking method | Rat model of cartilage defects | Promote formation of healthy hyaline cartilage | [98] |
| GO | PVA/HAP | 3D printing | In vitro model | Artificial cartilage replacement | [99,100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Liu, F.; Su, H.; Xiong, J.; Yang, L.; Xia, J.; Liang, Y. Advanced Nanocomposite Hydrogels for Cartilage Tissue Engineering. Gels 2022, 8, 138. https://doi.org/10.3390/gels8020138
Huang J, Liu F, Su H, Xiong J, Yang L, Xia J, Liang Y. Advanced Nanocomposite Hydrogels for Cartilage Tissue Engineering. Gels. 2022; 8(2):138. https://doi.org/10.3390/gels8020138
Chicago/Turabian StyleHuang, Jianghong, Fei Liu, Haijing Su, Jianyi Xiong, Lei Yang, Jiang Xia, and Yujie Liang. 2022. "Advanced Nanocomposite Hydrogels for Cartilage Tissue Engineering" Gels 8, no. 2: 138. https://doi.org/10.3390/gels8020138
APA StyleHuang, J., Liu, F., Su, H., Xiong, J., Yang, L., Xia, J., & Liang, Y. (2022). Advanced Nanocomposite Hydrogels for Cartilage Tissue Engineering. Gels, 8(2), 138. https://doi.org/10.3390/gels8020138






