TiO2 Nanoparticle-Loaded Poly(NIPA-co-NMA) Fiber Web for the Adsorption and Photocatalytic Degradation of 4-Isopropylphenol
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Poly(NIPA-co-NMA)
2.2. Preparation and Characterization of Composite Fibers
2.3. Adsorption and Photocatalytic Degradation of 4-Isopropylphenol
3. Results and Discussion
3.1. Morphology of the TiO2 Nanoparticle-Loaded Fiber
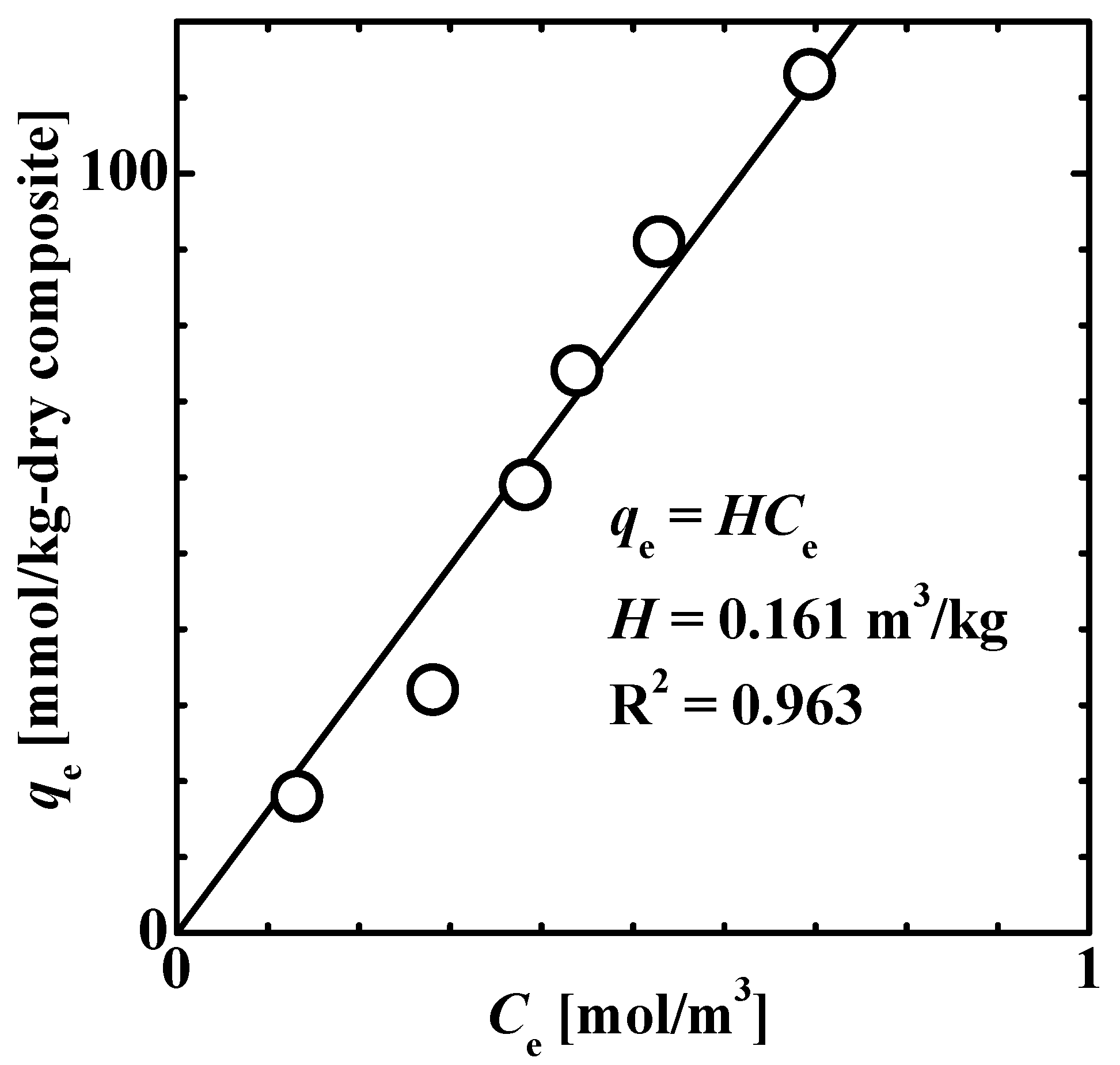
3.2. Adsorption Properties
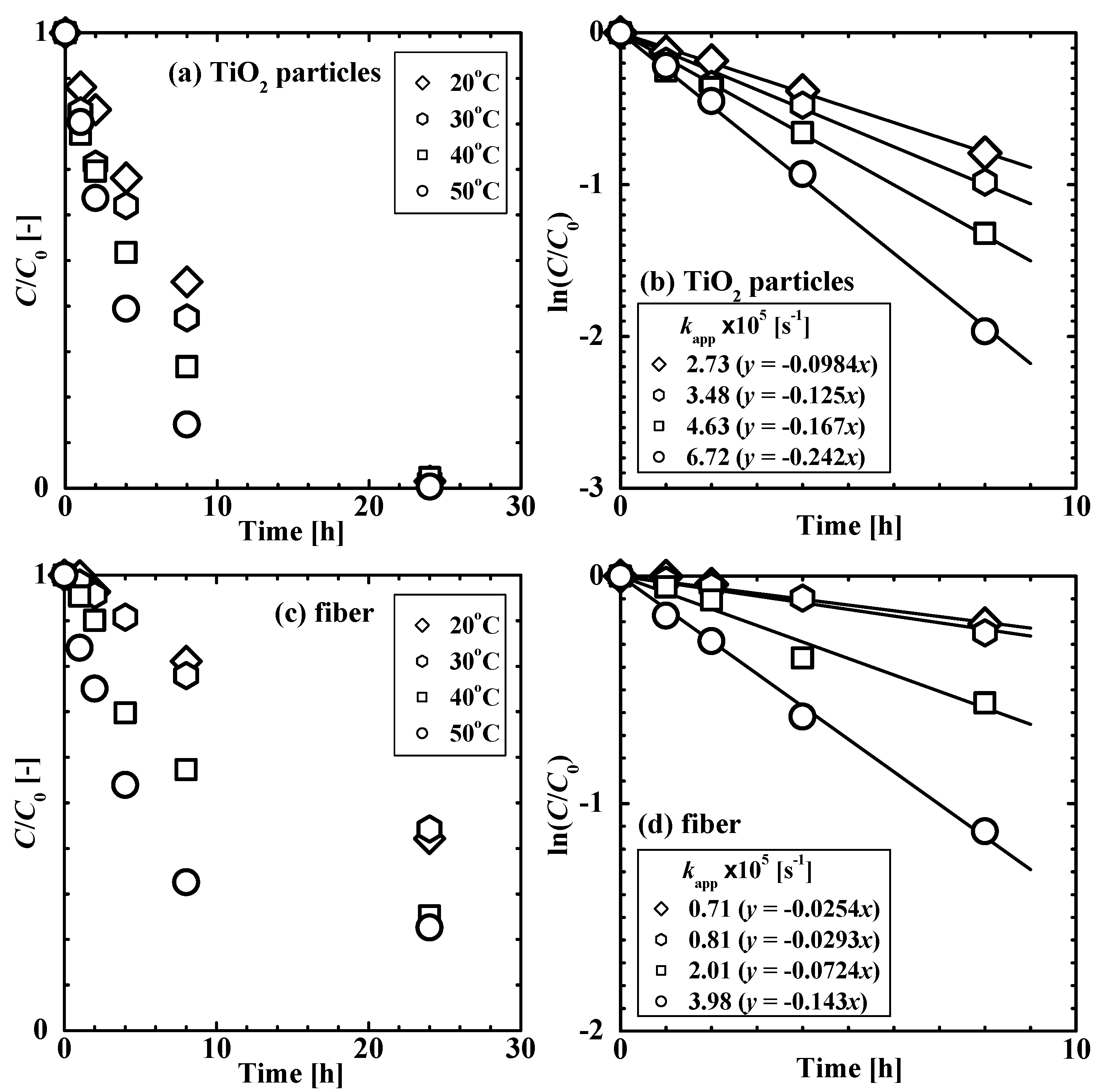
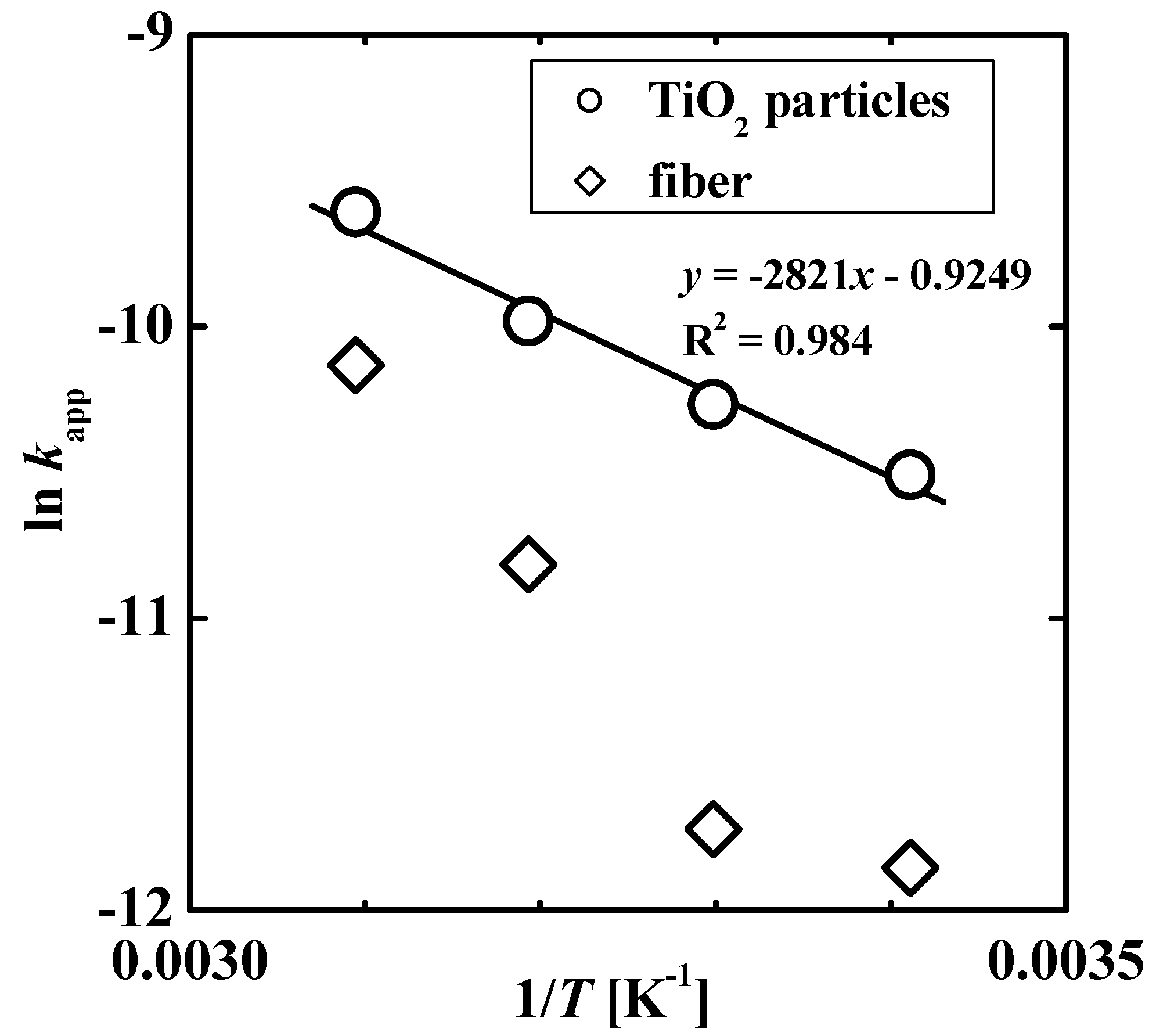
3.3. Photocatalytic Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatnagar, A.; Anastopoulos, I. Adsorptive removal of bisphenol A (BPA) from aqueous solution: A review. Chemosphere 2017, 168, 885–902. [Google Scholar] [CrossRef]
- Watanabe, N.; Horikoshi, S.; Kawabe, H.; Sugie, Y.; Zhao, J.; Hidaka, H. Photodegradation mechanism for bisphenol A at the TiO2/H2O interfaces. Chemosphere 2003, 52, 851–859. [Google Scholar] [CrossRef]
- Wang, R.; Ren, D.; Xia, S.; Zhang, Y.; Zhao, J. Photocatalytic degradation of Bisphenol A (BPA) using immobilized TiO2 and UV illumination in a horizontal circulating bed photocatalytic reactor (HCBPR). J. Hazard. Mater. 2009, 169, 926–932. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Lee, M.-K.; Su, T.-Y.; Chang, Y.-M. Photodegradation of bisphenol-A in a batch TiO2 suspension reactor. J. Hazard. Mater. 2009, 168, 269–275. [Google Scholar] [CrossRef]
- He, J.; Kumar, A.; Khan, M.; Lo, I.M.C. Critical review of photocatalytic disinfection of bacteria: From noble metals- and carbon nanomaterials-TiO2 composites to challenges of water characteristics and strategic solutions. Sci. Total Environ. 2021, 758, 143953. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S.; Yoshimura, C. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: A critical review of recent literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wang, X.; Liu, W. The synthesis strategies and photocatalytic performances of TiO2/MOFs composites: A state-of-the-art review. Chem. Eng. J. 2020, 391, 123601. [Google Scholar] [CrossRef]
- Wang, S.Q.; Liu, Q.L.; Zhu, A.M. Preparation of multisensitive poly (N-isopropylacrylamide-co-acrylic acid)/TiO2 composites for degradation of methyl orange. Eur. Polym. J. 2011, 47, 1168–1175. [Google Scholar] [CrossRef]
- Lučić, M.; Milosavljević, N.; Radetić, M.; Šaponjić, Z.; Radoičić, M.; Krušić, M.K. The potential application of TiO2/hydrogel nanocomposite for removal of various textile azo dyes. Sep. Purif. Technol. 2014, 122, 206–216. [Google Scholar] [CrossRef]
- Zhou, J.; Hao, B.; Wang, L.; Ma, J.; Cheng, W. Preparation and characterization of nano-TiO2/chitosan/poly(N-isopropylacrylamide) composite hydrogel and its application for removal of ionic dyes. Sep. Purif. Technol. 2017, 176, 193–199. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Deng, B. Electrospinning and in situ nitrogen doping of TiO2/PAN nanofibers with photocatalytic activation in visible lights. Mater. Lett. 2012, 82, 102–104. [Google Scholar] [CrossRef]
- Su, J.; Yang, G.; Cheng, C.; Huang, C.; Xu, H.; Ke, Q. Hierarchically structured TiO2/PAN nanofibrous membranes for high-efficiency air filtration and toluene degradation. J. Colloid Interface Sci. 2017, 507, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Im, J.S.; Kim, M.I.; Lee, Y.-S. Preparation of PAN-based electrospun nanofiber webs containing TiO2 for photocatalytic degradation. Mater. Lett. 2008, 62, 3652–3655. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S. Multifunctionality of poly(vinyl alcohol) nanofiber webs containing titanium dioxide. J. Appl. Polym. Sci. 2012, 124, 4038–4046. [Google Scholar] [CrossRef]
- Sun, F.; Ren, H.-T.; Li, T.-T.; Huang, S.-Y.; Zhang, Y.; Lou, C.-W.; Lin, J.-H. Bioinspired design of underwater superoleophobic Poly(N-isopropylacrylamide)/polyacrylonitrile/TiO2 nanofibrous membranes for highly efficient oil/water separation and photocatalysis. Environ. Res. 2020, 186, 109494. [Google Scholar] [CrossRef]
- Song, M.; Pan, C.; Li, J.; Zhang, R.; Wang, X.; Gu, Z. Blends of TiO2 nanoparticles and poly (N-isopropylacrylamide)-co-polystyrene nanofibers as a means to promote the biorecognition of an anticancer drug. Talanta 2008, 75, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, Y.; Tanaka, T. Volume phase transition in a nonionic gel. J. Chem. Phys. 1984, 81, 6379–6380. [Google Scholar] [CrossRef]
- Tokuyama, H.; Mori, H.; Hamaguchi, R.; Kato, G. Prediction of the lower critical solution temperature of poly(N-isopropylacrylamide-co-methoxy triethyleneglycol acrylate) in aqueous salt solutions using support vector regression. Chem. Eng. Sci. 2021, 231, 116325. [Google Scholar] [CrossRef]
- Seida, Y.; Nakano, Y. Effect of salt on the property of adsorption in thermosensitive polymer hydrogel. J. Chem. Eng. Jpn. 1996, 29, 767–772. [Google Scholar] [CrossRef][Green Version]
- Morisada, S.; Suzuki, H.; Emura, S.; Hirokawa, Y.; Nakano, Y. Temperature-swing adsorption of aromatic compounds in water using polyampholyte gel. Adsorption 2008, 14, 621–628. [Google Scholar] [CrossRef]
- Tokuyama, H.; Iwama, T. Temperature-swing solid-phase extraction of heavy metals on poly(N-isopropylacrylamide) hydrogel. Langmuir 2007, 23, 13104–13108. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, H.; Iwama, T. Solid-phase extraction of indium(III) ions onto thermosensitive poly(N-isopropylacrylamide). Sep. Purif. Technol. 2009, 68, 417–421. [Google Scholar] [CrossRef]
- Chuang, W.-J.; Chiu, W.-Y. Thermo-responsive nanofibers prepared from poly(N-isopropylacrylamide-co-N-methylol acrylamide). Polymer 2012, 53, 2829–2838. [Google Scholar] [CrossRef]
- Sanchez, L.; Peral, J.; Domenech, X. Photocatalyzed destruction of aniline in UV-illuminated aqueous TiO2 suspensions. Electrochim. Acta 1997, 42, 1877–1882. [Google Scholar] [CrossRef]
- Peiró, A.M.; Ayllón, J.A.; Peral, J.; Doménech, X. T TIO2-photocatalyzed degradation of phenol and ortho-substituted phenolic compounds. Appl. Catal. B 2001, 30, 359–373. [Google Scholar] [CrossRef]
- Sauer, T.; Neto, G.C.; José, H.J.; Moreira, R.F.P.M. Kinetics of photocatalytic degradation of reactive dyes in a TiO2 slurry reactor. J. Photochem. Photobiol. A 2002, 149, 147–154. [Google Scholar] [CrossRef]
- Mills, A.; Morris, S. Photomineralization of 4-chlorophenol sensitized by titanium-dioxide—A study of the initial kinetics of carbon-dioxide photogeneration. J. Photochem. Photobiol. A 1993, 71, 75–83. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokuyama, H.; Hamaguchi, R. TiO2 Nanoparticle-Loaded Poly(NIPA-co-NMA) Fiber Web for the Adsorption and Photocatalytic Degradation of 4-Isopropylphenol. Gels 2022, 8, 137. https://doi.org/10.3390/gels8020137
Tokuyama H, Hamaguchi R. TiO2 Nanoparticle-Loaded Poly(NIPA-co-NMA) Fiber Web for the Adsorption and Photocatalytic Degradation of 4-Isopropylphenol. Gels. 2022; 8(2):137. https://doi.org/10.3390/gels8020137
Chicago/Turabian StyleTokuyama, Hideaki, and Ryosuke Hamaguchi. 2022. "TiO2 Nanoparticle-Loaded Poly(NIPA-co-NMA) Fiber Web for the Adsorption and Photocatalytic Degradation of 4-Isopropylphenol" Gels 8, no. 2: 137. https://doi.org/10.3390/gels8020137
APA StyleTokuyama, H., & Hamaguchi, R. (2022). TiO2 Nanoparticle-Loaded Poly(NIPA-co-NMA) Fiber Web for the Adsorption and Photocatalytic Degradation of 4-Isopropylphenol. Gels, 8(2), 137. https://doi.org/10.3390/gels8020137




