Catalytic Reduction of Environmental Pollutants with Biopolymer Hydrogel Cross-Linked Gelatin Conjugated Tin-Doped Gadolinium Oxide Nanocomposites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
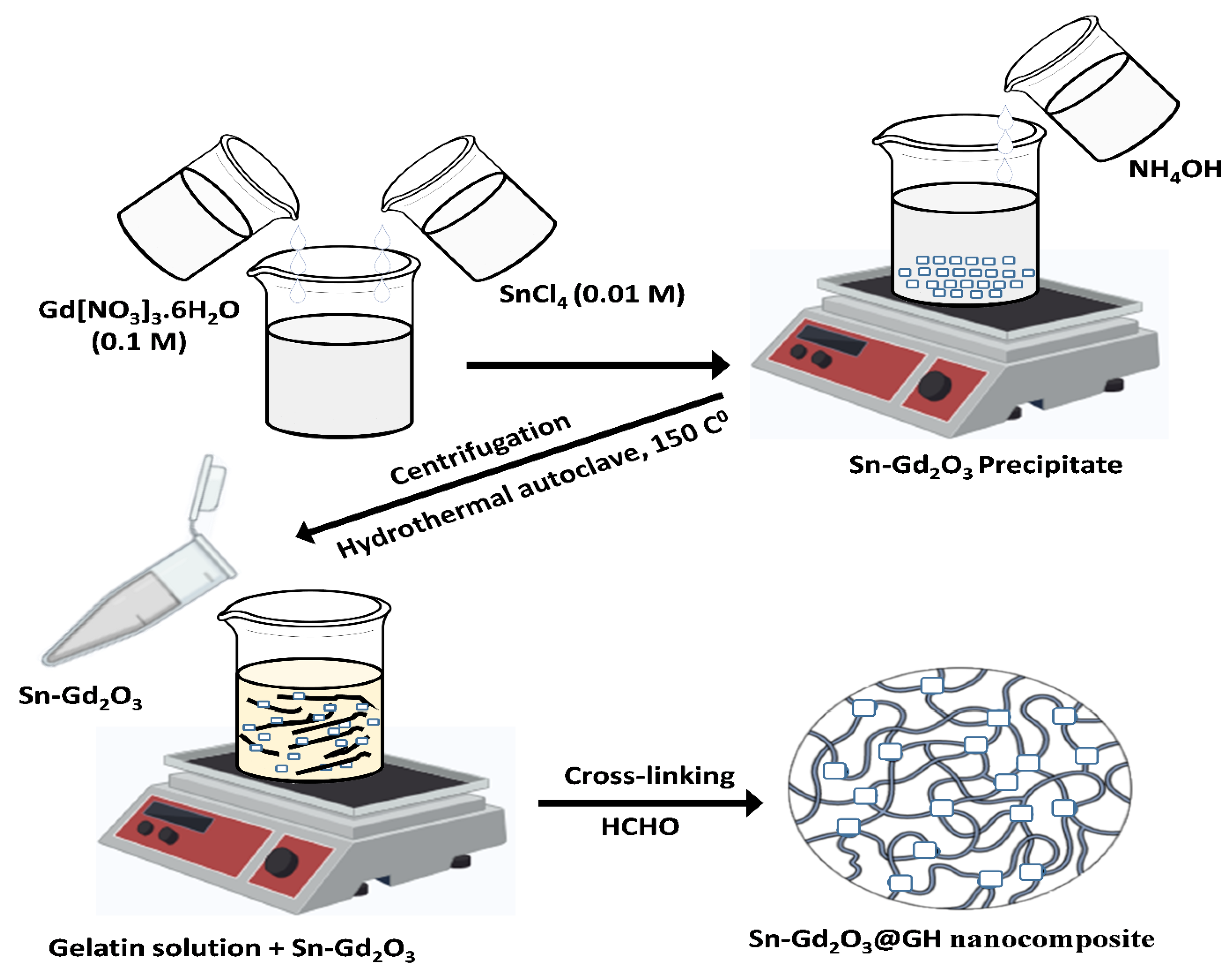
2.2. Synthesis of Sn-Gd2O3 Nanomaterial
2.3. Preparation of Gelatin Hydrogel Solution
2.4. Fabrication of Sn-Gd2O3@GH Nanocomposite
2.5. Preparation of Dyes Solution
3. Characterizations
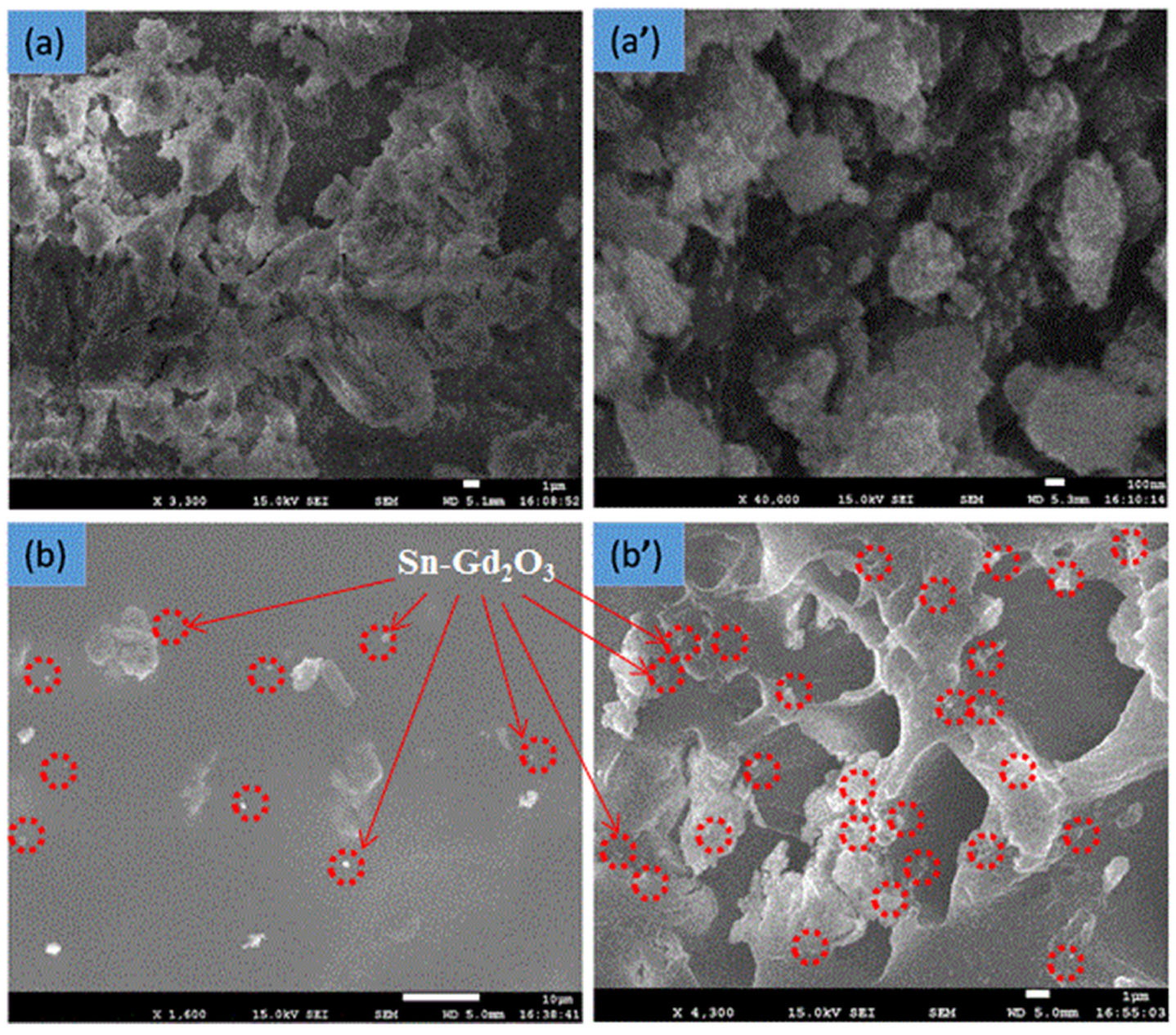
3.1. FESEM Analysis
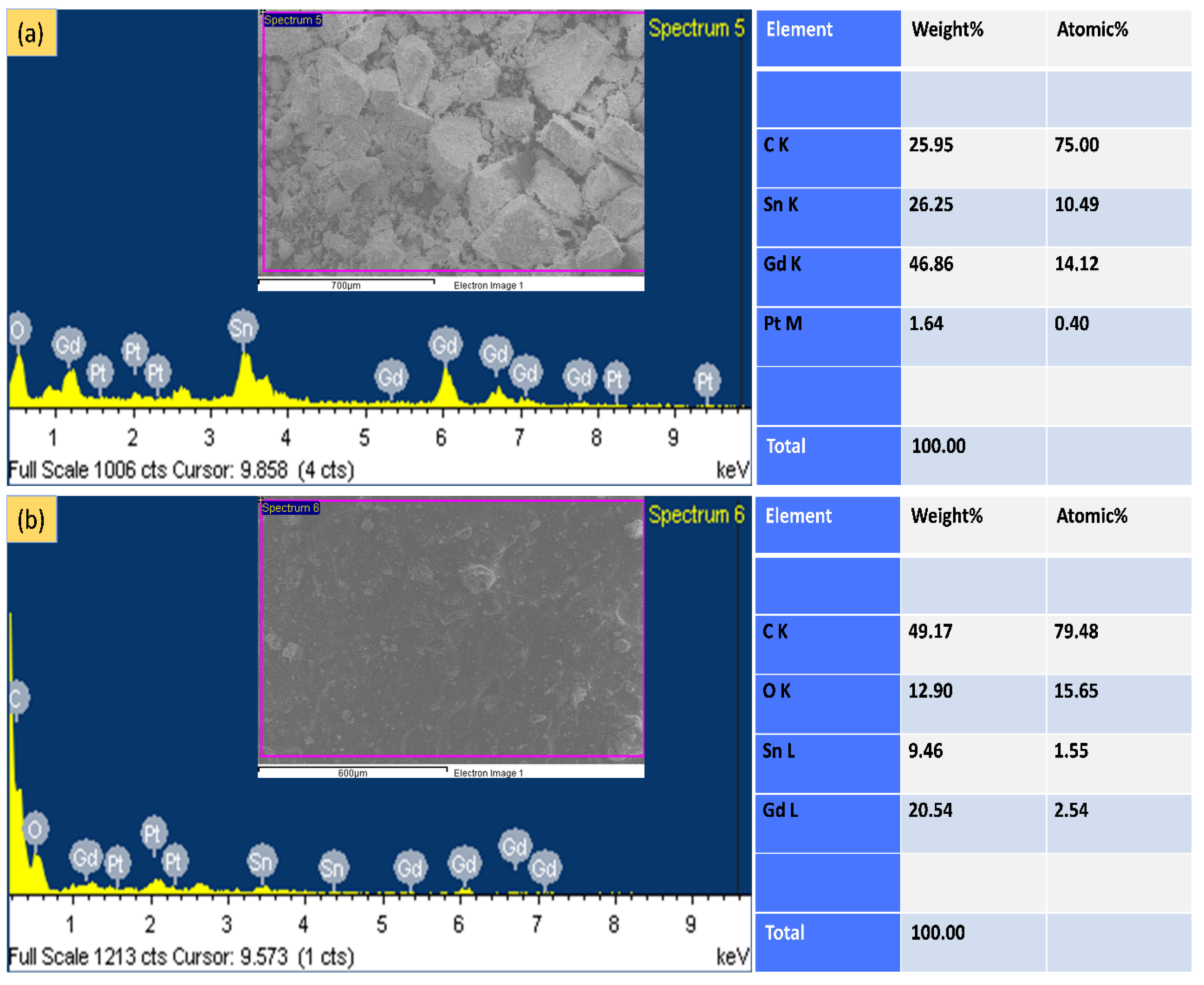
3.2. EDX Analysis
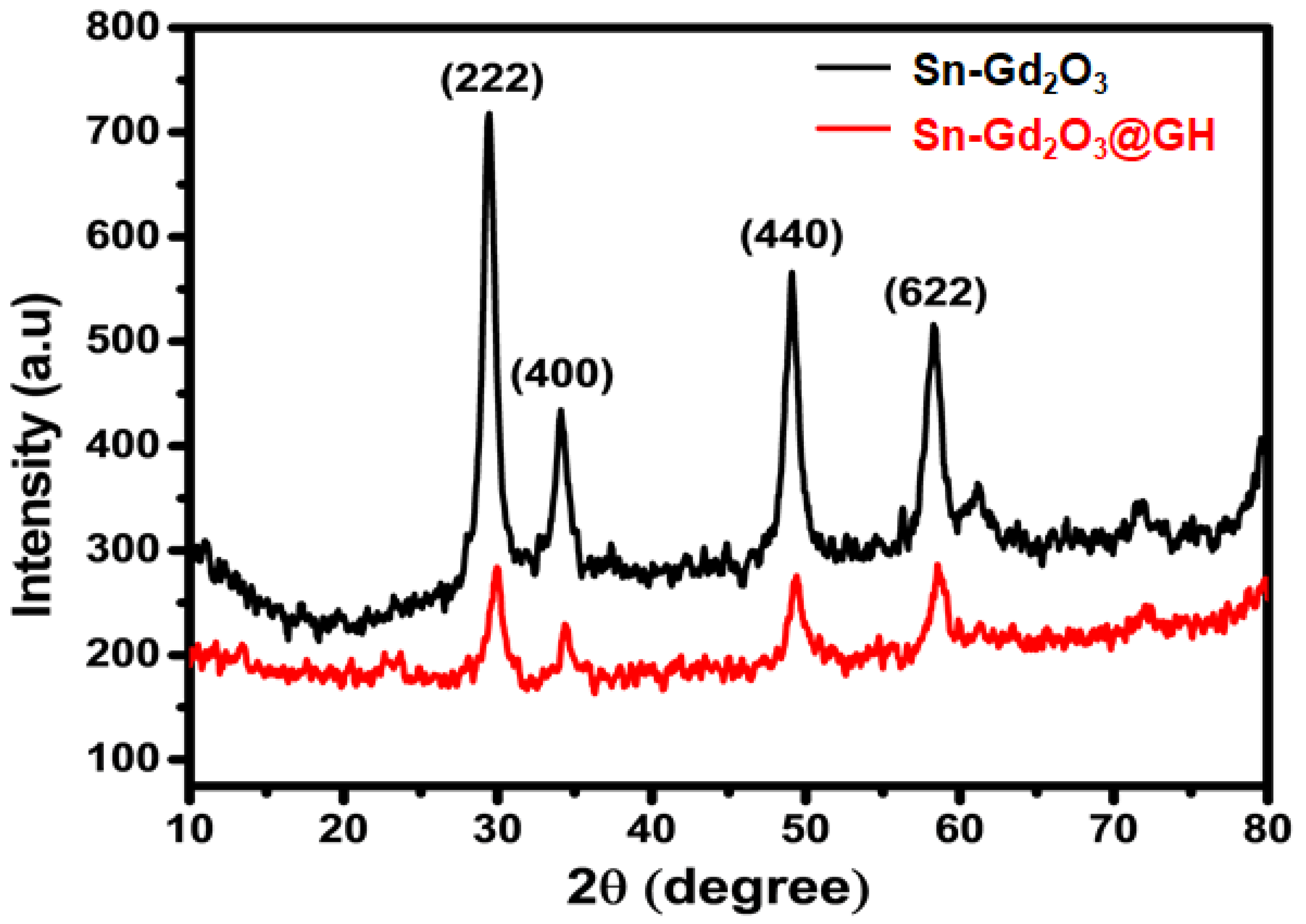
3.3. XRD Analysis
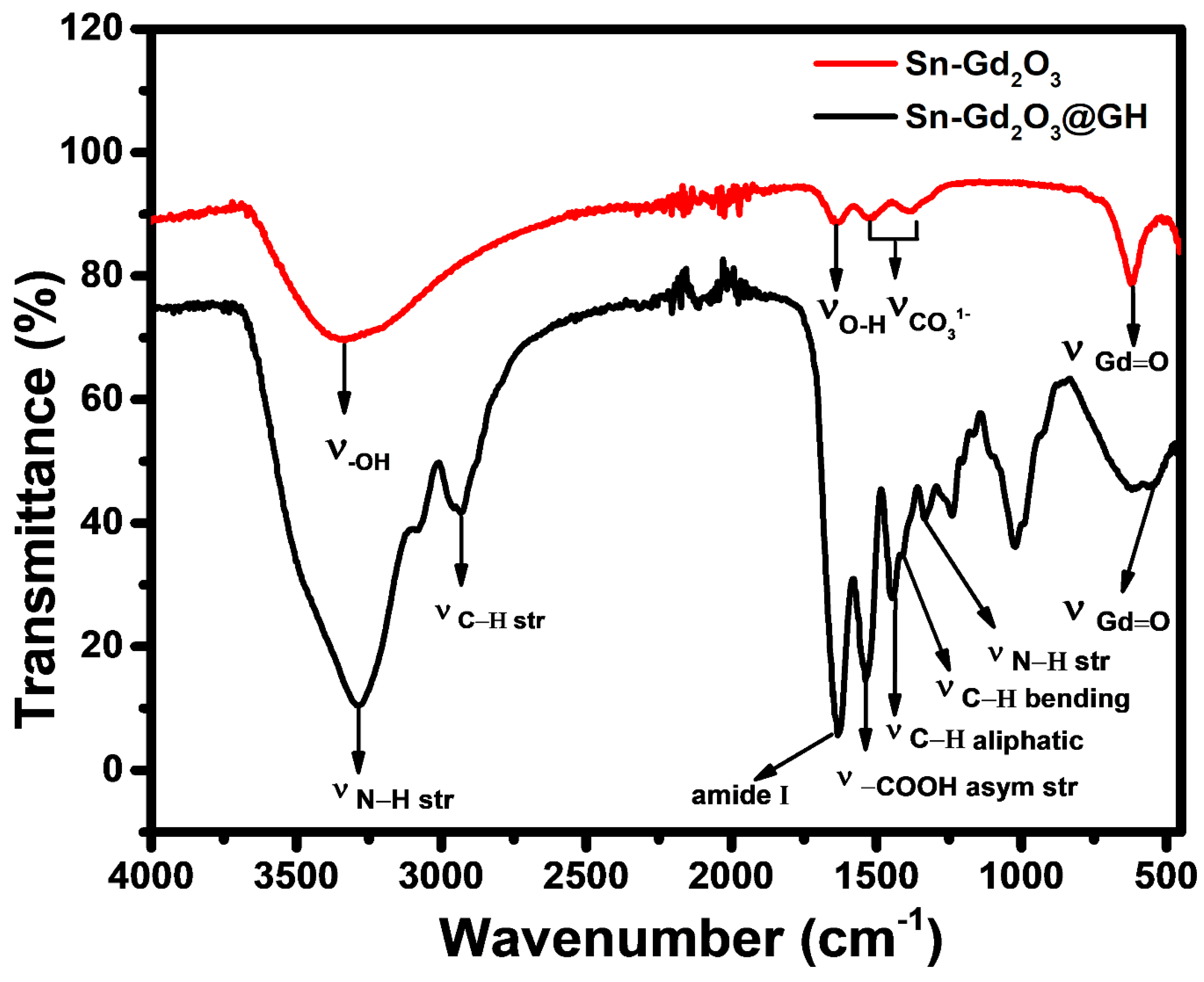
3.4. FTIR Analysis
4. Result and Discussion
4.1. Dyes Reduction
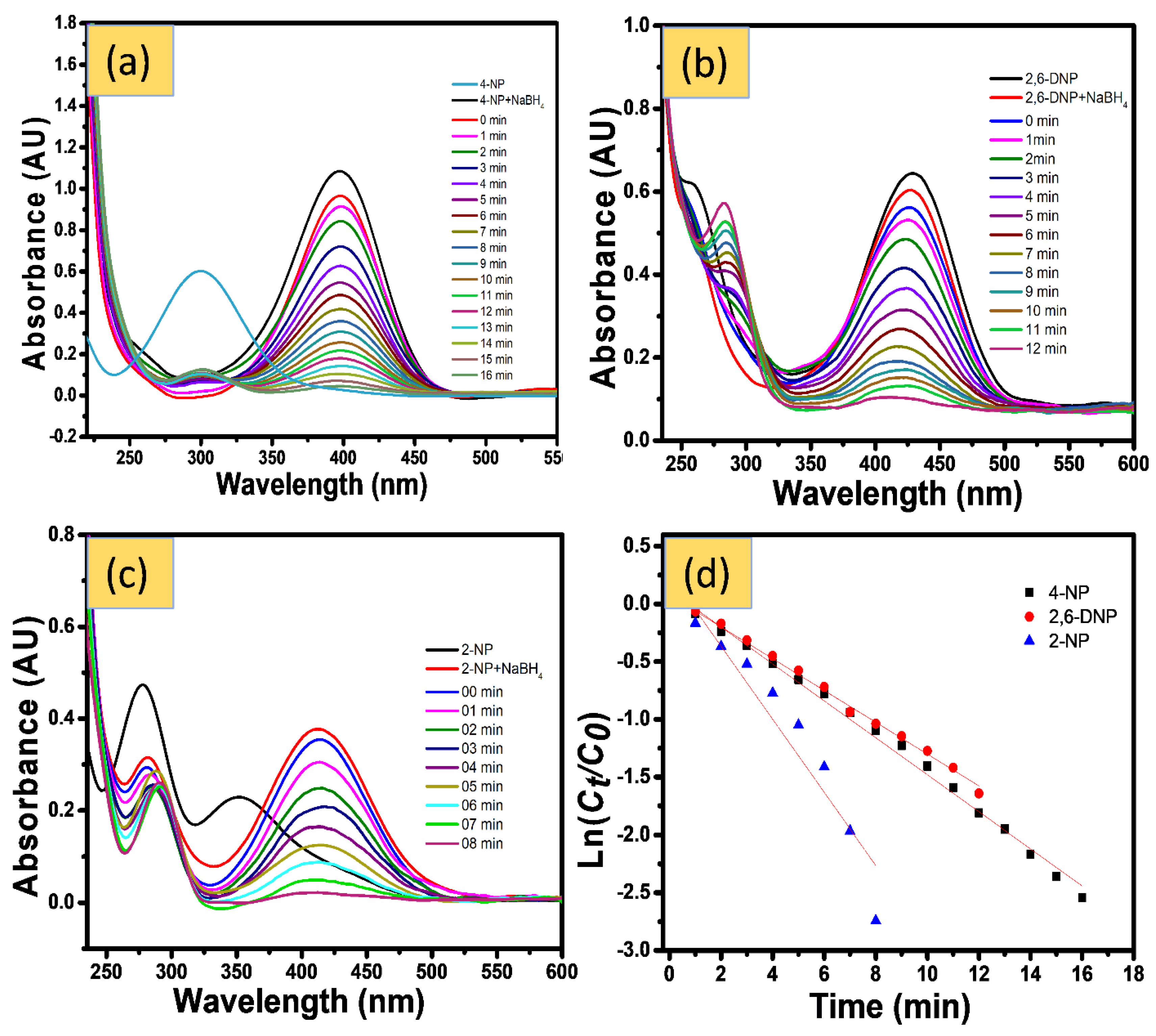
4.1.1. Catalytic Reduction of NPs
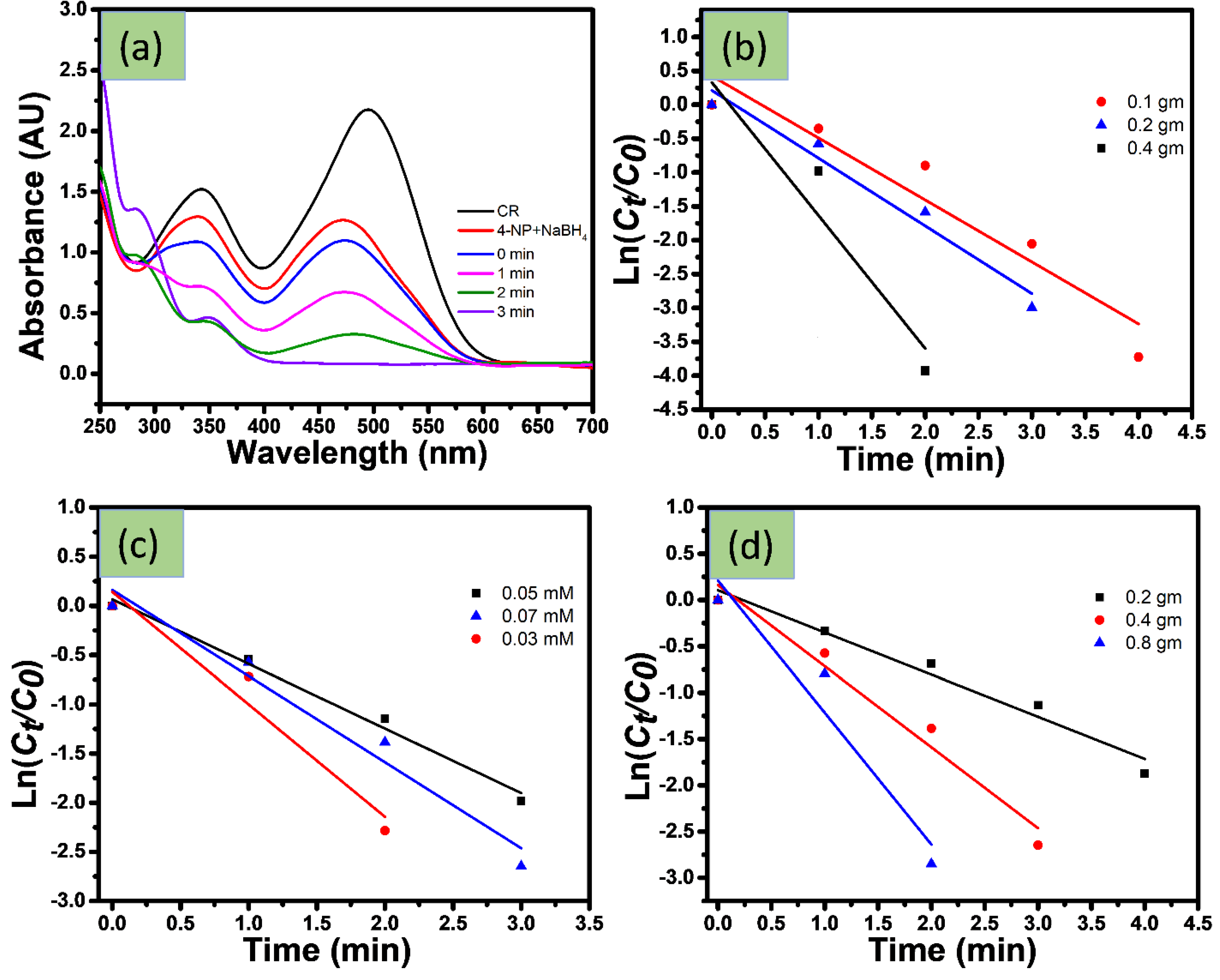
4.1.2. Catalytic Reduction of Azo Dyes
4.1.3. Catalytic Reduction Changing Experimental Parameter
5. Mechanism of Reduction
6. Reusability of Catalyst
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phipps, G.L.; Holcombe, G.W.; Fiandt, J.T. Acute toxicity of phenol and substituted phenols to the fathead minnow. Bull. Environ. Contam. Toxicol. 1981, 26, 585–593. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Ali, N.; Azeem, S.; Khan, A.; Khan, H.; Kamal, T.; Asiri, A.M. Experimental studies on removal of arsenites from industrial effluents using tridodecylamine supported liquid membrane. Environ. Sci Pollut. Res. 2020, 27, 11932–11943. [Google Scholar] [CrossRef]
- Devi, L.G.; Kumar, S.G.; Reddy, K.M.; Munikrishnappa, C. Photo degradation of Methyl Orange an azo dye by Advanced Fenton Process using zero valent metallic iron: Influence of various reaction parameters and its degradation mechanism. J. Hazard. Mater. 2009, 164, 459–467. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Kamal, T.; Khan, S.B.; Asiri, A.M. Nickel nanoparticles-chitosan composite coated cellulose filter paper: An efficient and easily recoverable dip-catalyst for pollutants degradation. Environ. Pollut. 2016, 218, 625–633. [Google Scholar] [CrossRef]
- Haider, S.; Kamal, T.; Khan, S.B.; Omer, M.; Haider, A.; Khan, F.U.; Asiri, A.M. Natural polymers supported copper nanoparticles for pollutants degradation. Appl. Surf. Sci. 2016, 387, 1154–1161. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, S.B.; Kamal, T.; Asiri, A.M. Visible light activated degradation of organic pollutants using zinc-iron selenide. J. Mol. Liq. 2017, 229, 429–435. [Google Scholar] [CrossRef]
- Kamal, T.; Ahmad, I.; Khan, S.B.; Ul-Islam, M.; Asiri, A.M. Microwave Assisted Synthesis and Carboxymethyl Cellulose Stabilized Copper Nanoparticles on Bacterial Cellulose Nanofibers Support for Pollutants Degradation. J. Polym. Environ. 2019, 27, 2867–2877. [Google Scholar] [CrossRef]
- Ahmad, I.; Kamal, T.; Khan, S.B.; Asiri, A.M. An efficient and easily retrievable dip catalyst based on silver nanoparticles/chitosan-coated cellulose filter paper. Cellulose 2016, 23, 3577–3588. [Google Scholar] [CrossRef]
- Kamal, T.; Ahmad, I.; Khan, S.B.; Asiri, A.M. Synthesis and catalytic properties of silver nanoparticles supported on porous cellulose acetate sheets and wet-spun fibers. Carbohydr. Polym. 2017, 157, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.J.; Khan, S.B.; Kamal, T.; Asiri, A.M. Agarose biopolymer coating on polyurethane sponge as host for catalytic silver metal nanoparticles. Polym. Test. 2019, 78, 105983. [Google Scholar] [CrossRef]
- Ali, N.; Ismail, M.; Khan, A.; Khan, H.; Haider, S.; Kamal, T. Spectrophotometric methods for the determination of urea in real samples using silver nanoparticles by standard addition and 2nd order derivative methods. Spectroc. Acta Part A Molec. Biomolec. Spectr. 2018, 189, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Kamal, T.; Ali, N.; Naseem, A.A.; Khan, S.B.; Asiri, A.M. Polymer Nanocomposite Membranes for Antifouling Nanofiltration. Recent Pat. Nanotechnol. 2016, 10, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Kamal, T.; Ul-Islam, S.; Shah, N.; Park, J.K. Recent Advancement in Cellulose based Nanocomposite for Addressing Environmental Challenges. Recent Pat. Nanotechnol. 2016, 10, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Kamal, T.; Khan, S.A.; Anwar, Y.; Saeed, M.T.; Asiri, A.M.; Khan, S.B. Assessment of Anti-bacterial Ni-Al/chitosan Composite Spheres for Adsorption Assisted Photo-Degradation of Organic Pollutants. Curr. Nanosci. 2016, 12, 569–575. [Google Scholar] [CrossRef]
- Kamal, T.; Khan, S.B.; Asiri, A.M. Synthesis of zero-valent Cu nanoparticles in the chitosan coating layer on cellulose microfibers: Evaluation of azo dyes catalytic reduction. Cellulose 2016, 23, 1911–1923. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, S.B.; Kamal, T.; Yasir, M.; Asiri, A.M. Antibacterial nanocomposites based on chitosan/Co-MCM as a selective and efficient adsorbent for organic dyes. Int. J. Biol. Macromol. 2016, 91, 744–751. [Google Scholar] [CrossRef]
- Ali, F.; Khan, S.B.; Kamal, T.; Anwar, Y.; Alamry, K.A.; Asiri, A.M. Anti-bacterial chitosan/zinc phthalocyanine fibers supported metallic and bimetallic nanoparticles for the removal of organic pollutants. Carbohydr. Polym. 2017, 173, 676–689. [Google Scholar] [CrossRef]
- Ali, F.; Khan, S.B.; Kamal, T.; Anwar, Y.; Alamry, K.A.; Asiri, A.M. Bactericidal and catalytic performance of green nanocomposite based on chitosan/carbon black fiber supported monometallic and bimetallic nanoparticles. Chemosphere 2017, 188, 588–598. [Google Scholar] [CrossRef]
- Ali, N.; Awais; Kamal, T.; Ul-Islam, M.; Khan, A.; Shah, S.J.; Zada, A. Chitosan-coated cotton cloth supported copper nanoparticles for toxic dye reduction. Int. J. Biol. Macromol. 2018, 111, 832–838. [Google Scholar] [CrossRef]
- Ali, F.; Khan, S.B.; Kamal, T.; Alamry, K.A.; Asiri, A.M.; Sobahi, T.R.A. Chitosan coated cotton cloth supported zero-valent nanoparticles: Simple but economically viable, efficient and easily retrievable catalysts. Sci. Rep. 2017, 7, 16957. [Google Scholar] [CrossRef] [Green Version]
- Kamal, T.; Ul-Islam, M.; Khan, S.B.; Asiri, A.M. Adsorption and photocatalyst assisted dye removal and bactericidal performance of ZnO/chitosan coating layer. Int. J. Biol. Macromol. 2015, 81, 584–590. [Google Scholar] [CrossRef]
- Khan, S.B.; Khan, S.A.; Marwani, H.M.; Bakhsh, E.M.; Anwar, Y.; Kamal, T.; Asiri, A.M.; Akhtar, K. Anti-bacterial PES-cellulose composite spheres: Dual character toward extraction and catalytic reduction of nitrophenol. RSC Adv. 2016, 6, 110077–110090. [Google Scholar] [CrossRef]
- Khan, S.B.; Ali, F.; Kamal, T.; Anwar, Y.; Asiri, A.M.; Seo, J. CuO embedded chitosan spheres as antibacterial adsorbent for dyes. Int. J. Biol. Macromol. 2016, 88, 113–119. [Google Scholar] [CrossRef]
- Kamal, T.; Anwar, Y.; Khan, S.B.; Chani, M.T.S.; Asiri, A.M. Dye adsorption and bactericidal properties of TiO2/chitosan coating layer. Carbohydr. Polym. 2016, 148, 153–160. [Google Scholar] [CrossRef]
- Kavitha, T.; Haider, S.; Kamal, T.; Ul-Islam, M. Thermal decomposition of metal complex precursor as route to the synthesis of Co3O4 nanoparticles: Antibacterial activity and mechanism. J. Alloys Compd. 2017, 704, 296–302. [Google Scholar] [CrossRef]
- Ali, F.; Khan, S.B.; Kamal, T.; Alamry, K.A.; Bakhsh, E.M.; Asiri, A.M.; Sobahi, T.R.A. Synthesis and characterization of metal nanoparticles templated chitosan-SiO2 catalyst for the reduction of nitrophenols and dyes. Carbohydr. Polym. 2018, 192, 217–230. [Google Scholar] [CrossRef]
- Kamal, T. Aminophenols formation from nitrophenols using agar biopolymer hydrogel supported CuO nanoparticles catalyst. Polym. Test. 2019, 77, 105896. [Google Scholar] [CrossRef]
- Khan, F.U.; Asimullah; Khan, S.B.; Kamal, T.; Asiri, A.M.; Khan, I.U.; Akhtar, K. Novel combination of zero-valent Cu and Ag nanoparticles@ cellulose acetate nanocomposite for the reduction of 4-nitro phenol. Int. J. Biol. Macromol. 2017, 102, 868–877. [Google Scholar] [CrossRef]
- Kamal, T.; Ahmad, I.; Khan, S.B.; Asiri, A.M. Agar hydrogel supported metal nanoparticles catalyst for pollutants degradation in water. Desalin. Water Treat. 2018, 136, 290–298. [Google Scholar] [CrossRef]
- Khan, M.S.J.; Khan, S.B.; Kamal, T.; Asiri, A.M. Catalytic Application of Silver Nanoparticles in Chitosan Hydrogel Prepared by a Facile Method. J. Polym. Environ. 2020, 28, 962–972. [Google Scholar] [CrossRef]
- Al-Mubaddel, F.S.; Haider, S.; Aijaz, M.O.; Haider, A.; Kamal, T.; Almasry, W.A.; Javid, M.; Khan, S.U.-D. Preparation of the chitosan/polyacrylonitrile semi-IPN hydrogel via glutaraldehyde vapors for the removal of Rhodamine B dye. Polym. Bull. 2017, 74, 1535–1551. [Google Scholar] [CrossRef]
- Islam, M.T.; Kamal, T.; Shin, T.; Seong, B.; Park, S.-Y. Self-assembly of a liquid crystal ABA triblock copolymer in a nematic liquid crystal solvent. Polymer 2014, 55, 3995–4002. [Google Scholar] [CrossRef]
- Kamal, T.; Ahmad, I.; Khan, S.B.; Asiri, A.M. Bacterial cellulose as support for biopolymer stabilized catalytic cobalt nanoparticles. Int. J. Biol. Macromol. 2019, 135, 1162–1170. [Google Scholar] [CrossRef]
- Klinger, J.M. A historical geography of rare earth elements: From discovery to the atomic age. Extr. Ind. Soc. 2015, 2, 572–580. [Google Scholar] [CrossRef]
- Chu, S. US Department of Energy “Critical Materials Strategy”; Technical Report; U.S. Department of Energy: Washington, DC, USA, 2011.
- Adachi, G.; Imanaka, N. The Binary Rare Earth Oxides. Chem. Rev. 1998, 98, 1479–1514. [Google Scholar] [CrossRef]
- Kaita, S.; Hou, Z.; Nishiura, M.; Doi, Y.; Kurazumi, J.; Horiuchi, A.C.; Wakatsuki, Y. Ultimately Specific 1,4-cis Polymerization of 1,3-Butadiene with a Novel Gadolinium Catalyst. Macromol. Rapid Commun. 2003, 24, 179–184. [Google Scholar] [CrossRef]
- Yang, J.; Li, C.; Cheng, Z.; Zhang, X.; Quan, Z.; Zhang, C.; Lin, J. Size-tailored synthesis and luminescent properties of one-dimensional Gd2O3: Eu3+ nanorods and microrods. J. Phys. Chem. C 2007, 111, 18148–18154. [Google Scholar] [CrossRef]
- Imtiaz, A.; Farrukh, M.A.; Khaleeq-ur-Rahman, M.; Adnan, R. Micelle-assisted synthesis of Al2O3 CaO nanocatalyst: Optical properties and their applications in photodegradation of 2, 4, 6-trinitrophenol. Sci. World J. 2013, 2013, 641420. [Google Scholar]
- Farrukh, M.A.; Teck, H.B.; Adnan, R. Surfactant-controlled aqueous synthesis of SnO_2 nanoparticles via the hydrothermal and conventional heating methods. Turk. J. Chem. 2010, 34, 537–550. [Google Scholar]
- Farrukh, M.A.; Tan, P.; Adnan, R. Influence of reaction parameters on the synthesis of surfactant-assisted tin oxide nanoparticles. Turk. J. Chem. 2012, 36, 303–314. [Google Scholar]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite Hydrogels: 3D Polymer–Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.; Lo, I.M.C. A holistic review of hydrogel applications in the adsorptive removal of aqueous pollutants: Recent progress, challenges, and perspectives. Water Res. 2016, 106, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Preparation and characterization of polyvinyl alcohol-gelatin hydrogel membranes for biomedical applications. AAPS PharmSciTech 2007, 8, E142–E146. [Google Scholar] [CrossRef]
- Madhumathi, K.; Shalumon, K.T.; Rani, V.V.D.; Tamura, H.; Furuike, T.; Selvamurugan, N.; Nair, S.V.; Jayakumar, R. Wet chemical synthesis of chitosan hydrogel–hydroxyapatite composite membranes for tissue engineering applications. Int. J. Biol. Macromol. 2009, 45, 12–15. [Google Scholar] [CrossRef]
- Kim, U.-J.; Park, J.; Li, C.; Jin, H.-J.; Valluzzi, R.; Kaplan, D.L. Structure and Properties of Silk Hydrogels. Biomacromolecules 2004, 5, 786–792. [Google Scholar] [CrossRef]
- Ali, F.; Khan, S.B.; Kamal, T.; Alamry, K.A.; Asiri, A.M. Chitosan-titanium oxide fibers supported zero-valent nanoparticles: Highly efficient and easily retrievable catalyst for the removal of organic pollutants. Sci. Rep. 2018, 8, 6260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haider, A.; Haider, S.; Kang, I.-K.; Kumar, A.; Kummara, M.R.; Kamal, T.; Han, S.S. A novel use of cellulose based filter paper containing silver nanoparticles for its potential application as wound dressing agent. Int. J. Biol. Macromol. 2018, 108, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Govender, P.P.; Mamo, M.A.; Tamulevicius, S.; Thakur, V.K. Recent progress in gelatin hydrogel nanocomposites for water purification and beyond. Vacuum 2017, 146, 396–408. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Cheng, Q.; Ye, D.; Chang, C. Tunicate cellulose nanocrystals reinforced nanocomposite hydrogels comprised by hybrid cross-linked networks. Carbohydr. Polym. 2017, 169, 139–148. [Google Scholar] [CrossRef]
- Lai, J.-Y. Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use. J. Mater. Sci. Mater. Med. 2010, 21, 1899–1911. [Google Scholar] [CrossRef]
- Karadağ, E.; Kundakcı, S. Application of highly swollen novel biosorbent hydrogels in uptake of uranyl ions from aqueous solutions. Fibers Polym. 2015, 16, 2165–2176. [Google Scholar] [CrossRef]
- Development of a Novel Tissue Adhesive using a Naturally-Derived Small Molecule. Available online: https://apps.dtic.mil/sti/citations/ADA429595 (accessed on 1 June 2003).
- Buhus, G.; Peptu, C.; Popa, M.; Desbrières, J. Controlled Release of Water Soluble Antibiotics by Carboxymethylcellulose- and Gelatin-based Hydrogels Crosslinked with Epichlorohydrin. Cellulose Chem. Technol. 2009, 43, 141–151. [Google Scholar]
- Zhang, X.; Do, M.D.; Casey, P.; Sulistio, A.; Qiao, G.G.; Lundin, L.; Lillford, P.; Kosaraju, S. Chemical Cross-Linking Gelatin with Natural Phenolic Compounds as Studied by High-Resolution NMR Spectroscopy. Biomacromolecules 2010, 11, 1125–1132. [Google Scholar] [CrossRef]
- Yi, Z.; Wen, B.; Qian, C.; Wang, H.; Rao, L.; Liu, H.; Zeng, S. Intense Red Upconversion Emission and Shape Controlled Synthesis of Gd2O3:Yb/Er Nanocrystals. Adv. Condens. Matter. Physics. 2013, 2013, 509374. [Google Scholar] [CrossRef] [Green Version]
- Aghazadeh, M. Preparation of Gd2O3 Ultrafine Nanoparticles by Pulse Electrodeposition Followed by Heat-treatment Method. J. Ultrafine Grained Nanostruct. Mater. 2016, 49, 80–86. [Google Scholar]
- Liu, H.; Liu, J. Hollow mesoporous Gd2O3: Eu 3+ spheres with enhanced luminescence and their drug releasing behavior. RSC Adv. 2016, 6, 99158–99164. [Google Scholar] [CrossRef]
- Fu, Y.; Huang, T.; Jia, B.; Zhu, J.; Wang, X. Reduction of nitrophenols to aminophenols under concerted catalysis by Au/g-C3N4 contact system. Appl. Catal. B Environ. 2017, 202, 430–437. [Google Scholar] [CrossRef]
- Jana, S.; Ghosh, S.K.; Nath, S.; Pande, S.; Praharaj, S.; Panigrahi, S.; Basu, S.; Endo, T.; Pal, T. Synthesis of silver nanoshell-coated cationic polystyrene beads: A solid phase catalyst for the reduction of 4-nitrophenol. Appl. Catal. A Gen. 2006, 313, 41–48. [Google Scholar] [CrossRef]
- Agarwal, S.; Tyagi, I.; Gupta, V.K.; Ghaedi, M.; Masoomzade, M.; Ghaedi, A.M.; Mirtamizdoust, B. RETRACTED: Kinetics and thermodynamics of methyl orange adsorption from aqueous solutions—Artificial neural network-particle swarm optimization modeling. J. Mol. Liq. 2016, 218, 354–362. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Alinezhad, H.; Nasrollahzadeh, M.; Kamali, T.A. Green synthesis of the Ag/HZSM-5 nanocomposite by using Euphorbia heterophylla leaf extract: A recoverable catalyst for reduction of organic dyes. J. Alloys Compd. 2016, 685, 258–265. [Google Scholar] [CrossRef]
- Ismail, M.; Gul, S.; Khan, M.I.; Khan, M.A.; Asiri, A.M.; Khan, S.B. Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange. Green Process. Synth. 2019, 8, 135–143. [Google Scholar] [CrossRef]
- Ilayaraja, M.; Krishnan, N.P.; Kannan, R.S. Adsorption of Rhodamine-B and Congo red dye from Aqueous Solution using Activated Carbon: Kinetics, Isotherms, and Thermodynamics. IOSR J. Environ. Sci. Toxicol. Food Technol. 2013, 5, 79–89. [Google Scholar]
- Dawood, S.; Sen, T.K. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, S.; Chatterjee, B.P.; Guha, A.K. Adsorptive removal of congo red, a carcinogenic textile dye by chitosan hydrobeads: Binding mechanism, equilibrium and kinetics. Colloids Surf. A Physicochem. Eng. Asp. 2007, 299, 146–152. [Google Scholar] [CrossRef]
- Indana, M.K.; Gangapuram, B.R.; Dadigala, R.; Bandi, R.; Guttena, V. A novel green synthesis and characterization of silver nanoparticles using gum tragacanth and evaluation of their potential catalytic reduction activities with methylene blue and Congo red dyes. J. Anal. Sci. Technol. 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Irfan, A. Removal of Congo red dye from aqueous medium by its catalytic reduction using sodium borohydride in the presence of various inorganic nano-catalysts: A review. J. Clean. Prod. 2018, 187, 296–307. [Google Scholar] [CrossRef]
- Khan, M.S.J.; Kamal, T.; Ali, F.; Asiri, A.M.; Khan, S.B. Chitosan-coated polyurethane sponge supported metal nanoparticles for catalytic reduction of organic pollutants. Int. J. Biol. Macromol. 2019, 132, 772–783. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marwani, H.M.; Ahmad, S.; Rahman, M.M. Catalytic Reduction of Environmental Pollutants with Biopolymer Hydrogel Cross-Linked Gelatin Conjugated Tin-Doped Gadolinium Oxide Nanocomposites. Gels 2022, 8, 86. https://doi.org/10.3390/gels8020086
Marwani HM, Ahmad S, Rahman MM. Catalytic Reduction of Environmental Pollutants with Biopolymer Hydrogel Cross-Linked Gelatin Conjugated Tin-Doped Gadolinium Oxide Nanocomposites. Gels. 2022; 8(2):86. https://doi.org/10.3390/gels8020086
Chicago/Turabian StyleMarwani, Hadi M., Shahid Ahmad, and Mohammed M. Rahman. 2022. "Catalytic Reduction of Environmental Pollutants with Biopolymer Hydrogel Cross-Linked Gelatin Conjugated Tin-Doped Gadolinium Oxide Nanocomposites" Gels 8, no. 2: 86. https://doi.org/10.3390/gels8020086






