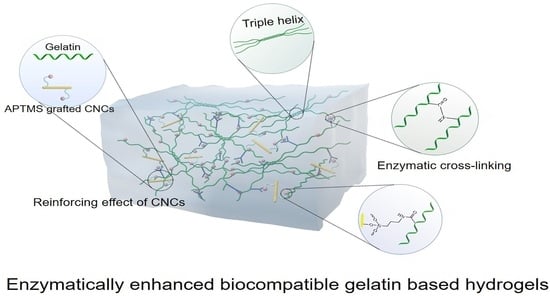
The Preparation and Properties of Composite Hydrogels Based on Gelatin and (3-Aminopropyl) Trimethoxysilane Grafted Cellulose Nanocrystals Covalently Linked with Microbial Transglutaminase
Abstract
:1. Introduction
2. Results and Discussion
2.1. FTIR Spectroscopy
2.2. XPS
2.3. Rheological Behavior
2.4. Gel Strength
2.5. SEM
2.6. Biocompatibility Assays
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Gel-TG-Si-CNCs Hydrogels
4.3. Characterization
4.4. Rheological Behavior
4.5. Gel Strength Test
4.6. Biocompatibility Assays
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Serafin, A.; Murphy, C.; Rubio, M.C.; Collins, M.N. Printable alginate/gelatin hydrogel reinforced with carbon nanofibers as electrically conductive scaffolds for tissue engineering. Mater. Sci. Eng. C 2021, 122, 111927. [Google Scholar] [CrossRef]
- Gritsch, L.; Motta, F.L.; Negrini, N.C.; Farè, S. Crosslinked gelatin hydrogels as carriers for controlled heparin release. Mater. Lett 2018, 228, 375–378. [Google Scholar] [CrossRef]
- Zhou, L.; Dai, C.; Fan, L.; Jiang, Y.; Liu, C.; Zhou, Z.; Guan, P.; Tian, Y.; Xing, J.; Li, X. Injectable Self-Healing Natural Biopolymer-Based Hydrogel Adhesive with Thermoresponsive Reversible Adhesion for Minimally Invasive Surgery. Adv. Funct. Mater. 2021, 31, 2007457. [Google Scholar] [CrossRef]
- Zhu, C.; Tang, N.; Gan, J.; Zhang, X.; Li, Y.; Jia, X.; Cheng, Y. A pH-sensitive semi-interpenetrating polymer network hydrogels constructed by konjac glucomannan and poly (γ-glutamic acid): Synthesis, characterization and swelling behavior. Int. J. Biol. Macromol. 2021. [Google Scholar] [CrossRef]
- Omata, K.; Matsuno, T.; Asano, K.; Hashimoto, Y.; Tabata, Y.; Satoh, T. Enhanced bone regeneration by gelatin–β-tricalcium phosphate composites enabling controlled release of bFGF. J. Tissue Eng. Regen. Med. 2014, 8, 604–611. [Google Scholar] [CrossRef]
- Del Valle, L.J.; Díaz, A.; Puiggalí, J. Hydrogels for biomedical applications: Cellulose, chitosan, and protein/peptide derivatives. Gels 2017, 3, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-based hydrogels for biomedical applications. Mrs Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Babić Radić, M.M.; Filipović, V.V.; Vukomanović, M.; Nikodinović Runić, J.; Tomić, S.L. Degradable 2-Hydroxyethyl Methacrylate/Gelatin/Alginate Hydrogels Infused by Nanocolloidal Graphene Oxide as Promising Drug Delivery and Scaffolding Biomaterials. Gels 2022, 8, 22. [Google Scholar] [CrossRef]
- Lu, T.; Yu, K.; Kuo, S.; Cheng, N.; Chuang, E.; Yu, J. Enzyme-crosslinked gelatin hydrogel with adipose-derived stem cell spheroid facilitating wound repair in the murine burn model. Polymers 2020, 12, 2997. [Google Scholar] [CrossRef]
- Dey, K.; Agnelli, S.; Borsani, E.; Sartore, L. Degradation-Dependent Stress Relaxing Semi-Interpenetrating Networks of Hydroxyethyl Cellulose in Gelatin-PEG Hydrogel with Good Mechanical Stability and Reversibility. Gels 2021, 7, 277. [Google Scholar] [CrossRef] [PubMed]
- Takei, T.; Yoshihara, R.; Danjo, S.; Fukuhara, Y.; Evans, C.; Tomimatsu, R.; Ohzuno, Y.; Yoshida, M. Hydrophobically-modified gelatin hydrogel as a carrier for charged hydrophilic drugs and hydrophobic drugs. Int. J. Biol. Macromol. 2020, 149, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Wisotzki, E.I.; Hennes, M.; Schuldt, C.; Engert, F.; Knolle, W.; Decker, U.; Käs, J.A.; Zink, M.; Mayr, S.G. Tailoring the material properties of gelatin hydrogels by high energy electron irradiation. J. Mater. Chem. B 2014, 2, 4297–4309. [Google Scholar] [CrossRef] [PubMed]
- Foox, M.; Zilberman, M. Drug delivery from gelatin-based systems. Expert Opin. Drug Del. 2015, 12, 1547–1563. [Google Scholar] [CrossRef] [PubMed]
- Lai, J. Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use. J. Mater. Sci. Mater. Med. 2010, 21, 1899–1911. [Google Scholar] [CrossRef]
- Fuchs, S.; Kutscher, M.; Hertel, T.; Winter, G.; Pietzsch, M.; Coester, C. Transglutaminase: New insights into gelatin nanoparticle cross-linking. J. Microencapsul. 2010, 27, 747–754. [Google Scholar] [CrossRef]
- Sperinde, J.J.; Griffith, L.G. Synthesis and characterization of enzymatically-cross-linked poly (ethylene glycol) hydrogels. Macromolecules 1997, 30, 5255–5264. [Google Scholar] [CrossRef]
- Besser, R.R.; Bowles, A.C.; Alassaf, A.; Carbonero, D.; Claure, I.; Jones, E.; Reda, J.; Wubker, L.; Batchelor, W.; Ziebarth, N. Enzymatically crosslinked gelatin–laminin hydrogels for applications in neuromuscular tissue engineering. Biomater. Sci. 2020, 8, 591–606. [Google Scholar] [CrossRef]
- Yu, T.; Guan, Y.; Xie, X.; Huang, Y.; Tang, J. Improved thrombin hemostat using the cross-linked gelatin by microbial transglutaminase. Int. J. Polym. Sci. 2015, 2015, 985286. [Google Scholar] [CrossRef] [Green Version]
- Broderick, E.P.; O’Halloran, D.M.; Rochev, Y.A.; Griffin, M.; Collighan, R.J.; Pandit, A.S. Enzymatic stabilization of gelatin-based scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 72B, 37–42. [Google Scholar] [CrossRef]
- Hivechi, A.; Bahrami, S.H.; Siegel, R.A. Investigation of morphological, mechanical and biological properties of cellulose nanocrystal reinforced electrospun gelatin nanofibers. Int. J. Biol. Macromol. 2019, 124, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Foston, M.; Ragauskas, A.J. Improving the mechanical and thermal properties of gelatin hydrogels cross-linked by cellulose nanowhiskers. Carbohyd. Polym. 2013, 91, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Ooi, S.Y.; Ahmad, I. Amin MCIM. Cellulose nanocrystals extracted from rice husks as a reinforcing material in gelatin hydrogels for use in controlled drug delivery systems. Ind. Crop. Prod. 2016, 93, 227–234. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, J.; Shi, H.; Zhang, Q.; Feng, C.; Xv, X. Preparation of cellulose nanocrystal/oxidized dextran/gelatin (CNC/OD/GEL) hydrogels and fabrication of a CNC/OD/GEL scaffold by 3D printing. J. Mater. Sci. 2020, 55, 2618–2635. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, S.; Lu, W.; Chen, N.; Zhu, D.; Li, Y. Preparation and characterization of enzymatically cross-linked gelatin/cellulose nanocrystal composite hydrogels. RSC Adv. 2021, 11, 10794–10803. [Google Scholar] [CrossRef]
- Robles, E.; Urruzola, I.; Labidi, J.; Serrano, L. Surface-modified nano-cellulose as reinforcement in poly (lactic acid) to conform new composites. Ind. Crop. Prod. 2015, 71, 44–53. [Google Scholar] [CrossRef]
- Shazali, N.A.H.; Zaidi, N.E.; Ariffin, H.; Abdullah, L.C.; Ghaemi, F.; Abdullah, J.M.; Takashima, I. Characterization and cellular internalization of spherical cellulose nanocrystals (CNC) into normal and cancerous fibroblasts. Materials 2019, 12, 3251. [Google Scholar] [CrossRef] [Green Version]
- Raquez, J.; Murena, Y.; Goffin, A.; Habibi, Y.; Ruelle, B.; DeBuyl, F.; Dubois, P. Surface-modification of cellulose nanowhiskers and their use as nanoreinforcers into polylactide: A sustainably-integrated approach. Compos. Sci. Technol. 2012, 72, 544–549. [Google Scholar] [CrossRef]
- Lu, J.; Askeland, P.; Drzal, L.T. Surface modification of microfibrillated cellulose for epoxy composite applications. Polymer 2008, 49, 1285–1296. [Google Scholar] [CrossRef]
- Cui, X.; Lee, J.J.; Chen, W.N. Eco-friendly and biodegradable cellulose hydrogels produced from low cost okara: Towards non-toxic flexible electronics. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, R.; Wang, W.; Wei, X.; Li, J.; Chen, X.; Liu, Y.; Lu, F.; Li, Y. Tunable physical and mechanical properties of gelatin hydrogel after transglutaminase crosslinking on two gelatin types. Int. J. Biol. Macromol. 2020, 162, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Hamad, W.Y. Critical insights into the reinforcement potential of cellulose nanocrystals in polymer nanocomposites. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100761. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, D.; Lee, H.; Kim, D.; Jung, S. Succinoglycan dialdehyde-reinforced gelatin hydrogels with toughness and thermal stability. Int. J. Biol. Macromol. 2020, 149, 281–289. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, S.; Cao, S.; Krecker, M.; Tsukruk, V.V.; Singamaneni, S. Protein-based functional nanocomposites. Mrs Bull. 2020, 45, 1017–1026. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Impacts of nanowhisker on formation kinetics and properties of all-cellulose composite gels. Carbohyd Polym. 2011, 83, 1937–1946. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Chuah, Y.J.; Kuddannaya, S.; Lee, M.H.A.; Zhang, Y.; Kang, Y. The effects of poly (dimethylsiloxane) surface silanization on the mesenchymal stem cell fate. Biomater. Sci. 2015, 3, 383–390. [Google Scholar] [CrossRef]
- Vuppaladadium, S.S.R.; Agarwal, T.; Kulanthaivel, S.; Mohanty, B.; Barik, C.S.; Maiti, T.K.; Pal, S.; Pal, K. Banerjee, I. Silanization improves biocompatibility of graphene oxide. Mater. Sci. Eng. C 2020, 110, 110647. [Google Scholar] [CrossRef]








| C1s | O1s | N1s | Si2p | O/C | |
|---|---|---|---|---|---|
| CNCs | 60.36 | 39.64 | 0.66 | ||
| CNCs–Si | 59.03 | 38.01 | 1.42 | 1.54 | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Chen, Z.; Dong, Y.; Lu, W.; Zhu, D. The Preparation and Properties of Composite Hydrogels Based on Gelatin and (3-Aminopropyl) Trimethoxysilane Grafted Cellulose Nanocrystals Covalently Linked with Microbial Transglutaminase. Gels 2022, 8, 146. https://doi.org/10.3390/gels8030146
Zhao S, Chen Z, Dong Y, Lu W, Zhu D. The Preparation and Properties of Composite Hydrogels Based on Gelatin and (3-Aminopropyl) Trimethoxysilane Grafted Cellulose Nanocrystals Covalently Linked with Microbial Transglutaminase. Gels. 2022; 8(3):146. https://doi.org/10.3390/gels8030146
Chicago/Turabian StyleZhao, Shouwei, Zhiwei Chen, Yaqi Dong, Wenhui Lu, and Deyi Zhu. 2022. "The Preparation and Properties of Composite Hydrogels Based on Gelatin and (3-Aminopropyl) Trimethoxysilane Grafted Cellulose Nanocrystals Covalently Linked with Microbial Transglutaminase" Gels 8, no. 3: 146. https://doi.org/10.3390/gels8030146