Evaluation of the Physical Stability of Starch-Based Hydrogels Produced by High-Pressure Processing (HPP)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Conventional Evaluations
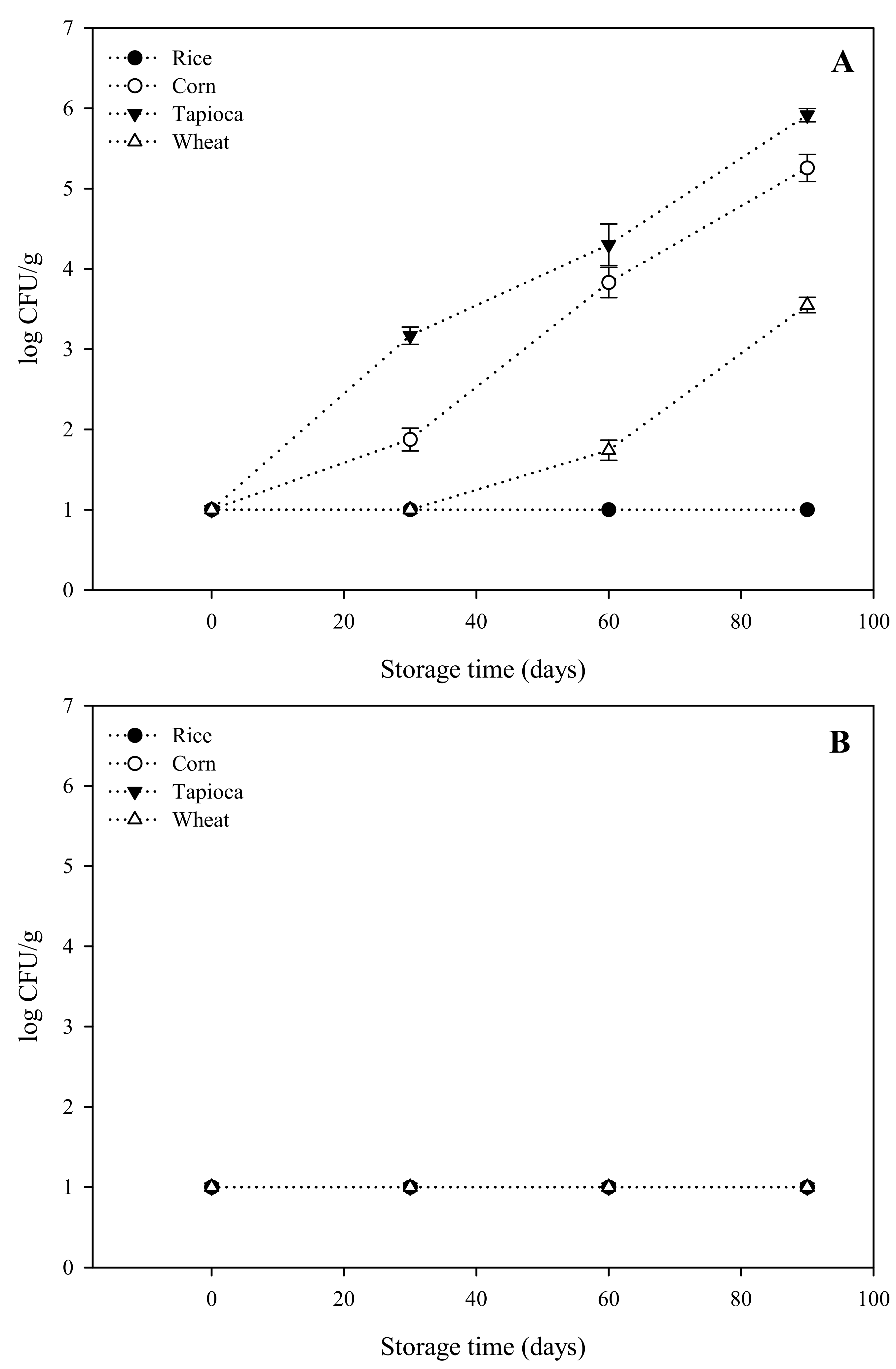
2.1.1. Microbiological Analysis
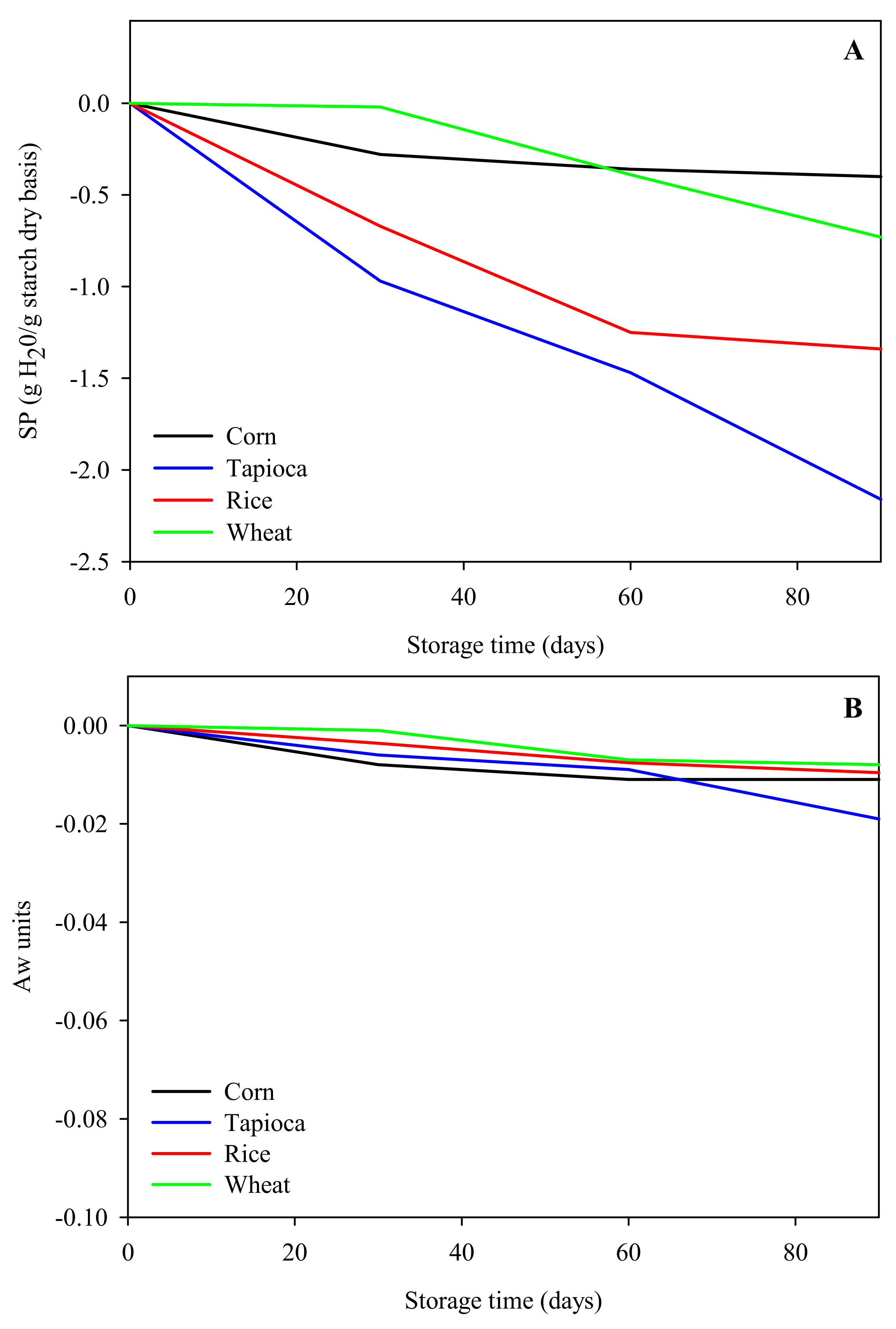
2.1.2. Swelling Stability
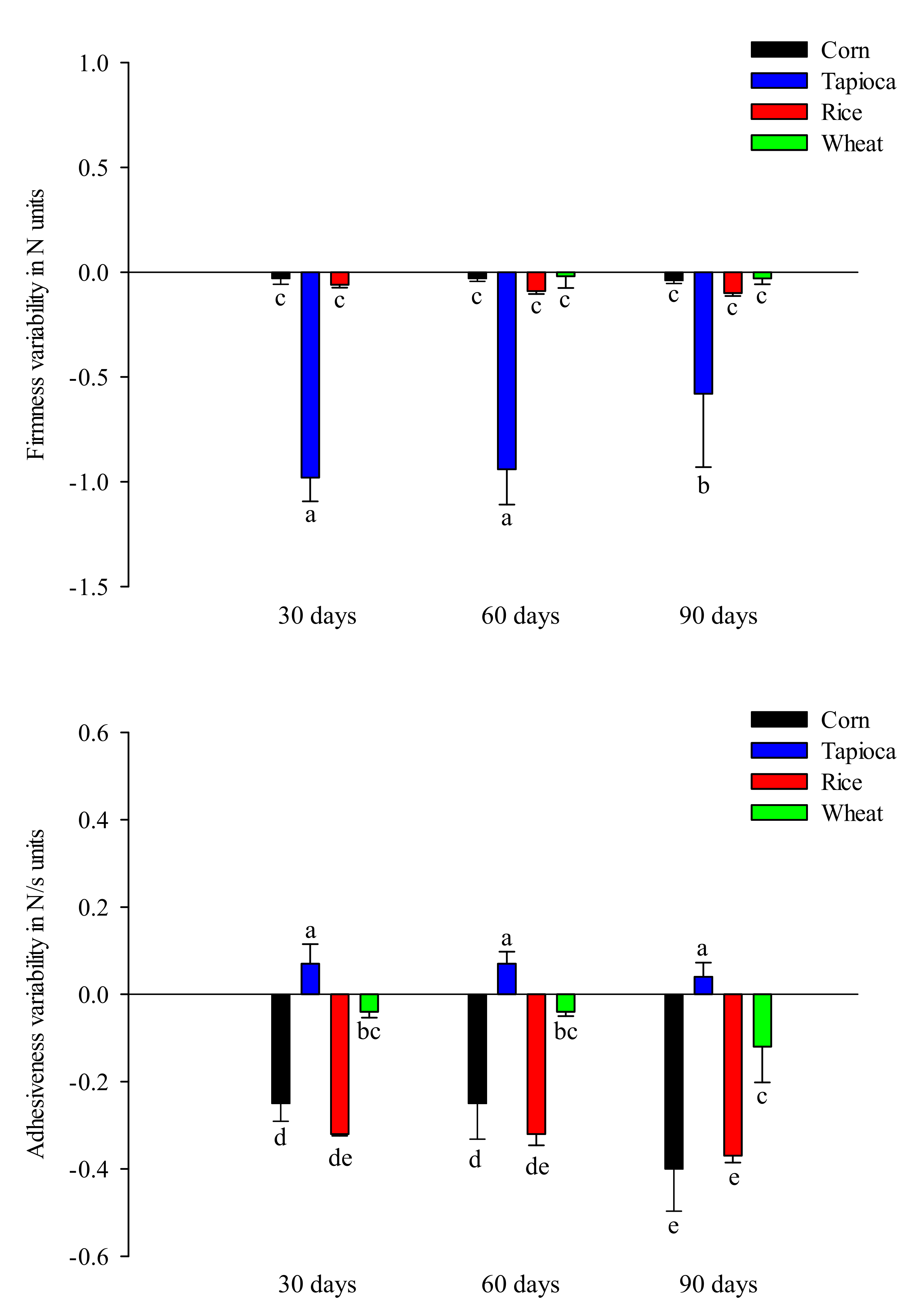
2.1.3. Texture Profile Analysis (TPA)
2.1.4. Organoleptic Property Evaluation
2.2. Accelerated Stability Tests
2.2.1. Temperature Cycling Test
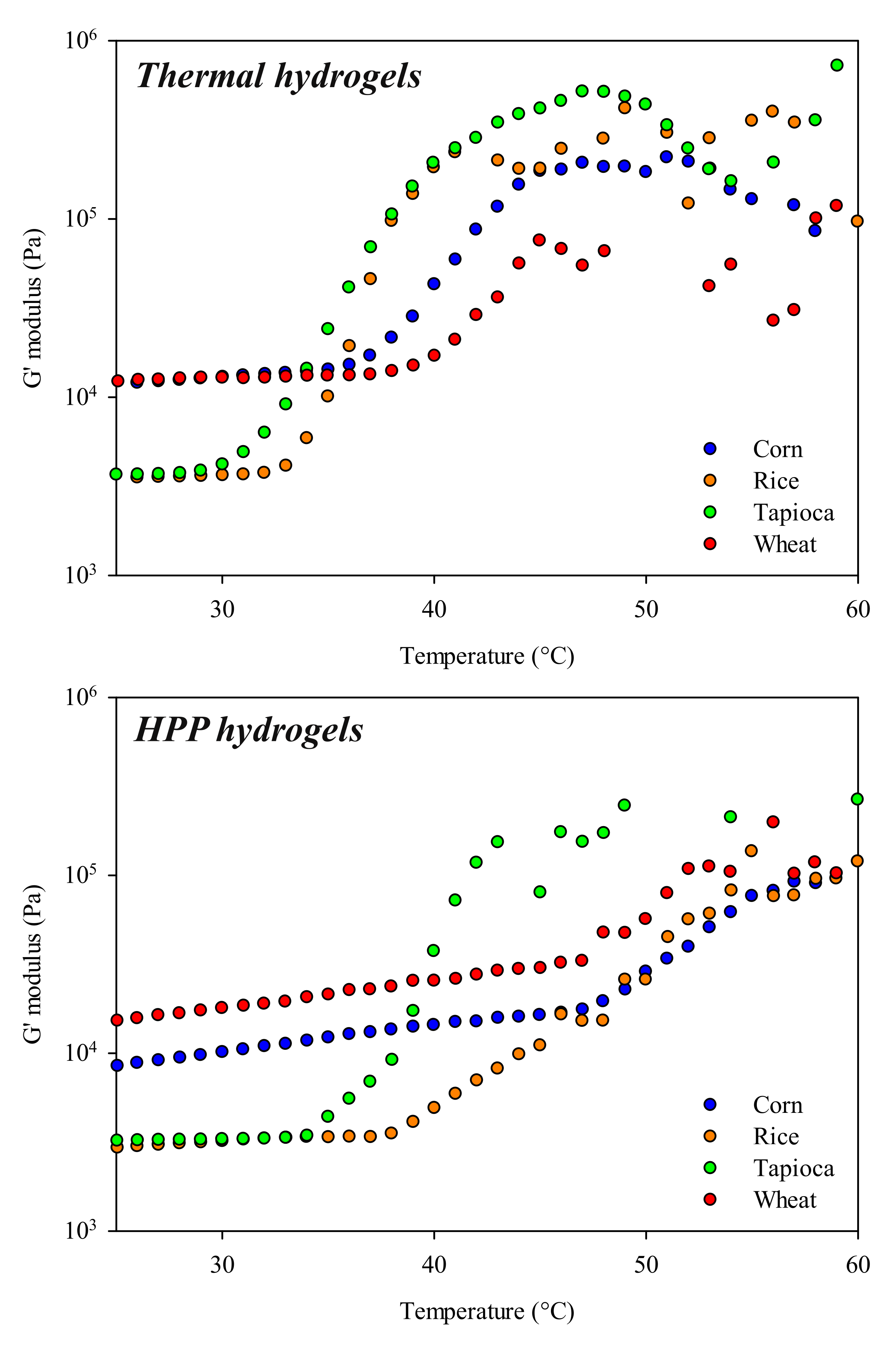
2.2.2. Rheology
Temperature Sweep Tests
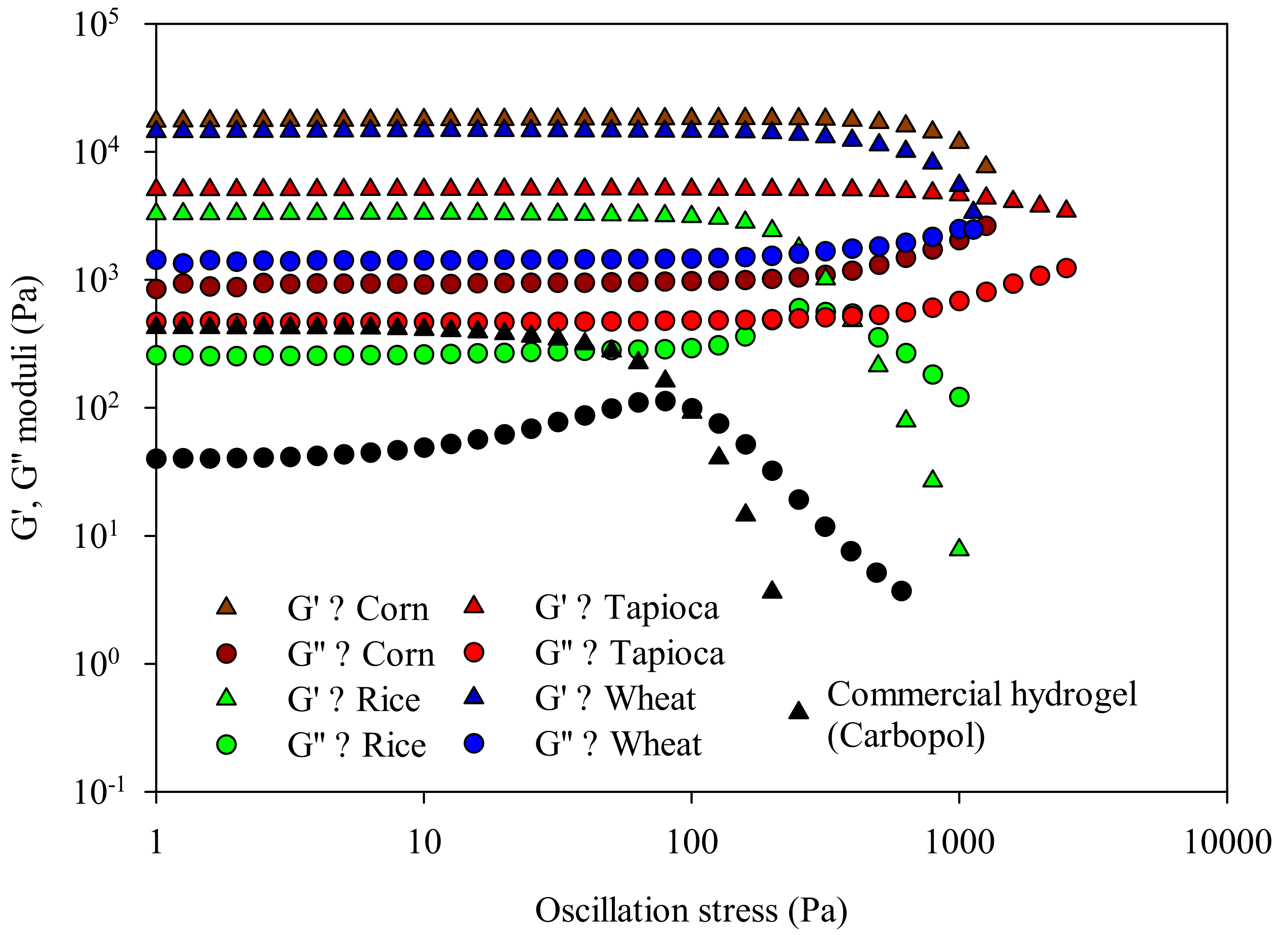
Stress Sweep Tests
3. Conclusions
4. Materials and Methods
4.1. Starch-Based Hydrogel Preparation via High-Pressure Processing (HPP)
4.1.1. Materials
4.1.2. Samples Preparation
4.1.3. High-Pressure Processing Treatments
4.2. Stability Measurements
4.2.1. Conventional Evaluations
Microbial Count
Evaluation of Organoleptic Properties
Swelling Properties
Determination of Water Activity (Aw)
Texture Profile Analysis (TPA)
4.2.2. Accelerated Evaluations
Cycling Test
Rheological Analysis
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biduski, B.; Max, W.; Colussi, R.; Lisie, S.; El, D.M.; Lim, L.; Renato, Á.; Dias, G.; Zavareze, R. International Journal of Biological Macromolecules Starch hydrogels: The in fl uence of the amylose content and gelatinization method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Mahinroosta, M.; Jomeh, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- McClements, D.J. Recent progress in hydrogel delivery systems for improving nutraceutical bioavailability. Food Hydrocoll. 2017, 68, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Mohammadinejad, R.; Maleki, H.; Larrañeta, E.; Fajardo, A.R.; Nik, A.B.; Shavandi, A.; Sheikhi, A.; Ghorbanpour, M.; Farokhi, M.; Govindh, P.; et al. Status and future scope of plant-based green hydrogels in biomedical engineering. Appl. Mater. Today 2019, 16, 213–246. [Google Scholar] [CrossRef] [Green Version]
- Ismail, H.; Irani, M.; Ahmad, Z. Starch-based hydrogels: Present status and applications. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 411–420. [Google Scholar] [CrossRef]
- Dong, G.; Mu, Z.; Liu, D.; Shang, L.; Zhang, W.; Gao, Y.; Zhao, M.; Zhang, X.; Chen, S.; Wei, M. Starch phosphate carbamate hydrogel based slow-release urea formulation with good water retentivity. Int. J. Biol. Macromol. 2021, 190, 189–197. [Google Scholar] [CrossRef]
- García-astrain, C.; Avérous, L. Synthesis and evaluation of functional alginate hydrogels based on click chemistry for drug delivery applications. Carbohydr. Polym. 2018, 190, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.; Kim, Y.R.; McClements, D.J. Control of β-carotene bioaccessibility using starch-based filled hydrogels. Food Chem. 2015, 173, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwenhove, I.; Salamon, A.; Adam, S.; Dubruel, P.; Van Vlierberghe, S.; Peters, K. Gelatin- and starch-based hydrogels. Part B: In vitro mesenchymal stem cell behavior on the hydrogels. Carbohydr. Polym. 2017, 161, 295–305. [Google Scholar] [CrossRef]
- Qi, X.; Wei, W.; Li, J.; Su, T.; Pan, X.; Zuo, G.; Zhang, J.; Dong, W. Design of Salecan-containing semi-IPN hydrogel for amoxicillin delivery. Mater. Sci. Eng. C 2017, 75, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yu, L.; Xie, F.; Bao, X.; Liu, H.; Ji, Z.; Chen, L. One-step method to prepare starch-based superabsorbent polymer for slow release of fertilizer. Chem. Eng. J. 2017, 309, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Larrea-Wachtendorff, D.; Di Nobile, G.; Ferrari, G. Effects of processing conditions and glycerol concentration on rheological and texture properties of starch-based hydrogels produced by high pressure processing (HPP). Int. J. Biol. Macromol. 2020, 159, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Larrea-Wachtendorff, D.; Tabilo-Munizaga, G.; Ferrari, G. Potato starch hydrogels produced by high hydrostatic pressure (HHP): A first approach. Polymers 2019, 11, 1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrea-Wachtendorff, D.; Sousa, I.; Ferrari, G. Starch-Based Hydrogels Produced by High-Pressure Processing (HPP): Effect of the Starch Source and Processing Time. Food Eng. Rev. 2020, 13, 622–633. [Google Scholar] [CrossRef]
- Barba, F.J.; Terefe, N.S.; Buckow, R.; Knorr, D.; Orlien, V. New opportunities and perspectives of high pressure treatment to improve health and safety attributes of foods. A review. Food Res. Int. 2015, 77, 725–742. [Google Scholar] [CrossRef]
- Knorr, D.; Heinz, V.; Buckow, R. High pressure application for food biopolymers. Biochim. Biophys. Acta-Proteins Proteom. 2006, 1764, 619–631. [Google Scholar] [CrossRef]
- Stute, R.; Klingler, R.W.; Boguslawski, S.; Eshtiaghi, M.N.; Knorr, D. Effects of high pressures treatment on starches. Starch/Starke 1996, 48, 399–408. [Google Scholar] [CrossRef]
- Błaszczak, W.; Fornal, J.; Kiseleva, V.I.; Yuryev, V.P.; Sergeev, A.I.; Sadowska, J. Effect of high pressure on thermal, structural and osmotic properties of waxy maize and Hylon VII starch blends. Carbohydr. Polym. 2007, 68, 387–396. [Google Scholar] [CrossRef]
- Błaszczak, W.; Valverde, S.; Fornal, J. Effect of high pressure on the structure of potato starch. Carbohydr. Polym. 2005, 59, 377–383. [Google Scholar] [CrossRef]
- Błaszczak, W.; Buciński, A.; Górecki, A.R. In vitro release of theophylline from starch-based matrices prepared via high hydrostatic pressure treatment and autoclaving. Carbohydr. Polym. 2015, 117, 25–33. [Google Scholar] [CrossRef]
- Buckow, R.; Heinz, V.; Knorr, D. High pressure phase transition kinetics of maize starch. J. Food Eng. 2007, 81, 469–475. [Google Scholar] [CrossRef]
- Katopo, H.; Song, Y.; Jane, J.L. Effect and mechanism of ultrahigh hydrostatic pressure on the structure and properties of starches. Carbohydr. Polym. 2002, 47, 233–244. [Google Scholar] [CrossRef]
- Kawai, K.; Fukami, K.; Yamamoto, K. Effect of temperature on gelatinization and retrogradation in high hydrostatic pressure treatment of potato starch—water mixtures. Carbohydr. Polym. 2012, 87, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tian, X.; Liu, L.; Wang, P.; Wu, G.; Zheng, J.; Ouyang, S.; Luo, Q.; Zhang, G. High pressure induced gelatinization of red adzuki bean starch and its effects on starch physicochemical and structural properties. Food Hydrocoll. 2015, 45, 132–139. [Google Scholar] [CrossRef]
- Li, W.; Bai, Y.; Mousaa, S.A.S.; Zhang, Q.; Shen, Q. Effect of High Hydrostatic Pressure on Physicochemical and Structural Properties of Rice Starch. Food Bioprocess Technol. 2012, 5, 2233–2241. [Google Scholar] [CrossRef]
- Bajaj, S.; Singla, D.; Sakhuja, N. Stability testing of pharmaceutical products. J. Appl. Pharm. Sci. 2012, 2, 129–138. [Google Scholar] [CrossRef]
- Reyes, J.E.; Guanoquiza, M.I.; Tabilo-Munizaga, G.; Vega-Galvez, A.; Miranda, M.; Pérez-Won, M. Microbiological stabilization of Aloe vera (Aloe barbadensis Miller) gel by high hydrostatic pressure treatment. Int. J. Food Microbiol. 2012, 158, 218–224. [Google Scholar] [CrossRef]
- Daryaei, H.; Yousef, A.E.; Balasubramaniam, V.M. Microbiological aspects of high pressure food processing: Inactivation of vegetative microorganisms and spores. In High Pressure Processing of Food; Springer: New York, NY, USA, 2016; pp. 271–294. [Google Scholar] [CrossRef]
- Reyes, J.E.; Tabilo-Munizaga, G.; Pérez-Won, M.; Maluenda, D.; Roco, T. Effect of high hydrostatic pressure (HHP) treatments on microbiological shelf-life of chilled Chilean jack mackerel (Trachurus murphyi). Innov. Food Sci. Emerg. Technol. 2015, 29, 107–112. [Google Scholar] [CrossRef]
- Modugno, C.; Peltier, C.; Simonin, H.; Dujourdy, L.; Capitani, F.; Sandt, C.; Perrier-Cornet, J.M. Understanding the Effects of High Pressure on Bacterial Spores Using Synchrotron Infrared Spectroscopy. Front. Microbiol. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Nguyen Thi Minh, H.; Dantigny, P.; Perrier-Cornet, J.M.; Gervais, P. Germination and inactivation of Bacillus subtilis spores induced by moderate hydrostatic pressure. Biotechnol. Bioeng. 2010, 107, 876–883. [Google Scholar] [CrossRef] [Green Version]
- Kawai, K.; Fukami, K.; Yamamoto, K. State diagram of potato starch-water mixtures treated with high hydrostatic pressure. Carbohydr. Polym. 2007, 67, 530–535. [Google Scholar] [CrossRef]
- Kawai, K.; Fukami, K.; Yamamoto, K. Effects of treatment pressure, holding time, and starch content on gelatinization and retrogradation properties of potato starch-water mixtures treated with high hydrostatic pressure. Carbohydr. Polym. 2007, 69, 590–596. [Google Scholar] [CrossRef]
- Chung, Y.L.; Lai, H.M. Water status of two gelatin gels during storage as determined by magnetic resonance imaging. J. Food Drug Anal. 2004, 12, 221–227. [Google Scholar] [CrossRef]
- Torres, M.D.; Fradinho, P.; Raymundo, A.; Sousa, I.; Falqué, E.; Domínguez, H. The key role of thermal waters in the development of innovative gelled starch-based matrices. Food Hydrocoll. 2021, 117, 106697. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Whistler, R. (Eds.) Starch: Chemistry and Technology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2009; ISBN 9780127462752. [Google Scholar]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- BeMiller, J.N. Carbohydrate Chemistry for Food Scientists; Woodhead Publishing and AACC International Press: Cambridge, UK, 2018; ISBN 9780128120699. [Google Scholar]
- Scott, W.J. Water Relations of Food Spoilage Microorganisms. Adv. Food Res. 1957, 7, 83–127. [Google Scholar] [CrossRef]
- Pycia, K.; Gałkowska, D.; Juszczak, L.; Fortuna, T.; Witczak, T. Physicochemical, thermal and rheological properties of starches isolated from malting barley varieties. J. Food Sci. Technol. 2015, 52, 4797–4807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Wang, P.; Tian, J.; Yu, X.; Chang, M.; Chu, X.; Wu, N. A new strategy to express the extracellular α-amylase from Pyrococcus furiosus in Bacillus amyloliquefaciens. Sci. Rep. 2016, 6, 22229. [Google Scholar] [CrossRef] [PubMed]
- Kommanaboyina, B.; Rhodes, C.T. Trends in stability testing, with emphasis on stability during distribution and storage. Drug Dev. Ind. Pharm. 1999, 25, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Almeida, I.F.; Bahia, M.F. Evaluation of the physical stability of two oleogels. Int. J. Pharm. 2006, 327, 73–77. [Google Scholar] [CrossRef]
- Carstensen, J.T.; Rhodes, C.T. Cyclic testing in stability programs. Drug Dev. Ind. Pharm. 1986, 12, 1219–1225. [Google Scholar] [CrossRef]
- Schirmer, M.; Jekle, M.; Becker, T. Starch gelatinization and its complexity for analysis. Starch/Starke 2015, 67, 30–41. [Google Scholar] [CrossRef]
- Taherian, A.R.; Fustier, P.; Ramaswamy, H.S. Steady and dynamic shear rheological properties, and stability of non-flocculated and flocculated beverage cloud emulsions. Int. J. Food Prop. 2008, 11, 24–43. [Google Scholar] [CrossRef]
- Abdul Rahman, M.N.; Qader, O.A.J.A.; Sukmasari, S.; Ismail, A.F.; Doolaanea, A.A. Rheological characterization of different gelling polymers for dental gel formulation. J. Pharm. Sci. Res. 2017, 9, 2633–2640. [Google Scholar]
- Doona, C.J.; Feeherry, F.E.; Baik, M.Y. Water dynamics and retrogradation of ultrahigh pressurized wheat starch. J. Agric. Food Chem. 2006, 54, 6719–6724. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, X.; Jin, Z.; Tian, Y.; Bai, Y.; Xie, Z. Retrogradation properties of rice starch gelatinized by heat and high hydrostatic pressure (HHP). J. Food Eng. 2011, 106, 262–266. [Google Scholar] [CrossRef]
- Yang, Z.; Chaib, S.; Gu, Q.; Hemar, Y. Food Hydrocolloids Impact of pressure on physicochemical properties of starch dispersions. Food Hydrocoll. 2017, 68, 164–177. [Google Scholar] [CrossRef]
- Biliaderis, C.G. Structural Transitions and Related Physical Properties of Starch. In Starch; Academic Press: Cambridge, MA, USA, 2009; pp. 293–372. ISBN 9780127462752. [Google Scholar]
- Fang, F.; Tuncil, Y.E.; Luo, X.; Tong, X.; Hamaker, B.R.; Campanella, O.H. Shear-thickening behavior of gelatinized waxy starch dispersions promoted by the starch molecular characteristics. Int. J. Biol. Macromol. 2019, 121, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.D.; Chenlo, F.; Moreira, R. Rheological Effect of Gelatinisation Using Different Temperature-Time Conditions on Potato Starch Dispersions: Mechanical Characterisation of the Obtained Gels. Food Bioprocess Technol. 2018, 11, 132–140. [Google Scholar] [CrossRef]
- Lapasin, R. Rheological Characterization of Hydrogels. In Polysaccharide Hydrogels; Matricardi, P., Alhaique, F., Coviello, T., Eds.; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2016; pp. 99–154. ISBN 9780429069338. [Google Scholar]
- De Maria, S.; Ferrari, G.; Maresca, P. Effect of high hydrostatic pressure on the enzymatic hydrolysis of bovine serum albumin. J. Sci. Food Agric. 2017, 97, 3151–3158. [Google Scholar] [CrossRef] [PubMed]
- Kusumayanti, H.; Handayani, N.A.; Santosa, H. Swelling Power and Water Solubility of Cassava and Sweet Potatoes Flour. Procedia Environ. Sci. 2015, 23, 164–167. [Google Scholar] [CrossRef] [Green Version]

| HHP Hydrogel | Storage Time (Days) | |||
|---|---|---|---|---|
| 0 | 30 | 60 | 90 | |
| Rice | Homogeneous Brilliant | N.M. | N.M. | More liquid |
| Corn | Homogeneous Opaque | N.M. | Yellowish | More liquid and yellowish |
| Tapioca | Homogeneous Compact | Evident Syneresis and Phase separation | Broken structure | |
| Wheat | Homogeneous opaque | N.M. | N.M. | Some lumps |
| HPP Hydrogel | Time (Day) | Firmness (N) | Adhesiveness (−N *s) |
|---|---|---|---|
| Corn | 0 | 0.11 ± 0.01 c | 0.82 ± 0.11 a |
| 7 | 0.06 ± 0.01 d | 0.36 ± 0.05 c | |
| Tapioca | 0 | 1.36 ± 0.09 a | – |
| 7 | 0.24 ± 0.07 b | 0.06 ± 0.00 e | |
| Rice | 0 | 0.17 ± 0.08 b | 0.85 ± 0.01 a |
| 7 | 0.06 ± 0.01 d | 0.50 ± 0.02 b | |
| Wheat | 0 | 0.11 ± 0.01 c | 0.44 ± 0.05 bc |
| 7 | 0.06 ± 0.00 d | 0.25 ± 0.02 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larrea-Wachtendorff, D.; Del Grosso, V.; Ferrari, G. Evaluation of the Physical Stability of Starch-Based Hydrogels Produced by High-Pressure Processing (HPP). Gels 2022, 8, 152. https://doi.org/10.3390/gels8030152
Larrea-Wachtendorff D, Del Grosso V, Ferrari G. Evaluation of the Physical Stability of Starch-Based Hydrogels Produced by High-Pressure Processing (HPP). Gels. 2022; 8(3):152. https://doi.org/10.3390/gels8030152
Chicago/Turabian StyleLarrea-Wachtendorff, Dominique, Vittoria Del Grosso, and Giovanna Ferrari. 2022. "Evaluation of the Physical Stability of Starch-Based Hydrogels Produced by High-Pressure Processing (HPP)" Gels 8, no. 3: 152. https://doi.org/10.3390/gels8030152
APA StyleLarrea-Wachtendorff, D., Del Grosso, V., & Ferrari, G. (2022). Evaluation of the Physical Stability of Starch-Based Hydrogels Produced by High-Pressure Processing (HPP). Gels, 8(3), 152. https://doi.org/10.3390/gels8030152








