1. Introduction
Water is an important liquid for human beings, the universe, and all life existing on earth [
1,
2]. This liquid can be easily polluted by different dyes [
3,
4]. Both water and dyes are still largely used in the textile industry, meaning that the wastewater after production is a mixture of dyes and water. Unfortunately, the elimination of the wastewater often occurs through discharge into rivers and other effluents [
5,
6,
7,
8,
9,
10,
11,
12].
This negative situation has motivated many researchers to publish many reports in the field of the treatment of water polluted with dyes [
13,
14,
15]. Several physical and chemical techniques have been developed to purify water from these compounds, including photocatalysis, oxidation, filtration, coagulation/flocculation, and adsorption [
16,
17,
18,
19,
20]. In particular, adsorption processes have been studied intensively because of their low cost, easy access, and effective dye removal, in which the dissolved dye compounds adsorb on the surface of suitable adsorbents [
21].
Many biodegradable materials and effective adsorbents obtained from natural resources have been used to remove dyes from aqueous solutions. Hydrogels were frequently applied for this purpose, consisting of a three-dimensional polymeric material with the capacity to uptake an important amount of water due to the presence of hydrophilic groups in their structure, such as –OH, –CONH, and –SO
3H [
22].
Copolymers based on acrylamide (AM) and acrylic acid (AA) have been applied to remove dyes. AM monomer is soluble in water, and linear poly(AM) finds many uses as water-soluble thickeners and flocculation agents [
23,
24,
25], whereas AA represents the simplest unsaturated carboxylic acid. This colorless liquid is miscible with water, alcohols, ethers, and chloroform [
26,
27,
28]. Solpan et al. [
29] used (poly(AM-
co-AA) hydrogels for the uptake of the cationic dyes, safranin-O and magenta. The diffusion of water and cationic dyes within hydrogels showed non-Fickian behavior. Corona-Rivera et al. [
30] applied poly(AM-
co-AA) crosslinked with N,N′-methylene bisacrylamide (NMBAM) for the removal of Remazol red dye from aqueous solutions, finding the maximum dye adsorption capacity for peculiar experimental conditions, with an adsorption mechanism well represented by the Langmuir model.
The diffusion of colored water inside the hydrogel depends on many factors, such as the dye structure and the functional groups on the polymer chains. The dye can generate an attraction through electrostatic interaction, which also represents an important key to removing dye from an aqueous medium, whereby a dye molecule and a receptor behave similarly to a ship and a harbor. In the field of biochemistry, the theory of docking was largely applied to study the interaction between ligand and protein, allowing us to explain the affinity between these components [
31,
32,
33,
34,
35,
36,
37]. In this study, the interactions between the dyes and polymer networks were investigated. The docking method was applied to analyze different interactions, with the receptor and ligand representing the polymer matrix and dye, respectively. This simulation method has the advantage of enabling us to predict the preferred orientation of one molecule to a second one when bound to each other to form a stable complex [
38,
39,
40]. Interestingly, this helps us to economize cost and time of research work.
In a previous paper [
41], the interaction between a polymer based on HEMA monomer and Eosin Y (EY) as a pollutant was discussed. It was found that the theoretical prediction correlates well with experimental results. In the literature, some authors report on poly(AM-
co-AA) crosslinked with NMBAM [
42,
43,
44,
45,
46]. In this work, AM and AA were copolymerized and crosslinked with HDDA since it contributes to the high level of conversion of acrylic double bonds [
47,
48]. Under the UV-visible light exposure in the presence of a suitable photoinitiator (Darocur 1173), a chemically crosslinked three-dimensional copolymer was successfully obtained. In contrast to thermal polymerization, which often requires elevated temperatures, photopolymerization can be performed at room temperature [
49]. In most reports on poly(AA), thermal polymerization was applied using a source of free radicals, together with a chemical stabilizer, such as ammonium persulfate and tetramethylethylenediamine [
50,
51,
52]. This method has disadvantages such as long polymerization times, unstable and toxic reagents, and tedious preparation steps. On the other hand, photopolymerization using an initiator sensitive to light represents a quicker method that is less tedious and less toxic. The field of the exploitation of these polymeric materials is, thus, enlarged to applications in which the elevation of temperature is not advised. The final properties of UV-polymerized gels depend on the UV-visible spectrum of the source, light intensity and uniformity, and exposure times [
53].
The model dyes studied in this report were Rose Bengal (RB), EY, and Methylene Blue (MB), presenting anionic and cationic natures. These dyes are widely used in many applications, thus increasing the probability that they contribute to water pollution, since even small quantities can easily affect the water quality [
54,
55,
56].
To understand the different interatomic interactions between dyes and polymers, the docking simulation method was exploited. Two model systems, crosslinked poly(AM)/dye and poly(AM-
co-AA)/dye, were considered using Avogadro software. These model systems were all energy-minimized, and the conformation of polymer/dye systems was simulated using Auto-Dock Vina software [
57,
58], and then visualized and analyzed using UCSF Chimera.
3. Conclusions
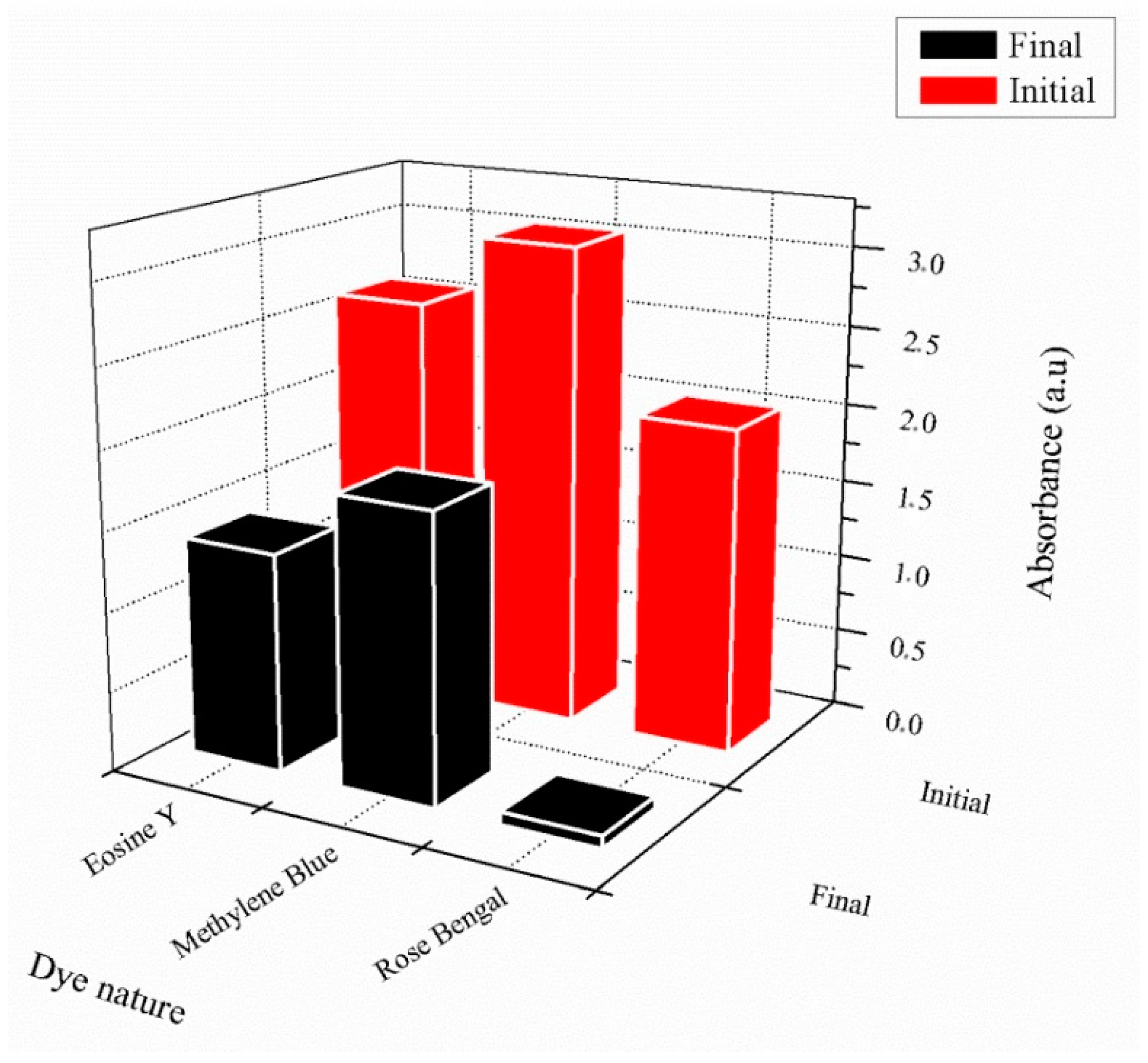
The UV photopolymerization technique in an aqueous medium was chosen to elaborate chemically crosslinked poly(AM) and poly(AM-co-AA) as dye adsorbent hydrogels. Experimental parameters, such as the optimal percentage of HDDA as a crosslinking agent, as well as the suitable dye concentration for analysis, were found to be1 wt% and 32 × 10−3 mg·mL−1, respectively. All dyes show negligible retention effects using the neutral poly(AM), and significant adsorption for the polyelectrolyte poly(AM-co-AA). It was found that 97% of RB was removed efficiently by the copolymer (MB: 45%, EY: 50%), which can be related to the presence of one more functional group compared to the other dyes, and also due to strong hydrogen bonding (O...H) with an interatomic distance of 2.24 Å, which plays a key role in interaction. As a consequence, this copolymer could be considered to be an efficient hydrogel with which to remove the considered dyes from a water medium.
The conformation of polymers and pollutants were analyzed via a docking simulation. Interestingly, halogen bonding could be neglected, whereas hydrogen bonding plays a key role for dye retention. The system composed of poly(AM-co-AA)/RB has a binding energy of −7.7 kcal/mol, which means that this system has the highest stability compared to the other investigated polymer/dye systems. An analysis of rotatable bonds shows that the AA repetition unit presents two rotatable bonds, whereas that of AM has one; therefore, poly(AM-co-AA) has more conformations than poly(AM), thus increasing dye retention.
4. Materials and Methods
4.1. Materials
The monomers used in this study were AM and AA (both from Sigma-Aldrich (Saint-Quentin-Fallavier, France), purity: 99%), the crosslinking agent was HDDA (from Cray Valley, Courbevoie, France), purity: 98%), and the photoinitiator was 2-hydroxy-2-methyl-1-phenyl-propane-1 (commercial designation: Darocur 1173) (from Ciba-Geigy, purity: 97%). RB (purity: 95%), EY (purity: 99%), and MB (purity: 70%) (all from Sigma-Aldrich) were applied as dyes. All products were used as received without purification. The chemical structures of the reagents are illustrated in
Table 6. Abbreviations are given in
Table S1.
4.2. Hydrogel Synthesis
First, the AM monomer was dissolved in distilled water. Then, 0.5 wt% of Darocur 1173 was added. To underline the crosslinker effect on the water uptake capacity of the obtained hydrogels, a set of three solutions with different percentages of HDDA (1 wt%, 4 wt%, and 7 wt%) was prepared. After steering for 24 h, the solutions were exposed to UV irradiation for 30 min, using a TL08 UV lamp, with a characteristic wavelength of λ = 365 nm and an intensity of 1.5 mW/cm2. For the sake of comparison, a hydrogel copolymer was generated. A stock solution of 50 wt%/50 wt% AM/AA was firstly prepared. In the second step, three solutions were prepared with different percentages of HDDA: 1 wt%, 4 wt%, and 7 wt%, with 98.5 wt%, 95.5 wt%, and 92.5 wt% of the AM/AA solution, respectively. Finally, Darocur 1173 as a photoinitiator was added to each of these solutions (0.5 wt%). All solutions were prepared at room temperature.
After the polymerization/crosslinking process (see also
Figure S5), all samples were obtained in pellet form (sample thickness: 3 mm, diameter: 2.5 cm) and washed in distilled water to remove all remaining residue.
4.3. Dye Retention Experiments
The selected cylindrical hydrogel (1.5 g), as an adsorbent, was immersed in 32 × 10−3 mg·mL−1 of dye solution at room temperature (T = 23 °C) for 24 h. Then, the hydrogel was separated by filtration, and the residual concentration of the considered dye solution was introduced in a glass flask and evaluated using a dual-beam ultraviolet–visible spectrometer (Specord 200 plus, Analytik Jena, Jena, Germany).
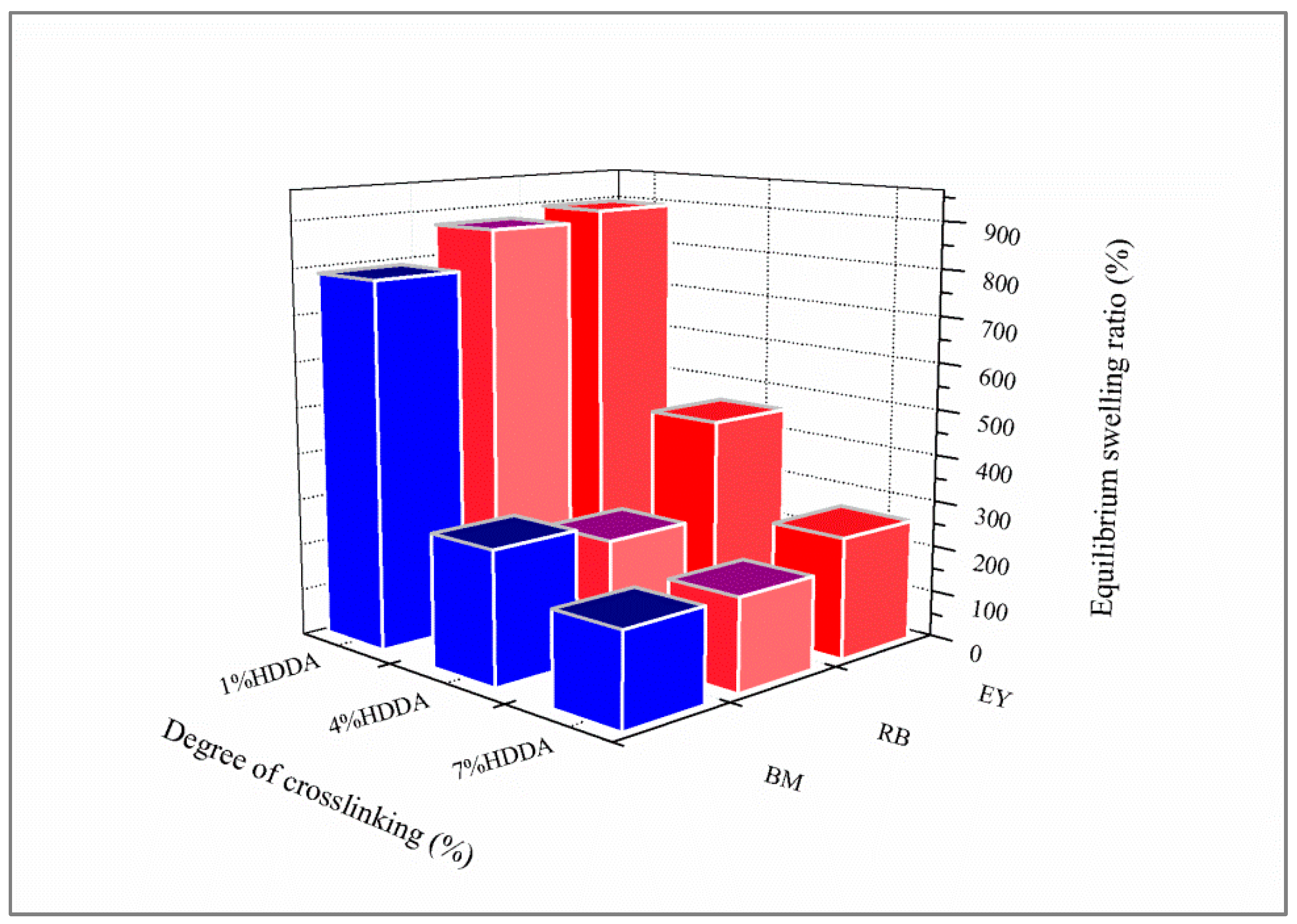
4.4. Equilibrium Swelling Measurements
In order to underline the equilibrium swelling of the elaborated hydrogels, the sample was weighed in the dry state and then immersed in a dye solution for 24 h under stirring. Then, the sample was wiped with a filter paper to remove free liquid on the surface before being weighed. The degree of swelling was calculated according to Equation (1).
where
τ(%) represents the degree of swelling,
mt stands for the weight of the swollen network at time
t, and
m0 is the weight of the initially dried network.
4.5. Model Proposition
Two model systems were proposed. The first one represents the crosslinked poly(AM)/HDDA system, based on three chains of poly(AM) containing ten units each. Chains were connected by three HDDA crosslinking nodes. The second model system, the crosslinked poly(AM-co-AA), was created similar to the first model. RB, EY, and MB were all presented in 3-D. All models were energy-minimized using auto-optimization with force field UFF and the steepest descent algorithm of the Avogadro software. The output simulation implies eight conformations; the best conformation of each hydrogel/dye system was illustrated based on their energy.
4.6. Software
Avogadro version 4.8.6 was used to visualize and optimize the model systems. The files were saved in the molecule file format pdb. AutoDock version 1.5.6, a molecular modeling simulation software, represents a suite of automated docking tools. It is designed to predict how dyes bind to polymer networks; the grid box allows the user to limit the space of interaction analysis (
Figure 15).
The simulation was conducted in dimensions of grid box points (x = y = z = 126 Å), and the grid box center dimensions were set as mentioned in
Table S2. The other parameters were maintained and were used as defaults. Finally, the output file (log.txt) was analyzed, and the best docking results regarding binding energies were selected and investigated with other software programs. The Chimera calculation software UCSF version 1.5.3 was used to analyze interatomic distances.