Low Concentrated Fractionalized Nanofibers as Suitable Fillers for Optimization of Structural–Functional Parameters of Dead Space Gel Implants after Rectal Extirpation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Nanofibers
2.2.2. Fractionalization of Nanofibers
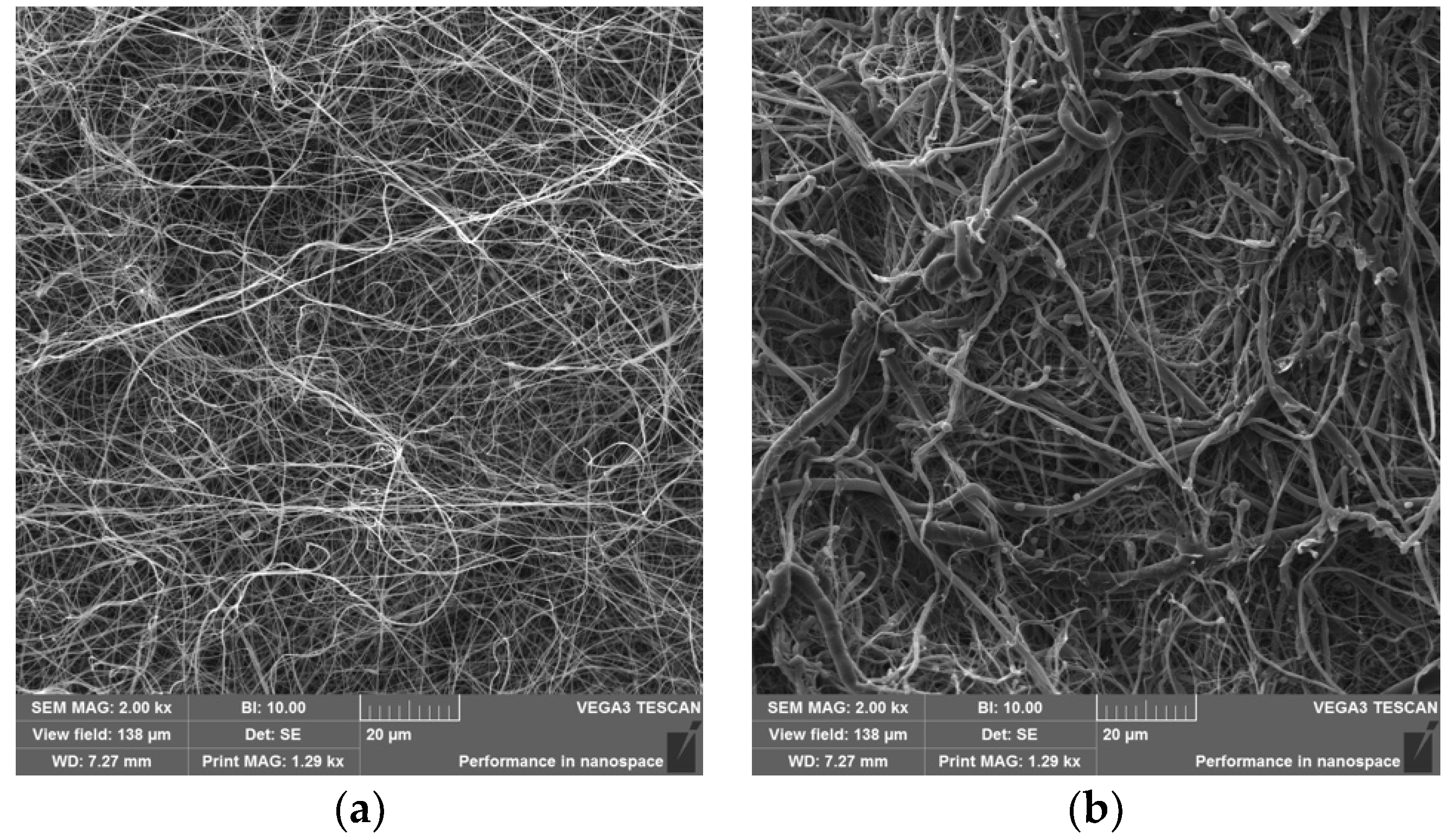
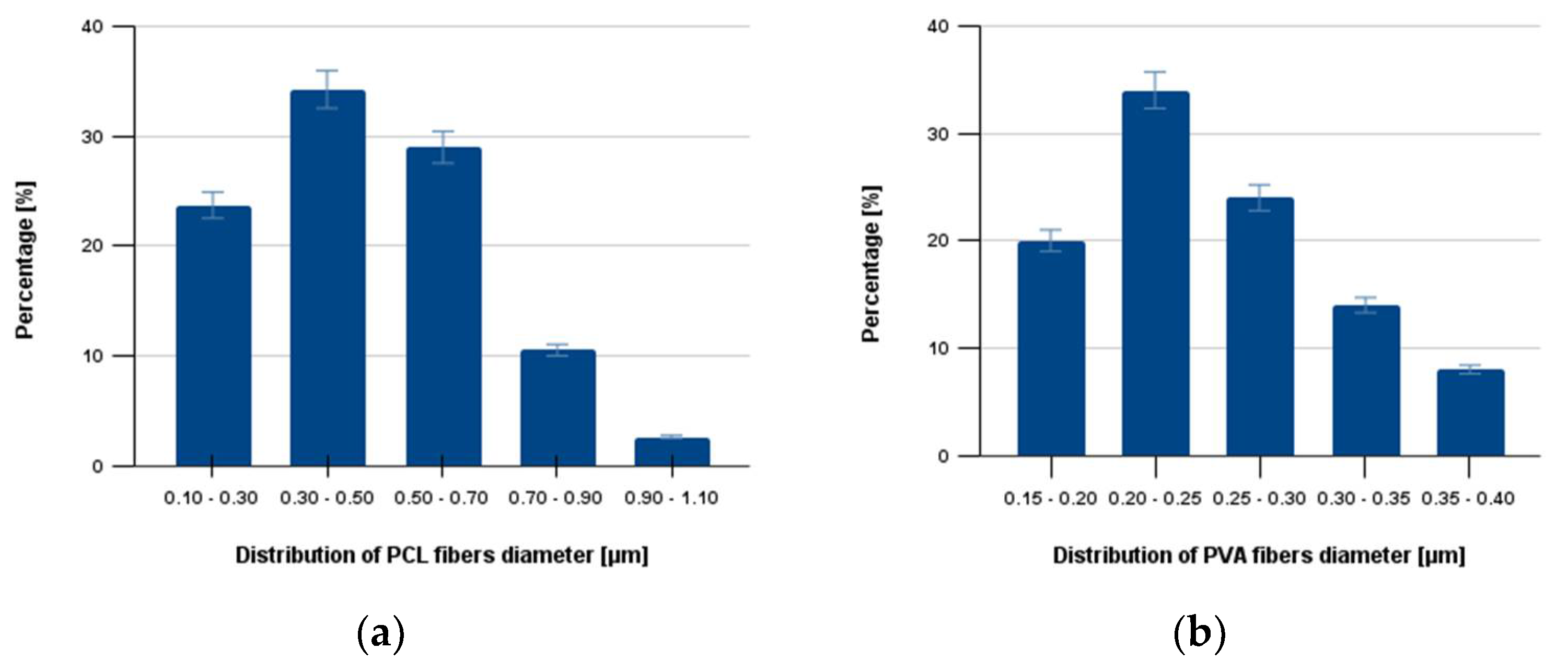
2.2.3. Visualization of Nanofibers and Diameter Determination
2.2.4. Gel Preparation and Its Functionalization
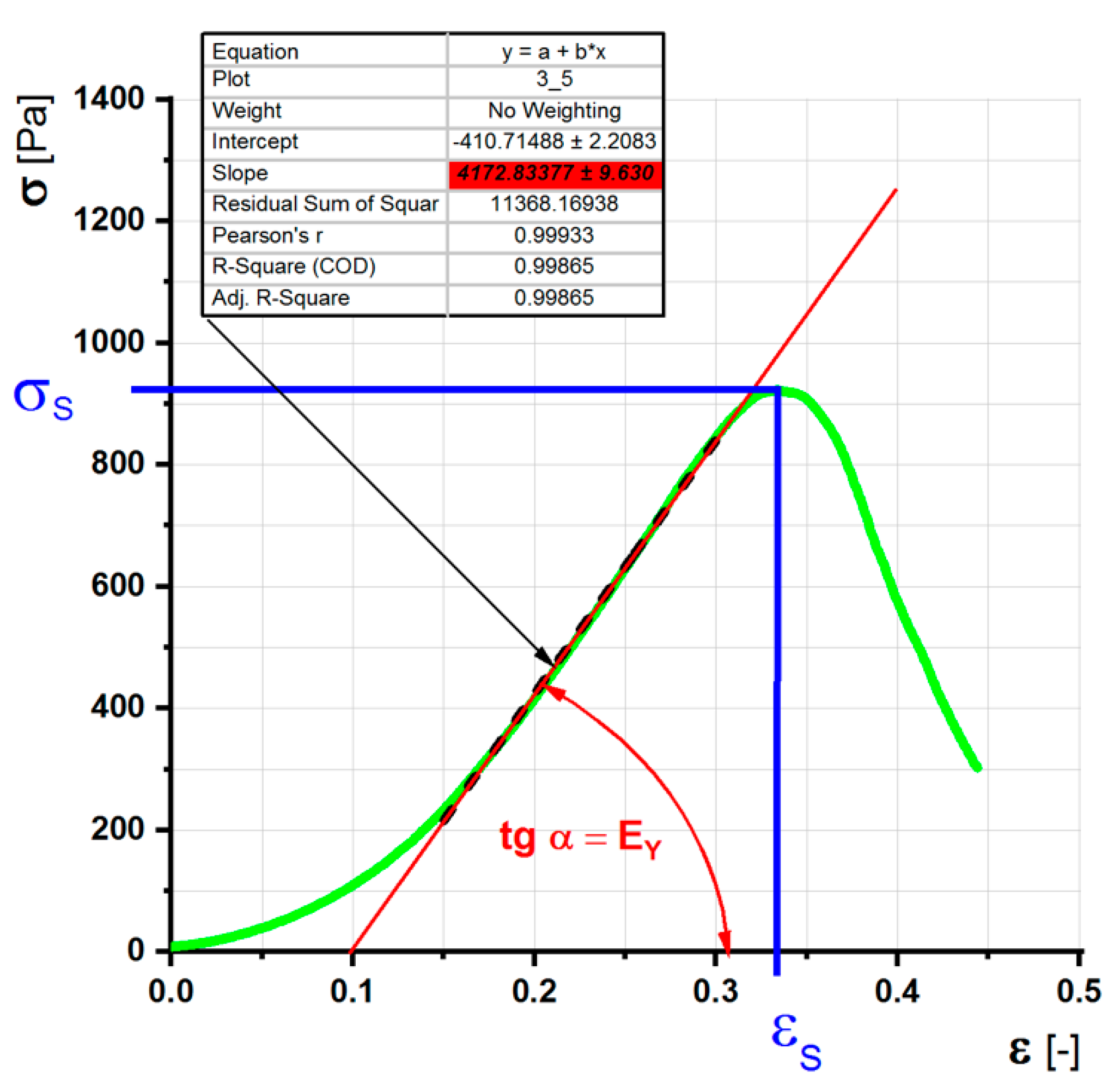
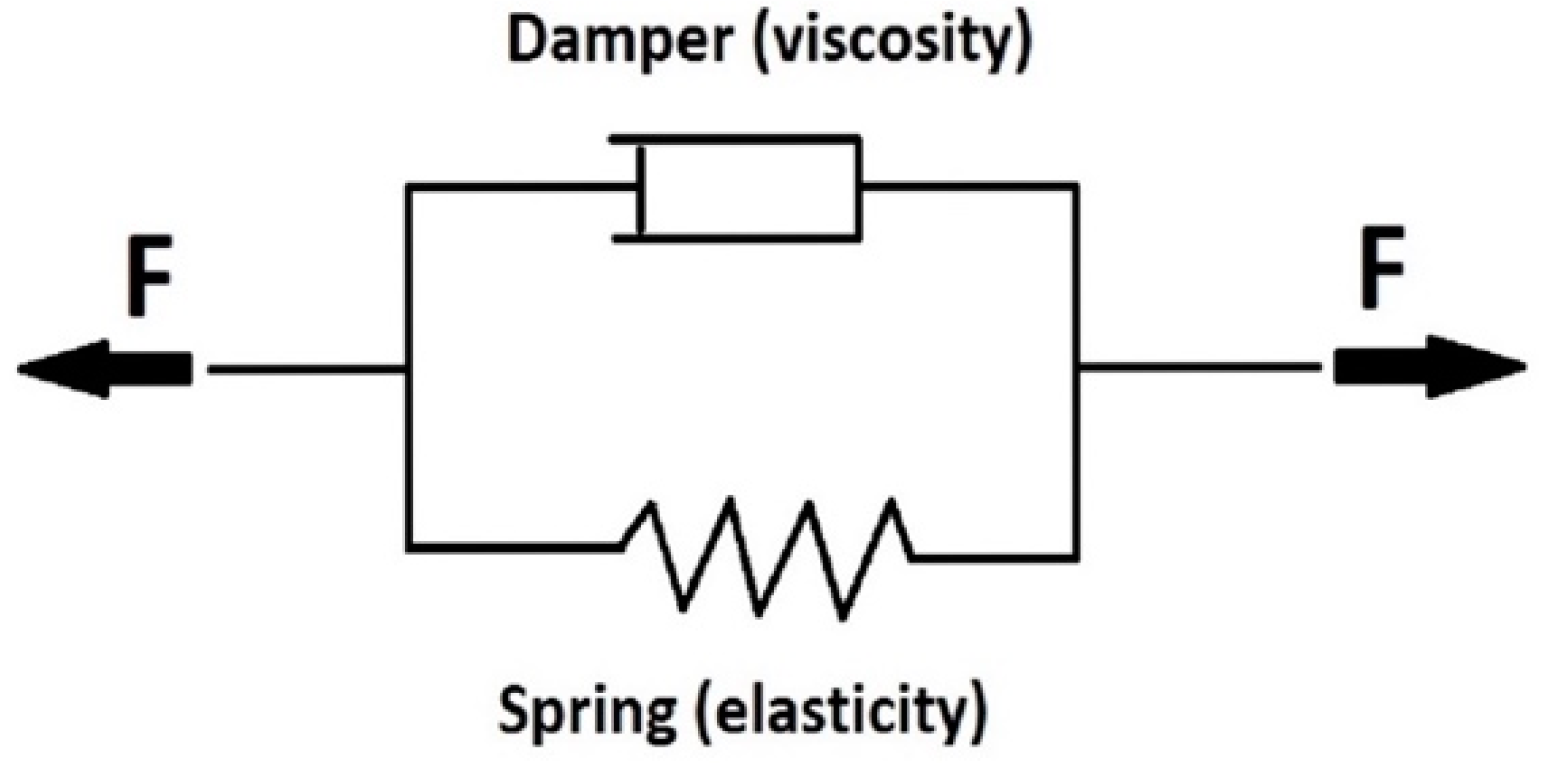
2.2.5. Determination of Mechanical Properties
- E [Pa], modulus of elasticity from Kelvin model
- η [-], viscosity from the Kelvin model
- τ [s], relaxation time from Kelvin model
- EY [Pa], Young’s modulus of elasticity of a simple pressure test
- σ [Pa], internal tension
- σS [Pa], limit of simple pressure test
- ε [-], relative elongation of the simple pressure test
- εS [-], maximum relative elongation of the simple pressure test
- T [mm], thickness of unloaded sample
- S [mm2], cross section of unloaded sample
- V [mm3], volume of the unloaded sample
Destructive Simple Pressure Test—Estimation of the Elongation and Strength Limits and Young’s Modulus
Constant Load Test (Creep)-Estimation of Modulus of Elasticity and Viscosity
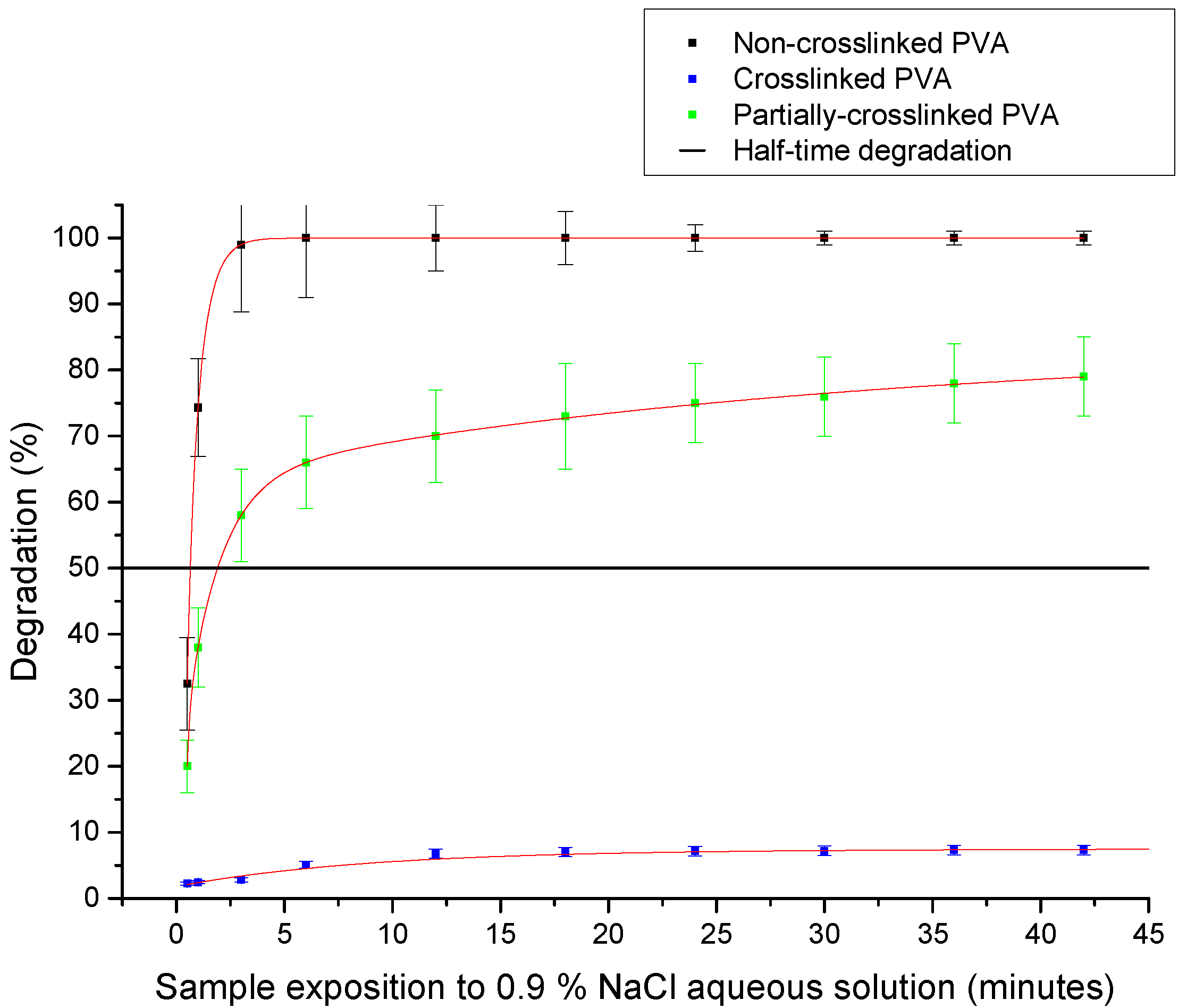
2.2.6. Degradation of PVA Nanofibers
2.2.7. Cell Proliferation Analysis
3. Results
3.1. Gel Maturation Is a Key Step for Biomechanical Parameters
3.2. Characterization of Fractionalized Nanofibers
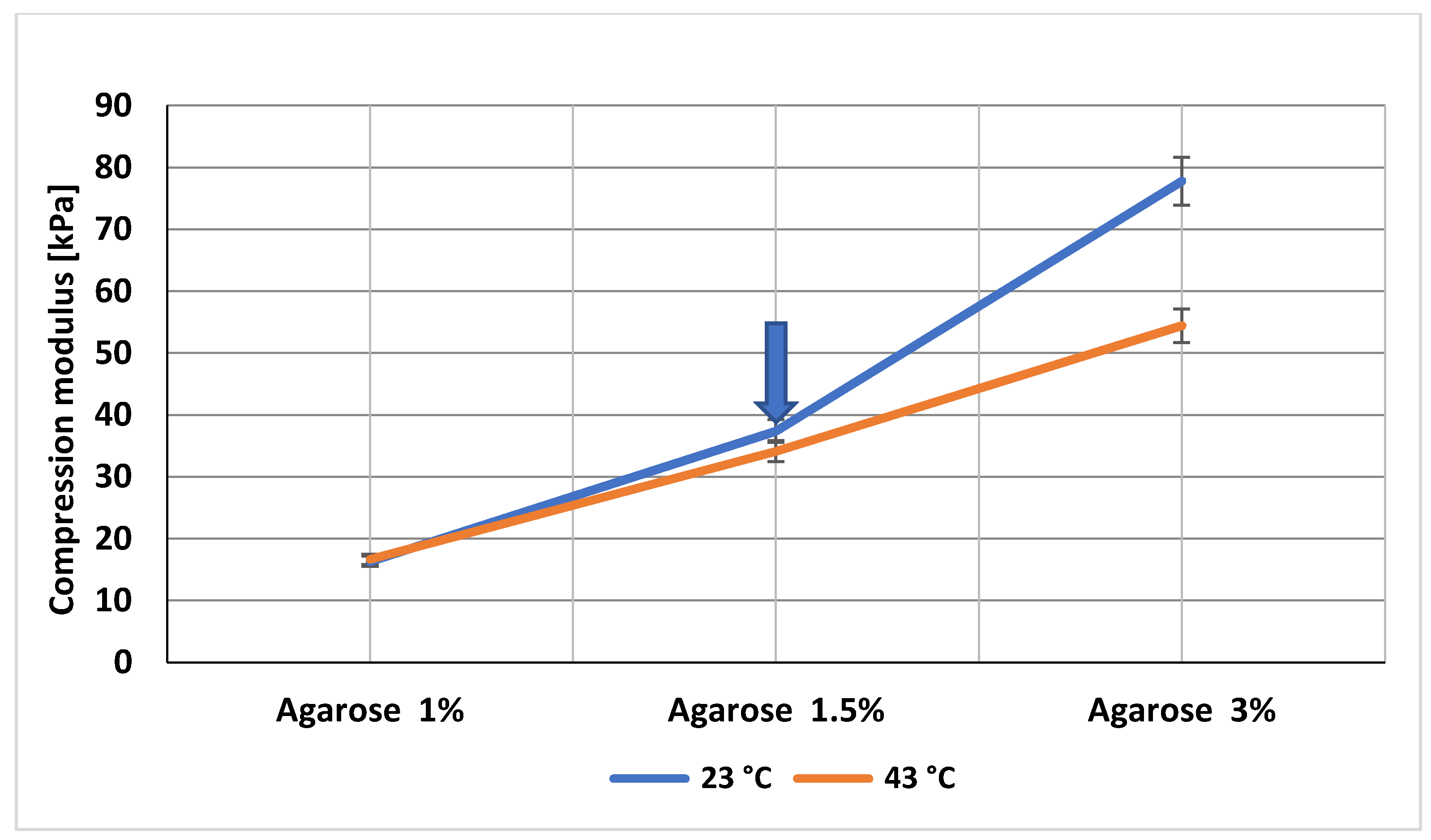
3.3. Fractionalized Nanofibers at Low Concentration Stiffen the Agarose Gels
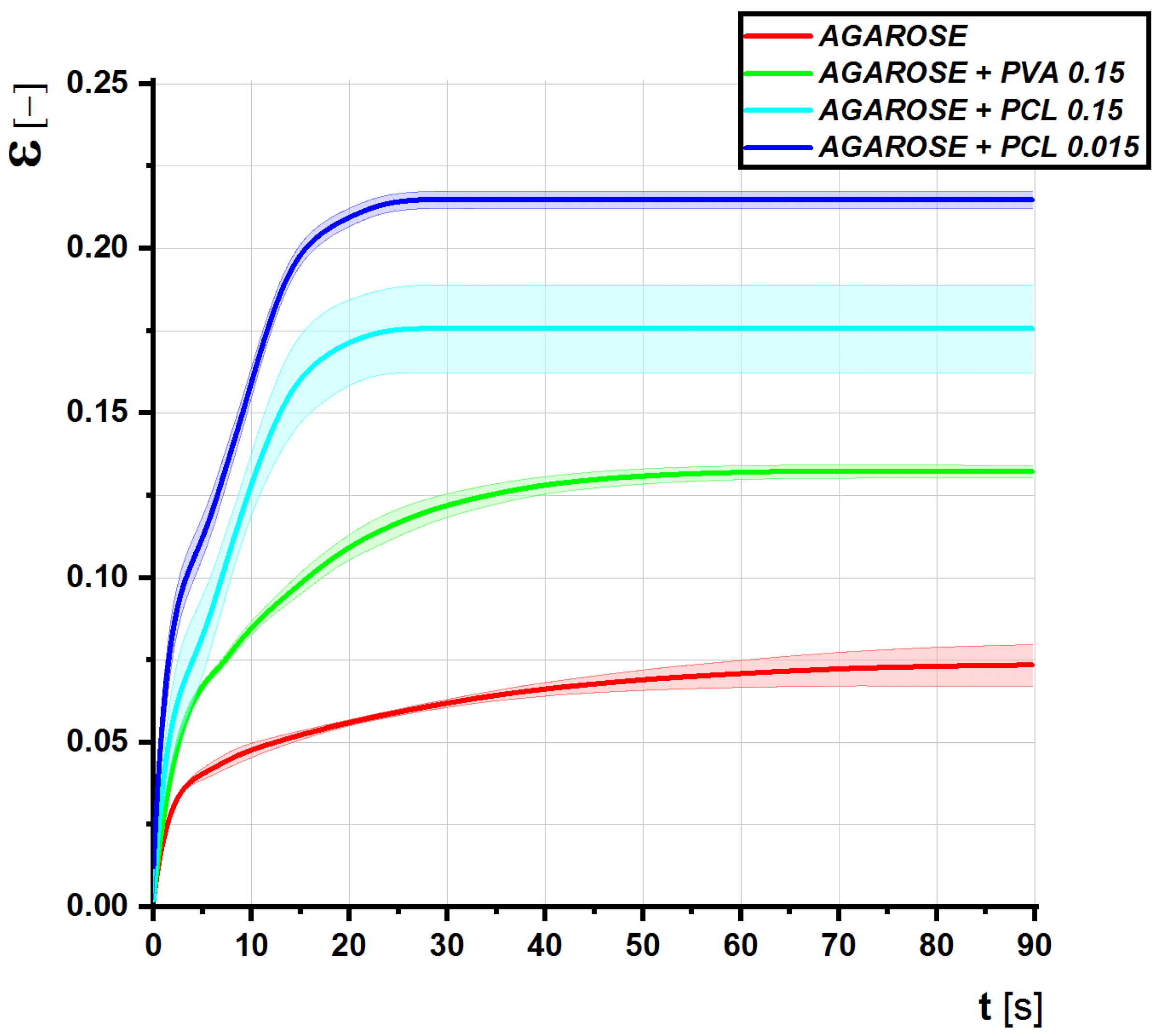
3.4. Fractionalized Nanofibers Serve as a System for a Controlled Drug Delivery
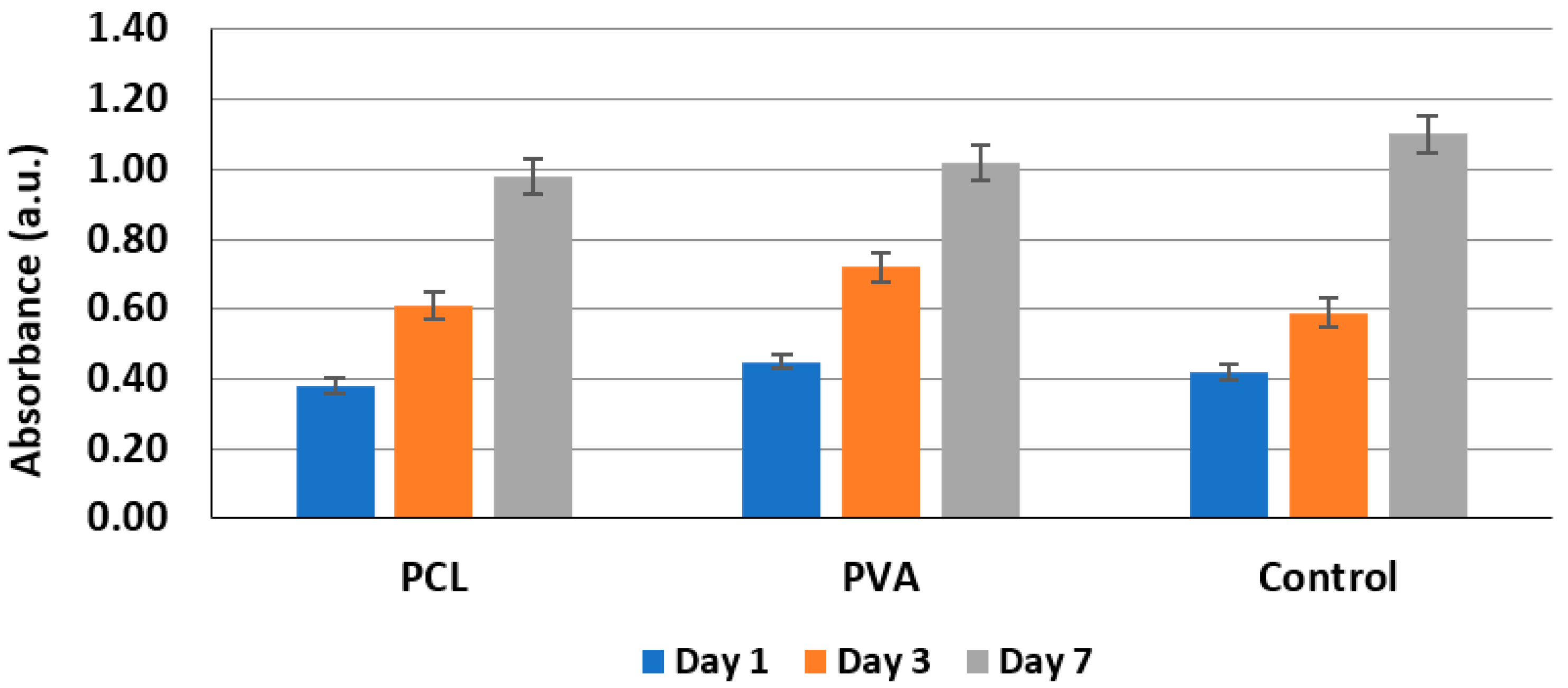
3.5. Biocompatibility Testing
4. Discussion
4.1. Low Concentration of Fractionalized Nanofibers Can Significantly Modify Hydrogel Structural Parameters
4.2. Fractionalized Nanofibers Could Serve as a Superior Drug Delivery System
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Scoring for Gel Fillers
- biomimetic with optimal biomechanical parameters in the range between 36 °C and 41 °C
- high biocompatibility
- minimal invasion by prokaryotic cells
- optimal colonization by eukaryotic cells
- optimal surface parameters (optimal interface between the gel and tissue)
- optimal degradation halftime
- 0–5 points for optimal biomechanical parameters between 31 °C to 46 °C (most importantly 37 °C)
- 0–3 points for high biocompatibility
- 0–3 points for optimal colonization by eukaryotic cells
- 0–3 points for minimal invasion by prokaryotic cells
- 0–5 points for optimal degradation halftime
- Possibility of functionalization by bioactive substances
- Y/N extra points for optimal surface parameters (optimal interface between the gel and tissue) for some types of fillers
- Y/N extra points for including antibiotics
References
- Killeen, S.; Devaney, A.; Mannion, M.; Martin, S.T.; Winter, D.C. Omental pedicle flaps following proctectomy: A systematic review. Colorectal Dis. 2013, 15, e634–e645. [Google Scholar] [CrossRef]
- Nilsson, P.J. Omentoplasty in abdominoperineal resection: A review of the literature using a systematic approach. Dis. Colon Rectum. 2006, 49, 1354–1361. [Google Scholar] [CrossRef]
- Blok, R.D.; Musters, G.D.; Borstlap, W.A.A.; Buskens, C.J.; Bemelman, W.A.; Tanis, P.J.; Collaborative Dutch Snapshot Research Group. Snapshot Study one the value of Omentoplasty in Abdominoperineal Resection with Primary Perineal Closure for Rectal Cancer. Ann. Surg. Oncol. 2018, 25, 729–736. [Google Scholar] [CrossRef]
- Brodbeck, R.; Horch, R.E.; Arkudas, A.; Beier, J.P. Plastic and Reconstructive Surgery in the Treatment of Oncological Perineal and Genital Defects. Front. Oncol. 2015, 5, 212. [Google Scholar] [CrossRef]
- Chong, T.W.; Balch, G.C.; Kehoe, S.M.; Margulis, V.; Saint-Cyr, M. Reconstruction of Large Perineal and Pelvic Wounds Using Gracilis Muscle Flaps. Ann. Surg. Oncol. 2015, 22, 3738–3744. [Google Scholar] [CrossRef]
- Devulapalli, C.; Jia Wei, A.T.; DiBiagio, J.R.; Baez, M.L.; Baltodano, P.A.; Seal, S.M.; Sacks, J.M.; Cooney, C.M.; Rosson, G.D. Primary versus Flap Closure of Perineal Defects following Oncologic Resection: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2016, 137, 1602–1613. [Google Scholar] [CrossRef]
- Johnstone, M.S. Vertical Rectus Abdominis Myocutaneous versus Alternative Flaps for Perineal Repair after Abdominoperineal Excision of the Rectum in the Era of Laparoscopic Surgery. Ann. Plast. Surg. 2017, 79, 101–106. [Google Scholar] [CrossRef]
- Cui, J.; Ma, J.P.; Xiang, J.; Luo, Y.X.; Cai, S.R.; Huang, Y.H.; Wang, J.P.; He, Y.L. Prospective study of reconstructing pelvic floor with GORE-TEX Dual Mesh in abdominoperineal resection. Chin. Med. J. 2009, 122, 2138–2141. [Google Scholar]
- Moreno-Sanz, C.; Manzanera-Diaz, M.; Cortina-Oliva, F.J.; De Pedro-Conal, J. Pelvic reconstruction after abdominoperineal resection: A pilot study using an absorbable synthetic prosthesis. Tech. Coloproctol. 2011, 15, 455–459. [Google Scholar] [CrossRef]
- Burns, N.K.; Jaffari, M.V.; Rios, C.N.; Mathur, A.B.; Butler, C.H.E. Non-Cross-Linked Porcine Acellular Dermal Matrices for Abdominal Wall Reconstruction. Plast. Reconstr. Surg. 2010, 125, 167–176. [Google Scholar] [CrossRef]
- Marshall, M.J.; Smart, N.J.; Daniels, I.R. Biologic meshes in perineal reconstruction following extra-levator abdominoperineal excision (ELAPE). Colorectal Dis. 2012, 3, 12–18. [Google Scholar] [CrossRef]
- Alam, N.N.; Narant, S.K.; Kockerling, F.; Danils, I.R.; Smart, N.J. Biologic Mesh Reconstruction of the Pelvic Floor after Extralevator Abdominoperineal Excision: A Systematic Review. Front. Surg. 2016, 3, 9. [Google Scholar] [CrossRef]
- Schilz, B.; Buchs, N.C.H.; Penna, M.; Scarpa, C.R.; Liot, E.; Morel, P.; Ris, F. Biological mesh reconstruction of the pelvic floor following abdominoperineal excision for cancer: A review. World J. Clin. Oncol. 2017, 8, 249–254. [Google Scholar] [CrossRef]
- Fitzgerald, J.F.; Kumar, A.S. Biologic versus Synthetic Mesh Reinforcement. What are the Pros and Cons? Clin. Colon Rectal. Surg. 2014, 27, 140–148. [Google Scholar]
- Matena, O. Antiadhesive agents in laparoscopic surgery. Prakt. Gyn. 2013, 17, 237–240. [Google Scholar]
- Alegre-Sánchez, A.; Bernárdez, C. A new non-hydrophilic agarose gel as subdermal filler for facial rejuvenation: Aesthetic results and patient satisfaction. J. Cosmet. Dermatol. 2020, 19, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cossío, S.; León-Mateos, A.; Sampedro, F.G.; Oreja, M.T.C. Biocompatibility of agarose gel as a dermal filler: Histologic evaluation of subcutaneous implants. Plast Reconstr. Surg. 2007, 120, 1161–1169. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, S.; Liu, X.; Ye, S.; Zhou, X.; Huang, Q.; Ren, L. Silk/agarose scaffolds with tunable properties via SDS assisted rapid gelation. RSC Adv. 2017, 7, 21740–21748. [Google Scholar] [CrossRef]
- Ishizaki, Y.; Bandai, Y.; Shimomura, K.; Abe, H.; Ohtomo, Y.; Idezuki, Y. Safe intraabdominal pressure of carbon dioxide pneumoperitoneum during laparoscopic surgery. Surgery 1993, 114, 549–554. [Google Scholar] [PubMed]
- Atkinson, T.M.; Giraud, G.D.; Togioka, B.M.; Jones, D.B.; Cigarroa, J.E. Cardiovascular and Ventilatory Consequences of Laparoscopic Surgery. Circulation 2017, 135, 700–710. [Google Scholar] [CrossRef]
- Kralovic, M.; Vjaclovsky, M.; Kestlerova, A.; Rustichelli, F.; Hoch, J.; Amler, E. Electrospun nanofibers as support for the healing of intestinal anastomoses. Physiol. Res. 2019, 68 (Suppl. 4), S517–S525. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.K.; Gupta, P. In vitro biocompatibility study of keratin/agar scaffold for tissue engineering. Int. J. Biol. Macromol. 2015, 81, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wester, K.; Andersson, A.C.; Ranefall, P.; Bengtsson, E.; Malmström, P.U.; Busch, C. Cultured human fibroblasts in agarose gel as a multi-functional control for immunohistochemistry. Standardization of Ki67 (MIB1) assessment in routinely processed urinary bladder carcinoma tissue. J. Pathol. 2000, 190, 503–511. [Google Scholar] [CrossRef]
- Varoni, E.; Tschon, M.; Palazzo, B.; Nitti, P.; Martini, L.; Rimondini, L. Agarose gel as biomaterial or scaffold for implantation surgery: Characterizaton, histological and histomorphometric study on soft tissue response. Connect. Tissue Res. 2012, 53, 548–554. [Google Scholar] [CrossRef]
- Alexandre, N.; Ribeiro, J.; Gärtner, A.; Pereira, T.; Amorim, I.; Fragoso, J.; Lopes, A.; Fernandes, J.; Costa, E.; Santos-Silva, A.; et al. Biocompatibility and hemocompatibility of polyvinyl alcohol hydrogel used for vascular grafting—In vitro and in vivo studies. J. Biomed. Mater. Res. A 2014, 102, 4262–4275. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, A.; Ladage, P.; Kodam, K.M.; Ottoor, D. Biodegradable and biocompatible agarose–poly (vinyl alcohol) hydrogel for the in vitro investigation of ibuprofen release. Chem. Pap. 2020, 74, 1965–1978. [Google Scholar] [CrossRef]
- Christen, M.O.; Vercesi, F. Polycaprolactone: How a Well-Known and Futuristic Polymer Has Become an Innovative Collagen-Stimulator in Esthetics. Clin. Cosmet. Investig. Dermatol. 2020, 13, 31–48. [Google Scholar] [CrossRef]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bonnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef]
- Levental, I.; Georges, P.C.; Janmey, P.A. Soft biological materials and their impact on cell function. Soft Matter 2007, 3, 299–306. [Google Scholar] [CrossRef]
- McKee, C.T.; Last, J.A.; Russell, P.; Murphy, C.J. Indentation versus tensile measurements of Young’s modulus for soft biological tissues. Tissue Eng. Part B Rev. 2011, 17, 155–164. [Google Scholar] [CrossRef]
- Ma, Z.; Kotaki, M.; Inai, R.; Ramakrishna, S. Potential of Nanofiber Matrix as Tissue-Engineering scaffolds. Tissue Eng. 2005, 11, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Plencner, M.; East, B.; Tonar, Z.; Otahal, M.; Prosecka, E.; Rampichova, M.; Krejci, T.; Litvinec, A.; Buzgo, M.; Mickova, A.; et al. Abdominal closure reinforcement by using polypropylene mesh functionalized with poly-ε-caprolactone nanofibers and growth factors for prevention of incisional hernia formation. Int. J. Nanomed. 2014, 9, 3263–3277. [Google Scholar] [CrossRef] [PubMed]
- Bölgen, N.; Korkusuz, V.P.; Menceloglu, Y.Z.; Piskin, E. In vivo performance of antibiotic embedded electrospun PCL membranes for prevention of abdominal adhesions. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 530–543. [Google Scholar] [CrossRef]
- Staffa, A.; Vocetkova, K.; Sovkova, V.; Rampichova, M.; Filova, E.; Amler, E. Polycaprolactone nanofiber mesh with adhered liposomes as a simple delivery system for bioactive growth factors. Transl. Med. Rep. 2017, 1, 58–63. [Google Scholar] [CrossRef][Green Version]
- Rampichova, M.; Buzgo, M.; Lukasova, V.; Mickova, A.; Vocetkova, K.; Sovkova, V.; Rustichelli, F.; Amler, E. Functionalization of 3D fibrous sca_olds prepared using centrifugal spinning with liposomes as a simple drug delivery system. Acta Polytechnol. CTU Proc. 2017, 8, 24–26. [Google Scholar] [CrossRef]


| Gel Composition Heated to 37 °C | ɛ =Δl/l (%) |
|---|---|
| Agarose 1.5% matured | 14 ± 1 |
| Agarose 1.5% fresh | 18 ± 2 |
| Agar-Agar 1.5% | 24 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocková, M.; Pashchenko, A.; Stuchlíková, S.; Kalábová, H.; Divín, R.; Novotný, P.; Kestlerová, A.; Jelen, K.; Kubový, P.; Firment, P.; et al. Low Concentrated Fractionalized Nanofibers as Suitable Fillers for Optimization of Structural–Functional Parameters of Dead Space Gel Implants after Rectal Extirpation. Gels 2022, 8, 158. https://doi.org/10.3390/gels8030158
Bocková M, Pashchenko A, Stuchlíková S, Kalábová H, Divín R, Novotný P, Kestlerová A, Jelen K, Kubový P, Firment P, et al. Low Concentrated Fractionalized Nanofibers as Suitable Fillers for Optimization of Structural–Functional Parameters of Dead Space Gel Implants after Rectal Extirpation. Gels. 2022; 8(3):158. https://doi.org/10.3390/gels8030158
Chicago/Turabian StyleBocková, Markéta, Aleksei Pashchenko, Simona Stuchlíková, Hana Kalábová, Radek Divín, Petr Novotný, Andrea Kestlerová, Karel Jelen, Petr Kubový, Peter Firment, and et al. 2022. "Low Concentrated Fractionalized Nanofibers as Suitable Fillers for Optimization of Structural–Functional Parameters of Dead Space Gel Implants after Rectal Extirpation" Gels 8, no. 3: 158. https://doi.org/10.3390/gels8030158
APA StyleBocková, M., Pashchenko, A., Stuchlíková, S., Kalábová, H., Divín, R., Novotný, P., Kestlerová, A., Jelen, K., Kubový, P., Firment, P., Fedačko, J., Jarošíková, T., Rulc, J., Rosina, J., Nečas, A., Amler, E., & Hoch, J. (2022). Low Concentrated Fractionalized Nanofibers as Suitable Fillers for Optimization of Structural–Functional Parameters of Dead Space Gel Implants after Rectal Extirpation. Gels, 8(3), 158. https://doi.org/10.3390/gels8030158






