Development and Evaluation of Ginkgo biloba/Sodium Alginate Nanocomplex Gel as a Long-Acting Formulation for Wound Healing
Abstract
1. Introduction
2. Results
2.1. Nanocomplex Gel Formulation and Optimization
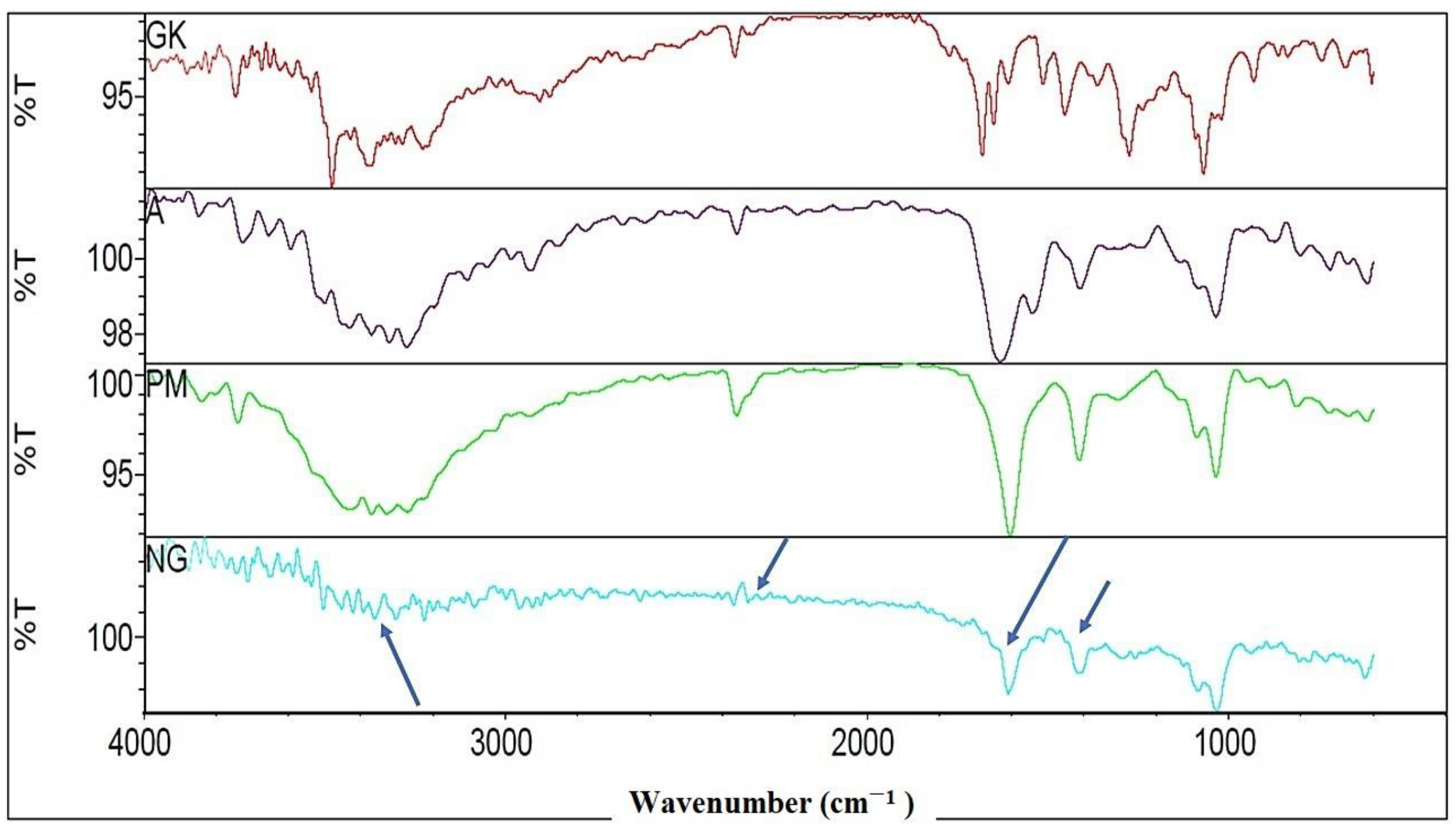
2.2. FTIR Characterizations of Nanocomplex Gel
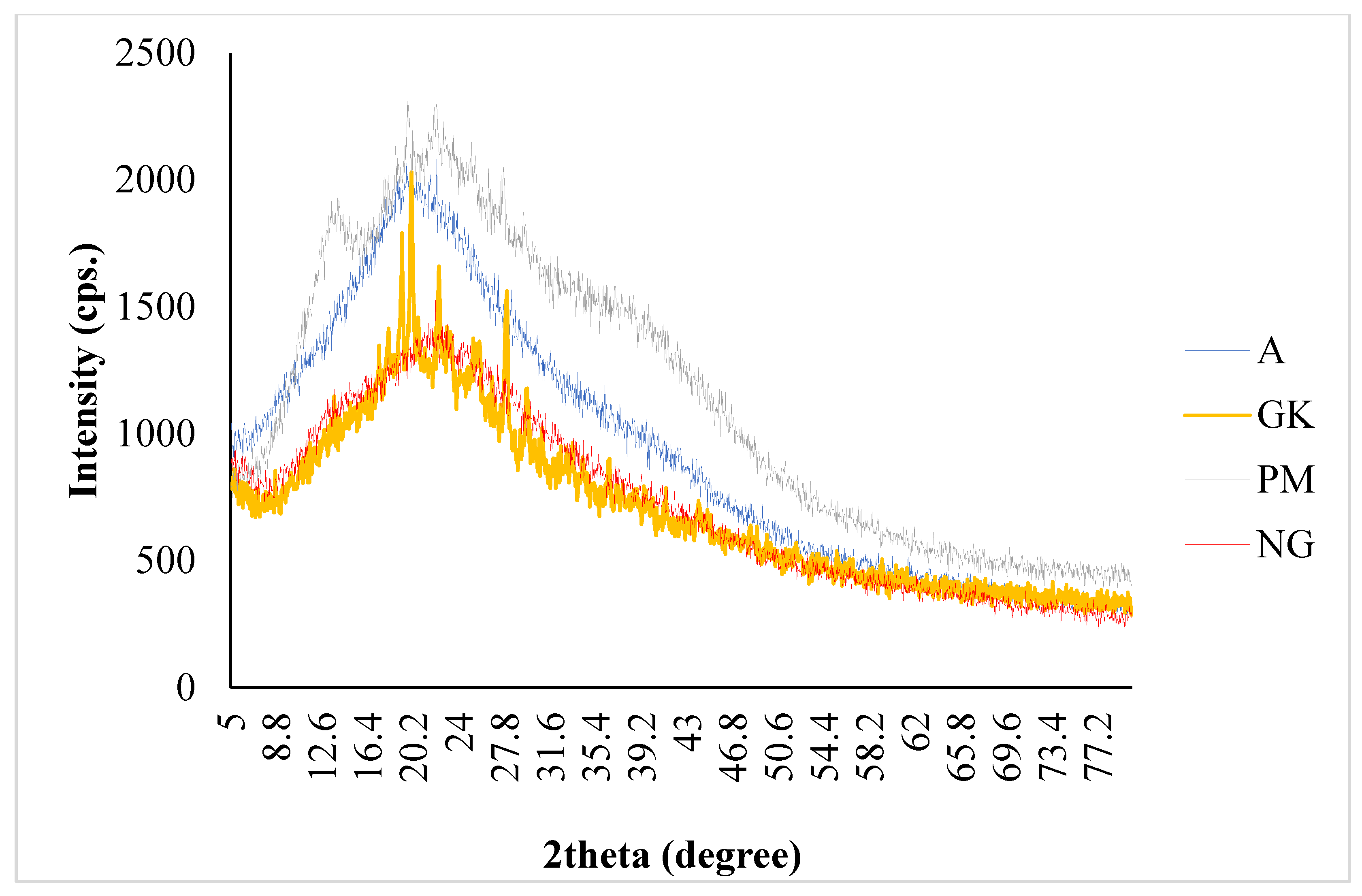
2.3. PXRD Characterizations of Nanocomplex Gel
2.4. SEM Characterizations of Nanocomplex Gel
2.5. TEM Characterization of Nanocomplex Gel
2.6. Rheology Study of Nanocomplex Gel
2.7. Nanocomplex Gel Diffusion Study
2.8. Swelling, Diffusibility, and Resilience Test
2.9. Effect of Nanocomplex Gel on Antioxidant Parameters
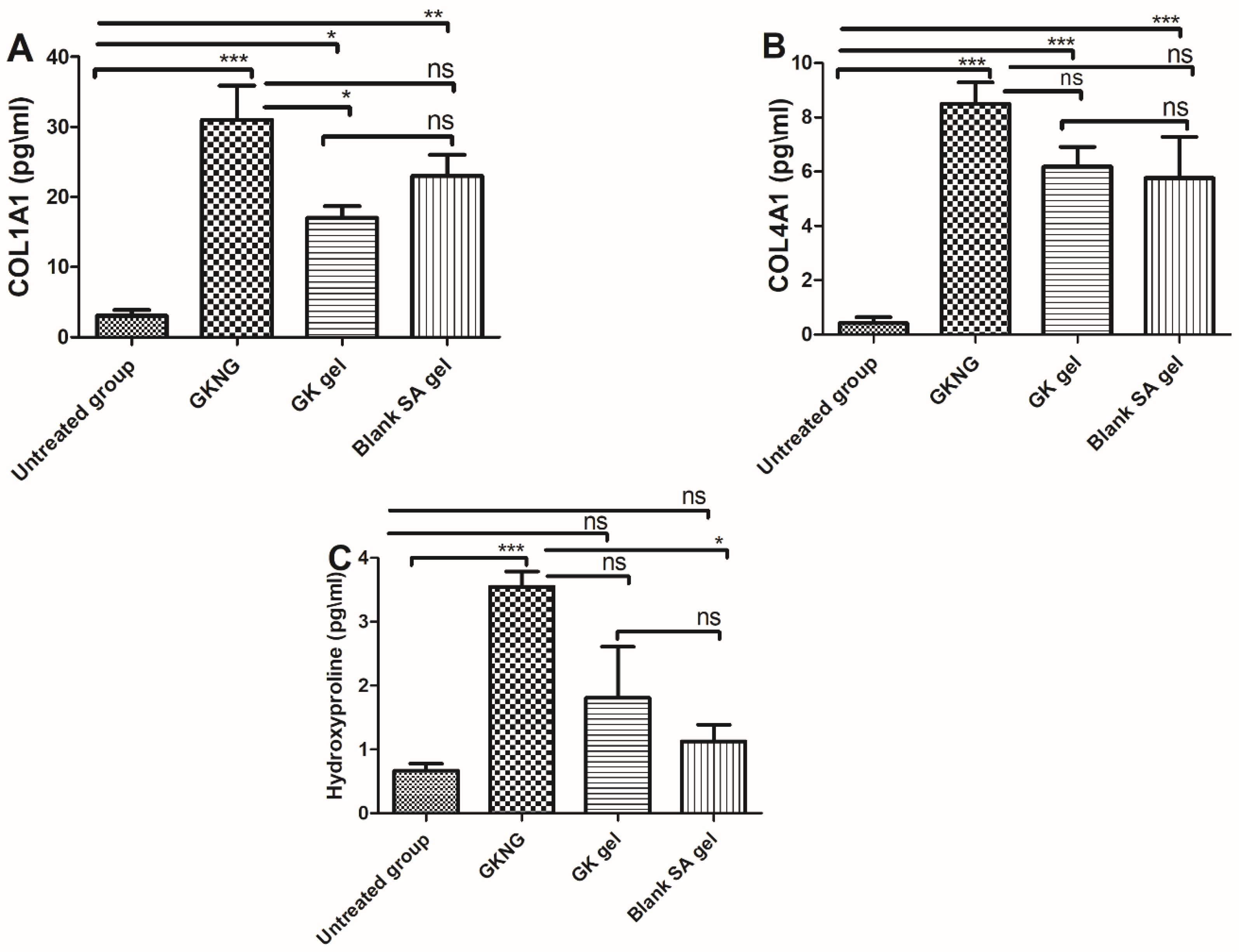
2.10. Effect of Nanocomplex Gel on Fibrotic Markers
2.11. Effect of Nanocomplex Gel on Angiogenic Markers and TGF-β1
2.12. Effect of Nanocomplex Gel on Wound Contraction
2.13. Effect of Nanocomplex Gel on Histopathological Changes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Drugs and Chemicals
5.2. Methods of Nanocomplex Gel Preparation
5.3. Characterization of Nanocomplex Gel
5.3.1. Determination of Particle Size, PDI, Zeta Potential, and Encapsulation Efficiency
5.3.2. FTIR Study
5.3.3. PXRD Study
5.3.4. Structural Analysis
5.3.5. Rheology Study
5.3.6. In Vitro Diffusion Study
5.3.7. Swelling, Diffusibility, and Resilience Test
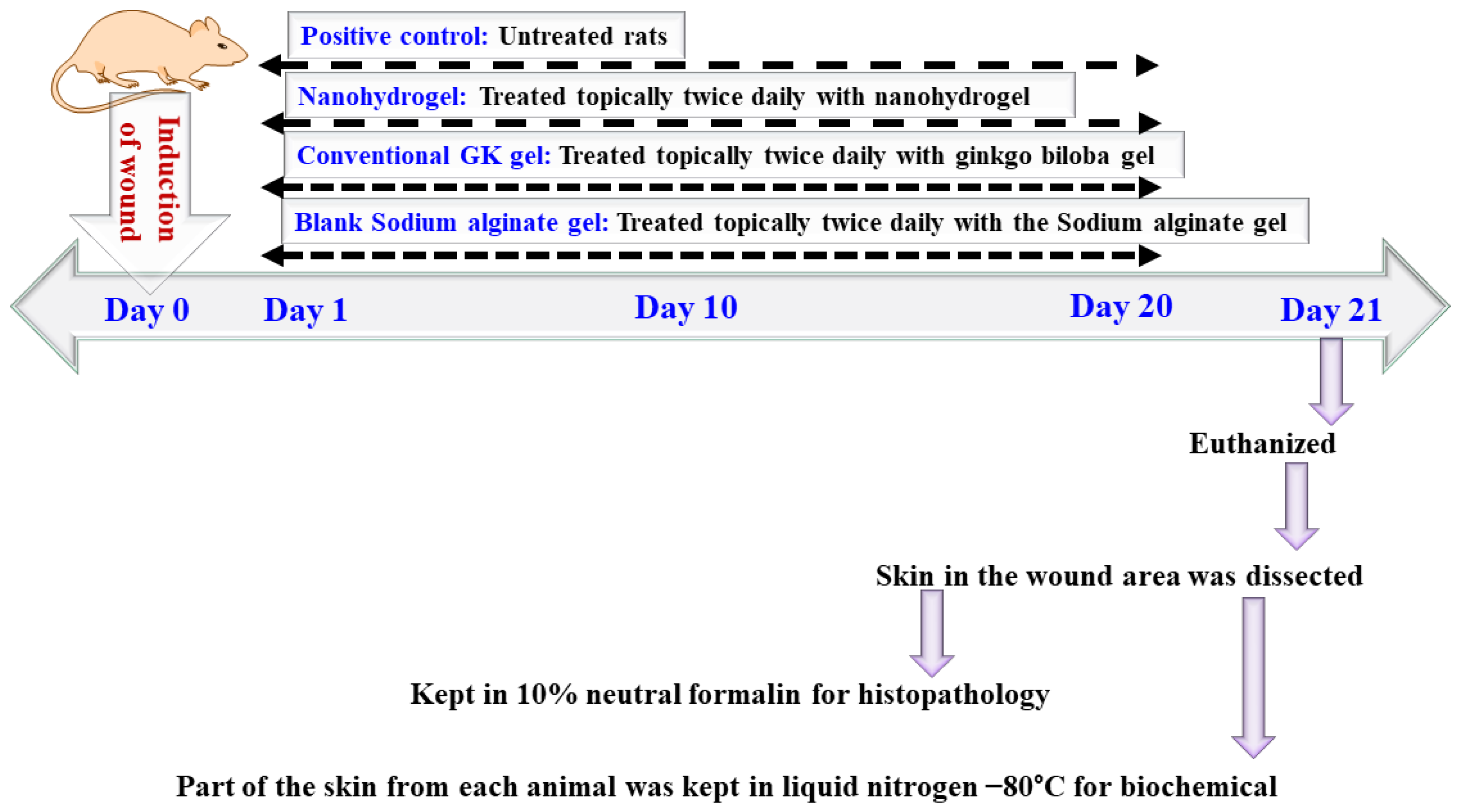
5.4. In Vivo Study
5.4.1. Treatment Protocol
5.4.2. Measurement of Wound
5.4.3. Histological Analysis
5.4.4. Tissue Homogenate Preparation
5.4.5. Effects of Nanocomplex Gel on Antioxidant Markers and Markers of Lipid Peroxidation
5.4.6. Effects of Nanocomplex Gel on the Levels of Collagen Formation (COL1A1 and COL4A1), Hydroxyproline, and Growth Factors (EGF, VEGF, and TGF-β1)
5.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iqubal, M.K.; Saleem, S.; Iqubal, A.; Chaudhuri, A.; Pottoo, F.H.; Ali, J.; Baboota, S. Natural, synthetic and their combinatorial nanocarriers based drug delivery system in the treatment paradigm for wound healing via dermal targeting. Curr. Pharm. Des. 2020, 26, 4551–4568. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, M.K.; Iqubal, A.; Anjum, H.; Gupta, M.M.; Ali, J.; Baboota, S. Determination of in vivo virtue of dermal targeted combinatorial lipid nanocolloidal based formulation of 5-fluorouracil and resveratrol against skin cancer. Int. J. Pharm. 2021, 610, 121179. [Google Scholar] [CrossRef] [PubMed]
- Gushiken, L.F.S.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous wound healing: An update from physiopathology to current therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.; Song, J.; Adams, V.; Jackson, C.J.; Pellizzon, C.H. Exploring prevalence of wound infections and related patient characteristics in homecare using natural language processing. Int. Wound J. 2021, 19, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Wadström, T.; Eliasson, I.; Holder, I.; Ljungh, A. Pathogenesis of Wound and Biomaterial-Associated Infections; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Yadav, S.; Mishra, A.P.; Kumar, S.; Negi, A.; Maurya, V.K. Herbal wound healing agents. In Preparation of Phytopharmaceuticals for the Management of Disorders; Elsevier: Amsterdam, The Netherlands, 2021; pp. 169–184. [Google Scholar]
- Bairy, K.; Rao, C. Wound healing profiles of Ginkgo biloba. J. Nat. Remedies 2001, 1, 25–27. [Google Scholar]
- Bardaa, S.; Makni, K.; Boudaouara, O.; Bardaa, T.; Ktari, N.; Hachicha, S.; Ben Salah, R.; Kallel, R.; Sahnoun, Z.; Boufi, S. Development and Evaluation of the Wound Healing Effect of a Novel Topical Cream Formula Based on Ginkgo biloba Extract on Wounds in Diabetic Rats. BioMed Res. Int. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Majumder, S.; Gupta, K.C.; Kumar, A. Dextran based amphiphilic nano-hybrid hydrogel system incorporated with curcumin and cerium oxide nanoparticles for wound healing. Colloids Surf. B Biointerfaces 2020, 195, 111263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, P.; Gao, X.; Chang, L.; Chen, Z.; Mei, X. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater. Sci. Eng. C 2021, 120, 111671. [Google Scholar] [CrossRef]
- Ahmad, Z.; Khuller, G. Alginate-based sustained release drug delivery systems for tuberculosis. Expert Opin. Drug Deliv. 2008, 5, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Bani-Jaber, A.; Abdullah, S. Development and characterization of novel ambroxol sustained-release oral suspensions based on drug-polymeric complexation and polymeric raft formation. Pharm. Dev. Technol. 2020, 25, 666–675. [Google Scholar] [CrossRef]
- Abdullah, S.; El Hadad, S.; Aldahlawi, A. In vitro optimization, characterization and anti-tumor evaluation against colorectal cancer of a novel 5-fluorouracil oral nanosuspension using soy protein, polysaccharides-protein complexation, and in-situ gel formation. J. Drug Deliv. Sci. Technol. 2022, 67, 102857. [Google Scholar] [CrossRef]
- Md, S.; Abdullah, S.; Alhakamy, N.A.; Alharbi, W.S.; Ahmad, J.; Shaik, R.A.; Ansari, M.J.; Ibrahim, I.M.; Ali, J. Development, Optimization, and In Vitro Evaluation of Novel Oral Long-Acting Resveratrol Nanocomposite In-Situ Gelling Film in the Treatment of Colorectal Cancer. Gels 2021, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Aprilliza, M. (Ed.) Characterization and properties of sodium alginate from brown algae used as an ecofriendly superabsorbent. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017. [Google Scholar]
- Song, R.; Zheng, J.; Liu, Y.; Tan, Y.; Yang, Z.; Song, X.; Yang, S.; Fan, R.; Zhang, Y.; Wang, Y. A natural cordycepin/chitosan complex hydrogel with outstanding self-healable and wound healing properties. Int. J. Biol. Macromol. 2019, 134, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Yin, L.; Yu, J.; Yin, C.; Pei, Y. Swelling behavior and biocompatibility of Carbopol-containing superporous hydrogel composites. J. Appl. Polym. Sci. 2007, 104, 2785–2791. [Google Scholar] [CrossRef]
- Farahani, M.; Shafiee, A. Wound healing: From passive to smart dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef] [PubMed]
- Salim, M.N.; Silvia, M.; Aliza, D.; Masyitha, D.; Iskandar, C.D.; Rusli, R.; Sugito, S.; Hasan, M.; Sabri, M.; Harris, A. (Eds.) Efficacy of Jatropha curcas latex cream in the epithelialization phase of wound healing in mice skin. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2020. [Google Scholar]
- Deal, H.E.; Brown, A.C.; Daniele, M.A. Microphysiological systems for the modeling of wound healing and evaluation of pro-healing therapies. J. Mater. Chem. B 2020, 8, 7062–7075. [Google Scholar] [CrossRef] [PubMed]
- Barman, P.K.; Koh, T.J. Macrophage dysregulation and impaired skin wound healing in diabetes. Front. Cell Dev. Biol. 2020, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Stefi, R.V.; Pasupuleti, M.; Paray, B.A.; Al-Sadoon, M.K.; Arockiaraj, J. Antioxidant molecular mechanism of adenosyl homocysteinase from cyanobacteria and its wound healing process in fibroblast cells. Mol. Biol. Rep. 2020, 47, 1821–1834. [Google Scholar] [CrossRef] [PubMed]
- El Ayadi, A.; Jay, J.W.; Prasai, A. Current approaches targeting the wound healing phases to attenuate fibrosis and scarring. Int. J. Mol. Sci. 2020, 21, 1105. [Google Scholar] [CrossRef] [PubMed]
- Yuniati, R.; Subchan, P.; Riawan, W.; Paray, B.A.; Al-Sadoon, M.K.; Arockiaraj, J. Topical ozonated virgin coconut oil improves wound healing and increases HSP90α, VEGF-A, EGF, bFGF, and CD34 in diabetic ulcer mouse model of wound healing. F1000Research 2021, 9, 580. [Google Scholar]
- Eid, B.G.; Alhakamy, N.A.; Fahmy, U.A.; Ahmed, O.A.; Md, S.; Abdel-Naim, A.B.; Caruso, G.; Caraci, F. Melittin and diclofenac synergistically promote wound healing in a pathway involving TGF-β1. Pharmacol. Res. 2021, 175, 105993. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, Z.; Sun, J. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-β/Smad signaling pathway. Stem Cell Res. Ther. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, S.; Sivamani, R.K.; Isseroff, R.R. Antioxidant therapies for wound healing: A clinical guide to currently commercially available products. Ski. Pharmacol. Physiol. 2011, 24, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; El Hadad, S.; Aldahlawi, A. The development of a novel oral 5-Fluorouracil in-situ gelling nanosuspension to potentiate the anticancer activity against colorectal cancer cells. Int. J. Pharm. 2022, 613, 121406. [Google Scholar] [CrossRef] [PubMed]
- Telange, D.R.; Patil, A.T.; Tatode, A.; Bhoyar, B. Development and Validation of UV Spectrophotometric Method for the Estimation of Kaempferol in Kaempferol: Hydrogenated Soy PhosphatidylCholine (HSPC) Complex. Pharm. Methods 2014, 5, 34–38. [Google Scholar] [CrossRef]
- Gusella, M.; Frigo, A.; Bolzonella, C.; Marinelli, R.; Barile, C.; Bononi, A.; Crepaldi, G.; Menon, D.; Stievano, L.; Toso, S. Predictors of survival and toxicity in patients on adjuvant therapy with 5-fluorouracil for colorectal cancer. Br. J. Cancer 2009, 100, 1549–1557. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, J.; Li, F.; Wang, X.; Yu, J.; Wu, D. Hyaluronic acid and polyethylene glycol hybrid hydrogel encapsulating nanogel with hemostasis and sustainable antibacterial property for wound healing. ACS Appl. Mater. Interfaces 2018, 10, 13304–13316. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Abdullah, S.T.; Alhakamy, N.A.; Bani-Jaber, A.; Radhakrishnan, A.K.; Karim, S.; Shahzad, N.; Gabr, G.A.; Alamoudi, A.J.; Rizg, W.Y. Ambroxol Hydrochloride Loaded Gastro-Retentive Nanosuspension Gels Potentiate Anticancer Activity in Lung Cancer (A549) Cells. Gels 2021, 7, 243. [Google Scholar] [CrossRef]
- Iqubal, A.; Sharma, S.; Ansari, M.A.; Najmi, A.K.; Syed, M.A.; Ali, J.; Alam, M.M.; Ahmad, S.; Haque, S.E. Nerolidol attenuates cyclophosphamide-induced cardiac inflammation, apoptosis and fibrosis in Swiss Albino mice. Eur. J. Pharmacol. 2019, 863, 172666. [Google Scholar] [CrossRef]
- Iqubal, A.; Syed, M.A.; Ali, J.; Najmi, A.K.; Haque, M.M.; Haque, S.E. Nerolidol protects the liver against cyclophosphamide-induced hepatic inflammation, apoptosis, and fibrosis via modulation of Nrf2, NF-κB p65, and caspase-3 signaling molecules in Swiss albino mice. BioFactors 2020, 46, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, A.; Syed, M.A.; Najmi, A.K.; Azam, F.; Barreto, G.E.; Iqubal, M.K.; Ali, J.; Haque, S.E. Nano-engineered nerolidol loaded lipid carrier delivery system attenuates cyclophosphamide neurotoxicity–Probable role of NLRP3 inflammasome and caspase-1. Exp. Neurol. 2020, 334, 113464. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liang, P.; Jiang, B.; Zhang, P.; Yu, W.; Duan, M.; Guo, L.; Cui, X.; Huang, M.; Huang, X. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 2020, 259, 118246. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Singh, K.; Muschhammer, J.; Schatz, S.; Sindrilaru, A.; Makrantonaki, E.; Qi, Y.; Wlaschek, M.; Scharffetter-Kochanek, K. MSCs rescue impaired wound healing in a murine LAD1 model by adaptive responses to low TGF-β1 levels. EMBO Rep. 2020, 21, e49115. [Google Scholar] [CrossRef] [PubMed]
- Berner, T.; Nakahara, K.; Kobayashi, E.; Tanaka, A.; Taniguchi, Y.; Iizuka, T.; Sawada, K. Investigating the effect of antiseptic solution on the release of interleukin-6 and transforming growth factor beta 1 from human gingival fibroblasts using wound healing assays. J. Oral Sci. 2020, 62, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, Z.; Xue, R.; Tanaka, A.; Taniguchi, Y.; Iizuka, T.; Sawada, K. TGF-β1 promoted MMP-2 mediated wound healing of anterior cruciate ligament fibroblasts through NF-κB. Connect. Tissue Res. 2011, 52, 218–225. [Google Scholar] [CrossRef]
- Baie, S.H.; Sheikh, K. The wound healing properties of Channa striatus-cetrimide cream-wound contraction and glycosaminoglycan measurement. J. Ethnopharmacol. 2000, 73, 15–30. [Google Scholar] [CrossRef]
- Montandon, D.; D’Andiran, G.; Gabbiani, G. The mechanism of wound contraction and epithelialization: Clinical and experimental studies. Clin. Plast. Surg. 1977, 4, 325–346. [Google Scholar] [CrossRef]
- Iqubal, M.K.; Iqubal, A.; Imtiyaz, K.; Rizvi, M.M.A.; Gupta, M.M.; Ali, J.; Baboota, S. Combinatorial lipid-nanosystem for dermal delivery of 5-fluorouracil and resveratrol against skin cancer: Delineation of improved dermatokinetics and epidermal drug deposition enhancement analysis. Eur. J. Pharm. Biopharm. 2021, 163, 223–239. [Google Scholar] [CrossRef]
- Garg, B.; Singh, R. 3, 5, 7, 4′-Tetrahydroxyflavone (kaempferol) as a chromogenic reagent for gallium and indium. Talanta 1971, 18, 761–766. [Google Scholar] [CrossRef]
- Iqubal, A.; Syed, M.A.; Haque, M.M.; Najmi, A.K.; Ali, J.; Haque, S.E. Effect of nerolidol on cyclophosphamide-induced bone marrow and hematologic toxicity in Swiss albino mice. Exp. Hematol. 2020, 82, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, A.; Sharma, S.; Sharma, K.; Bhavsar, A.; Hussain, I.; Iqubal, M.K.; Kumar, R. Intranasally administered pitavastatin ameliorates pentylenetetrazol-induced neuroinflammation, oxidative stress and cognitive dysfunction. Life Sci. 2018, 211, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]






| Combination. No. | Ginkgo Extract. (mg/mL) | Alginate Equivalent Amount (mg/mL) | Ginkgo: Alginate Ratio | Gel Total Volume (mL) | Particle Size (nm) | PDI |
|---|---|---|---|---|---|---|
| 1 | 10 | 30 | 1:3 | 10 | 450.14 ± 36.06 | 0.32 ± 0.12 |
| 2 | 20 | 2:3 | 580.25 ± 66.33 | 0.44 ± 0.06 | ||
| 3 | 30 | 3:3 | 860.33 ± 30.33 | 0.54 ± 0.91 | ||
| 4 | 40 | 4:3 | 1000.01 ± 29.33 | 0.59 ± 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Md, S.; Abdullah, S.; Alhakamy, N.A.; Shaik, R.A.; Eldakhakhny, B.M.; Omar, U.M.; Eid, B.G.; Ansari, A.R.; Alamoudi, A.J.; Rizg, W.Y.; et al. Development and Evaluation of Ginkgo biloba/Sodium Alginate Nanocomplex Gel as a Long-Acting Formulation for Wound Healing. Gels 2022, 8, 189. https://doi.org/10.3390/gels8030189
Md S, Abdullah S, Alhakamy NA, Shaik RA, Eldakhakhny BM, Omar UM, Eid BG, Ansari AR, Alamoudi AJ, Rizg WY, et al. Development and Evaluation of Ginkgo biloba/Sodium Alginate Nanocomplex Gel as a Long-Acting Formulation for Wound Healing. Gels. 2022; 8(3):189. https://doi.org/10.3390/gels8030189
Chicago/Turabian StyleMd, Shadab, Samaa Abdullah, Nabil A. Alhakamy, Rasheed A. Shaik, Basmah Medhat Eldakhakhny, Ulfat Mohammad Omar, Basma G. Eid, Akhalakur Rahman Ansari, Abdulmohsin J. Alamoudi, Waleed Y. Rizg, and et al. 2022. "Development and Evaluation of Ginkgo biloba/Sodium Alginate Nanocomplex Gel as a Long-Acting Formulation for Wound Healing" Gels 8, no. 3: 189. https://doi.org/10.3390/gels8030189
APA StyleMd, S., Abdullah, S., Alhakamy, N. A., Shaik, R. A., Eldakhakhny, B. M., Omar, U. M., Eid, B. G., Ansari, A. R., Alamoudi, A. J., Rizg, W. Y., Riadi, Y., Venkateswaran, S. P., & Rashid, M. A. (2022). Development and Evaluation of Ginkgo biloba/Sodium Alginate Nanocomplex Gel as a Long-Acting Formulation for Wound Healing. Gels, 8(3), 189. https://doi.org/10.3390/gels8030189









