Preparation of Styrene-Butadiene Rubber (SBR) Composite Incorporated with Collagen-Functionalized Graphene Oxide for Green Tire Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Composites
2.3. Characterization
2.3.1. Morphology
2.3.2. Porosity
2.3.3. Chemical Functionality
2.3.4. Thermal Property
2.3.5. Mechanical Property
2.3.6. Biodegradability
3. Results and Discussion
3.1. Morphology, Porosity, and Elemental Composition
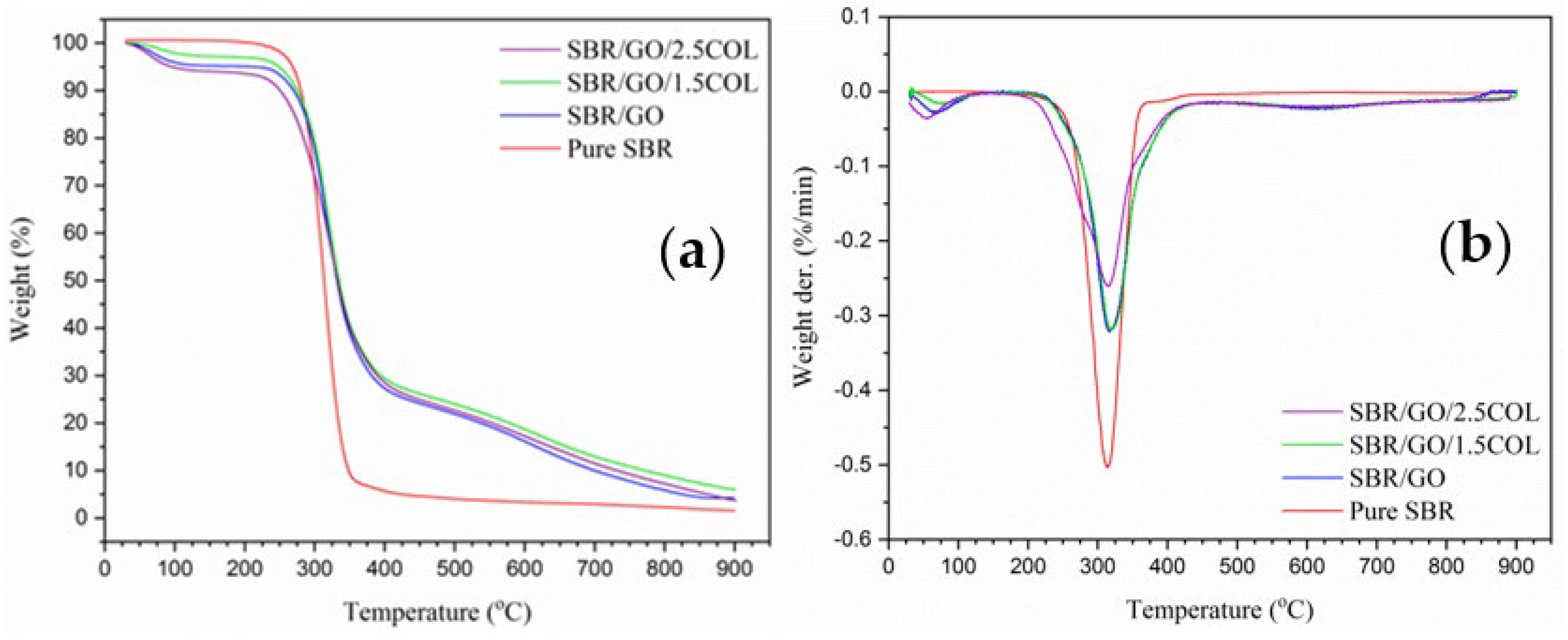
3.2. Thermal Stability
3.3. FTIR
3.4. Mechanical and Biodegradability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohanty, A.K.; Vivekanandhan, S.; Pin, J.M.; Misra, M. Composites from renewable and sustainable resources: Challenges and innovations. Science 2018, 362, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, K.; Stallwood, B.; Hart, A.G. Tire rubber recycling and bioremediation: A review. Bioremediat. J. 2008, 12, 1–11. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene: The new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef] [Green Version]
- Bai, H.; Li, C.; Wang, X.; Shi, G. On the gelation of graphene oxide. J. Phys. Chem. C 2011, 115, 5545–5551. [Google Scholar] [CrossRef]
- Yan, L.; Zheng, Y.B.; Zhao, F.; Li, S.; Gao, X.; Xu, B.; Weiss, P.S.; Zhao, Y. Chemistry and physics of a single atomic layer: Strategies and challenges for functionalization of graphene and graphene-based materials. Chem. Soc. Rev. 2012, 41, 97–114. [Google Scholar] [CrossRef]
- Wei, W.; Qu, X. Extraordinary physical properties of functionalized graphene. Small 2012, 8, 2138–2151. [Google Scholar] [CrossRef]
- Scharfenberg, S.; Rocklin, D.Z.; Chialvo, C.; Weaver, R.L.; Goldbart, P.M.; Mason, N. Probing the mechanical properties of graphene using a corrugated elastic substrate. Appl. Phys. Lett. 2011, 98, 091908. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Adamcik, J.; Mezzenga, R. Biodegradable nanocomposites of amyloid fibrils and graphene with shape-memory and enzyme-sensing properties. Nat. Nanotechnol. 2012, 7, 421–427. [Google Scholar] [CrossRef]
- Guntur, N.P.R.; Yadav, S.G.; Gopalan, S. Effect of titanium carbide as a filler on the mechanical properties of styrene butadiene rubber. Mater. Today Proc. 2020, 24, 1552–1560. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Chang, S.K.C. Isolation and characterization of collagen extracted from channel catfish (Ictalurus punctatus) skin. Food Chem. 2018, 242, 147–155. [Google Scholar] [CrossRef]
- Liu, H.; Huang, K. Structural characteristics of extracted collagen from Tilapia (Oreochromis mossambicus) bone: Effects of ethylenediaminetetraacetic acid solution and hydrochloric acid treatment. Int. J. Food Prop. 2016, 19, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.M.; Kishimura, H.; Benjakul, S. Extraction efficiency and characteristics of acid and pepsin soluble collagens from the skin of golden carp (Probarbus Jullieni) as affected by ultrasonication. Process Biochem. 2018, 66, 237–244. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y.; Luo, Y.; Jia, D.; Liu, L. Styrene butadiene rubber/carbon black composites modified by imidazole derivatives. Int. J. Polym. Anal. Charact. 2016, 21, 447–457. [Google Scholar] [CrossRef]
- Mao, Y.; Wen, S.; Chen, Y.; Zhang, F.; Panine, P.; Chan, T.W.; Zhang, L.; Liang, Y.; Liu, L. High performance graphene oxide based rubber composites. Sci. Rep. 2013, 3, 2508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahamad, A.; Kumar, P. Effect of reinforcing ability of halloysite nanotubes in styrene-butadiene rubber nanocomposites. Compos. Commun. 2020, 22, 100440. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.; Chen, Z. Improvement of short-term aging resistance of styrene-butadiene rubber modified asphalt by Sasobit and epoxidized soybean oil. Constr. Build. Mater. 2021, 271, 121870. [Google Scholar] [CrossRef]




| Samples of Composite | Formulation | ||
|---|---|---|---|
| SBR (wt%) | GO (wt%) | COL (wt%) | |
| SBR | 100.0 | - | - |
| SBR/GO | 95.0 | 5.0 | - |
| SBR/GO/1.5COL | 93.5 | 5.0 | 1.5 |
| SBR/GO/2.5COL | 92.5 | 5.0 | 2.5 |
| Samples | Carbon (%) a | Oxygen (%) a | Nitrogen (%) a | Surface Area (m2/g) b | Pore Volume (cm3/g) b | Pore Size (nm) b |
|---|---|---|---|---|---|---|
| Pure SBR | 100.0 | - | - | 2.76 | 0.345 | 249.75 |
| SBR/GO | 92.73 | 7.27 | - | 3.91 | 0.168 | 85.83 |
| SBR/GO/1.5COL | 91.40 | 8.04 | 0.56 | 4.51 | 0.186 | 82.68 |
| SBR/GO/2.5COL | 87.70 | 11.71 | 0.58 | 4.10 | 0.325 | 158.49 |
| Membranes | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (GPa) |
|---|---|---|---|
| Pure SBR | 39.4 | 5.8 | 1.34 |
| SBR/GO | 44.2 | 5.9 | 1.46 |
| SBR/GO/1.5COL | 47.0 | 7.2 | 1.51 |
| SBR/GO/2.5COL | 33.8 | 5.4 | 1.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Kian, L.K.; Jawaid, M.; Khan, A.A.P.; Alotaibi, M.M.; Asiri, A.M.; Marwani, H.M. Preparation of Styrene-Butadiene Rubber (SBR) Composite Incorporated with Collagen-Functionalized Graphene Oxide for Green Tire Application. Gels 2022, 8, 161. https://doi.org/10.3390/gels8030161
Khan A, Kian LK, Jawaid M, Khan AAP, Alotaibi MM, Asiri AM, Marwani HM. Preparation of Styrene-Butadiene Rubber (SBR) Composite Incorporated with Collagen-Functionalized Graphene Oxide for Green Tire Application. Gels. 2022; 8(3):161. https://doi.org/10.3390/gels8030161
Chicago/Turabian StyleKhan, Anish, Lau Kia Kian, Mohammad Jawaid, Aftab Aslam Parwaz Khan, Maha Moteb Alotaibi, Abdullah M. Asiri, and Hadi M. Marwani. 2022. "Preparation of Styrene-Butadiene Rubber (SBR) Composite Incorporated with Collagen-Functionalized Graphene Oxide for Green Tire Application" Gels 8, no. 3: 161. https://doi.org/10.3390/gels8030161







