Mechanochemical Effect on Controlled Drug Release of Konjac Glucomannan Matrix Tablets during Dry Grinding
Abstract
:1. Introduction
2. Results
2.1. Change in the Physicochemical Properties of the Ground KGM and Ca(OH)2 Mixed Powder
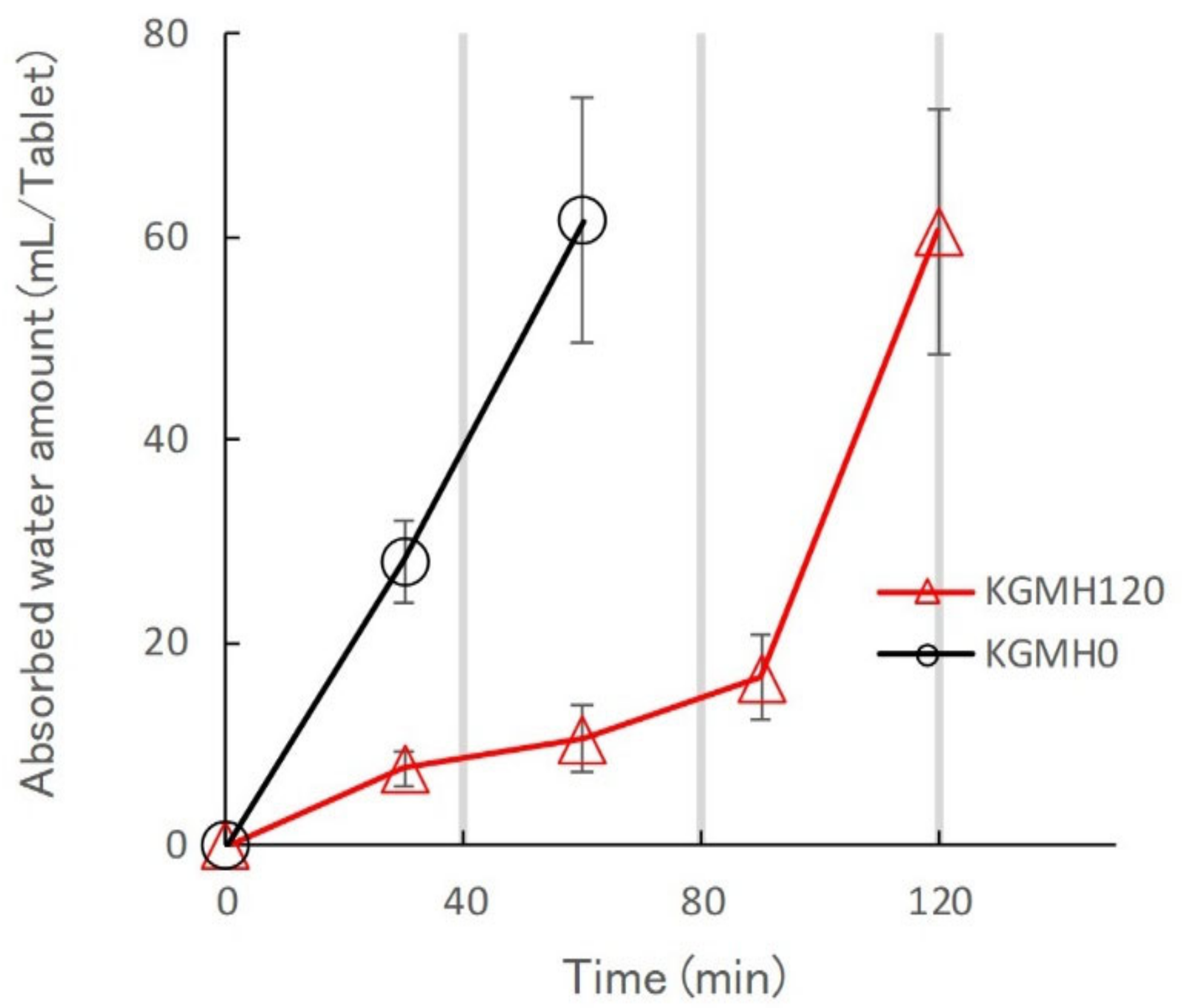
2.2. Effect of Grinding on the Drug Release Profiles of the KGM Matrix Tablets
3. Discussion
3.1. Mechanochemical Reaction of KGM with Ca(OH)2 during Grinding
3.2. Effect of Grinding on the Drug Release Kinetics of KGM Matrix Tablets and Hydrogel Formation Mechanism
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Grinding Treatment
5.3. Scanning Electron Microscopy with Energy-Dispersive X-ray Spectroscopy
5.4. Powder X-ray Diffraction (XRD)
5.5. Fourier-Transform Infrared (FT-IR) Spectroscopy
5.6. Tablet Preparation
5.7. Drug Release Kinetics Measured by the Dissolution Test
5.8. Water Penetration into Tablets
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Colombo, P.; Bettini, R.; Santi, P.; Peppas, N.A. Swellable matrices for controlled drug delivery: Gel-layer behaviour, mechanisms and optimal performance. Pharm. Sci. Technol. Today 2000, 3, 198–204. [Google Scholar] [CrossRef]
- Kiortsis, S.; Kachrimanis, K.; Broussali, T.; Malamataris, S. Drug release from tableted wet granulations comprising cellulosic (HPMC or HPC) and hydrophobic component. Eur. J. Pharm. Biopharm. 2005, 59, 73–83. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Kumar§, N. Polymeric Controlled Drug-Delivery Systems: Perspective Issues and Opportunities. Drug Dev. Ind. Pharm. 2001, 27, 1–30. [Google Scholar] [CrossRef]
- Levina, M.; Rajabi-Siahboomi, A.R. The Influence of Excipients on Drug Release from Hydroxypropyl Methylcellulose Matrices. J. Pharm. Sci. 2004, 93, 2746–2754. [Google Scholar] [CrossRef] [PubMed]
- Melia, C.D. Hydrophilic matrix sustained release systems based on polysaccharide carriers. Crit. Rev. Ther. Drug Carr. Syst. 1991, 8, 395–421. [Google Scholar]
- Bown, D. Aroids: Plants of the Arum Family; Timber Press: Portland, OR, USA, 2010. [Google Scholar]
- Talbott, S.M. A Guide to Understanding Dietary Supplements, 1st ed.; Haworth Press: Philadelphia, PA, USA, 2003. [Google Scholar] [CrossRef]
- Yang, D.; Yuan, Y.; Wang, L.; Wang, X.; Mu, R.; Pang, J.; Xiao, J.; Zheng, Y. A Review on Konjac Glucomannan Gels: Microstructure and Application. Int. J. Mol. Sci. 2017, 18, 2250. [Google Scholar] [CrossRef] [PubMed]
- Keithley, J.; Swanson, B. Glucomannan and obesity: A critical review. Altern. Ther. Health Med. 2005, 11, 30–34. [Google Scholar]
- Shevkar, B.; Ahirrao, S.; Bhavsar, G.; Patel, A.; Rajurkar, V.; Amale, P. Konjac Glucomannan Matrix Tablet for Extended Release of Diclofenac Sodium. Int. J. Adv. Pharm. Sci. 2014, 5, 2098–2108. [Google Scholar]
- Alvarez-Manceñido, F.; Landin, M.; Martínez-Pacheco, R. Konjac glucomannan/xanthan gum enzyme sensitive binary mixtures for colonic drug delivery. Eur. J. Pharm. Biopharm. 2008, 69, 573–581. [Google Scholar] [CrossRef]
- Annable, P.; Williams, P.A.; Nishinari, K. Interaction in Xanthan-Glucomannan Mixtures and the Influence of Electrolyte. Macromolecules 1994, 27, 4204–4211. [Google Scholar] [CrossRef]
- Goycoolea, F.M.; Richardson, R.K.; Morris, E.R.; Gidley, M.J. Stoichiometry and Conformation of Xanthan in Synergistic Gelation with Locust Bean Gum or Konjac Glucomannan: Evidence for Heterotypic Binding. Macromolecules 1995, 28, 8308–8320. [Google Scholar] [CrossRef]
- Wang, K.; He, Z. Alginate–konjac glucomannan–chitosan beads as controlled release matrix. Int. J. Pharm. 2002, 244, 117–126. [Google Scholar] [CrossRef]
- Boldyrev, V. Mechanical activation of solids and its application to technology. J. Chim. Phys. 1986, 83, 821–829. [Google Scholar] [CrossRef]
- Hattori, Y.; Takaku, T.; Otsuka, M. Mechanochemical effect on swelling and drug release of natural polymer matrix tablets by X-ray computed tomography. Int. J. Pharm. 2018, 539, 31–38. [Google Scholar] [CrossRef]
- Takacs, L. What Is Unique About Mechanochemical Reactions? Acta Phys. Pol. A 2014, 126, 1040–1043. [Google Scholar] [CrossRef]
- Doi, N.; Yamauchi, Y.; Ikegami, R.; Kuzuya, M.; Sasai, Y.; Kondo, S.-I. Photo-responsive polymer micelles from o-nitrobenzyl ester-based amphiphilic block copolymers synthesized by mechanochemical solid-state copolymerization. Polym. J. 2020, 52, 1375–1385. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hattori, Y.; Sasaki, T.; Nishizawa, J.-I.; Otsuka, M. Characteristic Evaluation of the Pseu-do-Polymorphism of Amorphous Atorvastatin Calcium Hydrates by Terahertz Spectroscopy. Colloids Surf. A Physi-Cochem. Eng. Asp. 2021, 127551. [Google Scholar] [CrossRef]
- Otsuka, Y.; Ito, A.; Takeuchi, M.; Sasaki, T.; Tanaka, H. Effects of Temperature on Terahertz Spectra of Caf-feine/Oxalic Acid 2:1 cocrystal and Its Solid-State Density Functional Theory. J. Drug Deli Sci. Technol. 2020, 56, 101215. [Google Scholar] [CrossRef]
- Otsuka, Y.; Ito, A.; Takeuchi, M.; Tanaka, H. Dry Mechanochemical Synthesis of Caffeine/Oxalic Acid Cocrystals and Their Evaluation by Powder X-Ray Diffraction and Chemometrics. J. Pharm. Sci. 2017, 106, 3458–3464. [Google Scholar] [CrossRef]
- Takaku, T.; Hattori, Y.; Sasaki, T.; Sakamoto, T.; Otsuka, M. Evaluation of swelling properties and drug release from mechanochemical pre-gelatinized glutinous rice starch matrix tablets by near infrared spectroscopy. J. Near Infrared Spectrosc. 2021, 29, 92–101. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Wu, C.; Peng, S.; Wen, C.; Wang, X.; Fan, L.; Deng, R.; Pang, J. Structural characterization and properties of konjac glucomannan/curdlan blend films. Carbohydr. Polym. 2012, 89, 497–503. [Google Scholar] [CrossRef]
- Rey, C.; Collins, B.; Goehl, T.; Dickson, I.R.; Glimcher, M.J. The carbonate environment in bone mineral: A resolution-enhanced fourier transform infrared spectroscopy study. Calcif. Tissue Int. 1989, 45, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Merck Home Page, IR Spectrum Table & Chart. Available online: https://www.sigmaaldrich.com/JP/ja/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 25 December 2021).
- National Institute of Standards and Technology (NIST). Chemistry WebBook, SRD 69, Carbon Dioxide. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C124389&Units=SI (accessed on 25 December 2021).
- Home Page of Shimizu Chemical Co. Ltd. General Food/Functional Food Material “Leorex Series”. Available online: https://www.ipros.jp/product/detail/2000524072/ (accessed on 26 December 2021).
- Zhang, T.; Li, Z.; Wang, Y.; Xue, Y.; Xue, C. Effects of konjac glucomannan on heat-induced changes of physicochemical and structural properties of surimi gels. Food Res. Int. 2016, 83, 152–161. [Google Scholar] [CrossRef]
- Du, X.; Li, J.; Chen, J.; Li, B. Effect of degree of deacetylation on physicochemical and gelation properties of konjac glucomannan. Food Res. Int. 2012, 46, 270–278. [Google Scholar] [CrossRef]
- Otsuka, M.; Matsumoto, T.; Kaneniwa, N. Effect of environmental temperature on polymorphic solid-state transformation of indomethacin during grinding. Chem. Pharm. Bull. 1986, 34, 1784–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, Y.; Sugata, M.; Kamata, H.; Nagata, M.; Nagato, T.; Hasegawa, K.; Otsuka, M. Real-time monitoring of the tablet-coating process by near-infrared spectroscopy—Effects of coating polymer concentrations on pharmaceutical properties of tablets. J. Drug Deliv. Sci. Technol. 2018, 46, 111–121. [Google Scholar] [CrossRef]
- Takayama, K.; Nambu, N.; Nagai, T. Factors affecting the dissolution of indomethacin dispersed in various water-soluble polymers. Chem. Pharm. Bull. 1982, 30, 673–678. [Google Scholar] [CrossRef] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okazaki, F.; Hattori, Y.; Sasaki, T.; Otsuka, M. Mechanochemical Effect on Controlled Drug Release of Konjac Glucomannan Matrix Tablets during Dry Grinding. Gels 2022, 8, 181. https://doi.org/10.3390/gels8030181
Okazaki F, Hattori Y, Sasaki T, Otsuka M. Mechanochemical Effect on Controlled Drug Release of Konjac Glucomannan Matrix Tablets during Dry Grinding. Gels. 2022; 8(3):181. https://doi.org/10.3390/gels8030181
Chicago/Turabian StyleOkazaki, Fuminori, Yusuke Hattori, Tetsuo Sasaki, and Makoto Otsuka. 2022. "Mechanochemical Effect on Controlled Drug Release of Konjac Glucomannan Matrix Tablets during Dry Grinding" Gels 8, no. 3: 181. https://doi.org/10.3390/gels8030181





