1. Introduction
High-energy photons, especially X-rays and gamma rays, have been increasingly utilized in various applications, including material characterization [
1,
2,
3], medical diagnostic and therapy [
4,
5], quality control in industrial products [
6], plant mutation breeding [
7], and national security [
8]. Despite their great benefits, excessive exposures to X-rays and gamma rays pose serious biological effects on human populations, which could lead to a reduction in the immune system, the initiation of brain cancers, and the increase in mutation rates [
9,
10,
11]. To cope with possible radiation illnesses and other side effects, a concept for radiation safety, namely, “As Low As Reasonably Achievable” or “ALARA”, consisting of the management of (1) exposure time, (2) distance between radiation sources and users, and (3) utilization of sufficient and appropriate shielding equipment, must be strictly practiced in all nuclear and radiation facilities [
12].
Specifically for users who are required to work in a proximity to radiation sources for extended time periods, utilization of effective shielding equipment has become a necessity to prevent the users and the public from excessive exposure to radiations. In the case of X-rays and gamma rays, shielding equipment mostly relies on the use of heavy metals, especially lead (Pb) and lead oxide (PbO) [
13], mainly due to their relatively higher interaction probabilities between the incident photons and Pb atoms, which significantly improved photon attenuation capabilities of the materials/composites as well as their economical availability [
14]. Nonetheless, the toxicity of Pb has raised serious safety concerns as excessive exposure to Pb potentially leads to an increase in blood pressure, slow nerve conduction, fatigue, drowsiness, fertility disorders, encephalopathy, and death [
15]. Furthermore, the spread of Pb in water resources and forests could negatively affect animals and plants. As a result, significant efforts to replace the toxic Pb with safer compounds have been emphasized and discussed in recent years. Among several potential candidates, bismuth oxide (Bi
2O
3), tungsten oxide (WO
3), and barium sulfate (BaSO
4) have shown promising possibilities to serve for such purposes due to the high atomic numbers (Z) of Bi, W, and Ba (Z = 83, 74, and 56, respectively) as well as their high densities (ρ = 8.9, 7.16, and 4.5 g/cm
3, respectively), resulting in substantial enhancement of photon attenuation after being added to the main matrix [
16]. For instance, Poltabtim et al. reported that natural rubber (NR) composites containing Bi
2O
3 numerically exhibited comparable X-ray and gamma shielding properties as those containing Pb; for instance, the µ
m values of Pb/NR and Bi
2O
3/NR composites were 0.094 and 0.092 cm
2/g, respectively, determined at the filler content of 80 phr and the photon energy of 0.662 MeV) [
17]. Maleksadeh et al. also confirmed the usability of WO
3 and BaSO
4 as Pb alternatives, evidenced by just a slight decrease in the attenuation capabilities of silicone-based composites containing WO
3 and BaSO
4 in comparison with those containing PbO (determined at the same filler content and photon energy) [
18]. These reports clearly implied that Bi
2O
3, WO
3, and BaSO
4 could be utilized as safe and effective alternatives to Pb and Pb compounds in the production of high-energy photon equipment.
Additionally, the types of the main matrix used for the production of the shielding materials are one of key factors that could define other important characteristics and properties. For instance, materials based on polyethylene (PE) and polyvinyl chloride (PVC) such as Bi
2O
3/UHMWPE and Bi
2O
3/wood/PVC composites exhibited exceptional strength and rigidity, which were suitable for use as construction parts or movable equipment in nuclear facilities [
19,
20]. On the other hand, materials based on natural rubber (NR) and synthetic rubber (SR) such as Bi
2O
3/NR, WO
3/NR, and Bi
2O
3/EPDM composites could be used as personal protective equipment (PPE) and covers of transporting casks due to their exceptional flexibility and elongation at break [
21,
22,
23]. Nonetheless, the mentioned materials had some limitations due to the lack of self-healing capabilities, which may result in increased costs and procedures needed for repairment, replacement, and waste management of the damaged products. To alleviate such drawbacks, materials based on the autonomously self-healing poly(vinyl alcohol) (PVA) hydrogels have been recently developed to be used as radiation shielding materials [
24,
25]. An example of some PVA-based shielding materials is nano-Bi
2O
3/PVA hydrogels, which could attenuate gamma rays having the energies of 1.17 MeV and 1.33 MeV by 35% and 30%, respectively, while providing the percentage of recoverable strength (%Recovery) of 88.6% after being brought together for just 1 min [
26]. Furthermore, these developed Bi
2O
3/PVA hydrogels were also lead-free, which additionally improve the health safety of users and the public with respect to the toxic lead. Another use of self-healing PVA hydrogels is Sm
2O
3/PVA and Gd
2O
3/PVA composites for neutron attenuation, for which both 1-cm-thick PVA hydrogels containing either 10.5 wt.% of Sm
2O
3 or Gd
2O
3 could reduce the initial intensity of incident thermal neutrons by almost 70%, while providing the %Recovery up to 70% after being brought into contact for 6 h [
27]. These self-healing abilities of PVA hydrogels were due to the capabilities of PVA polymer chains to diffuse across fractured surfaces and initiate hydrogen bonds between two polymers (without any external stimuli after the damage) [
26]. These two examples clearly indicated the great potentials of utilizing PVA hydrogels as autonomously self-healing and effective radiation shielding materials.
Despite the promising self-healable capabilities of PVA hydrogels, the addition of fillers, especially radiation protective fillers that tend to improve shielding properties of the composites with their increasing contents, may result in the decrease of %Recovery and, thus, the self-healing capabilities. This limitation was evidenced by the reports of Tiamduangtawan et al., which indicated that the increase in nano-Bi
2O
3 contents from 0 to 20 and 40 wt.% lowered the %Recovery of the hydrogels from 96.3% to 95.9% and 88.6%, respectively, while the increases in the Sm
2O
3 and Gd
2O
3 contents from 0 to 10.5 wt.% decreased the %Recovery of the self-healed hydrogels from 85% in pristine samples to ~65% and ~60%, respectively [
26,
27]. These negative relationships between filler contents and %Recovery were mainly due to increases in the cross-linking network of the PVA chains from physical interactions between the hydroxyl groups of PVA and the fillers, leading to the increase in overall crystallinity of the hydrogels that subsequently obstructed the chain diffusion and the initiation of self-healing mechanisms [
24,
26]. As a result, due to the competing roles of the fillers in enhancing photon attenuation capabilities and obstructing self-healing properties of the hydrogels, the least filler contents that provide the materials with sufficient photon shielding, while preserving the self-healing capabilities of the hydrogels, must be thoroughly determined.
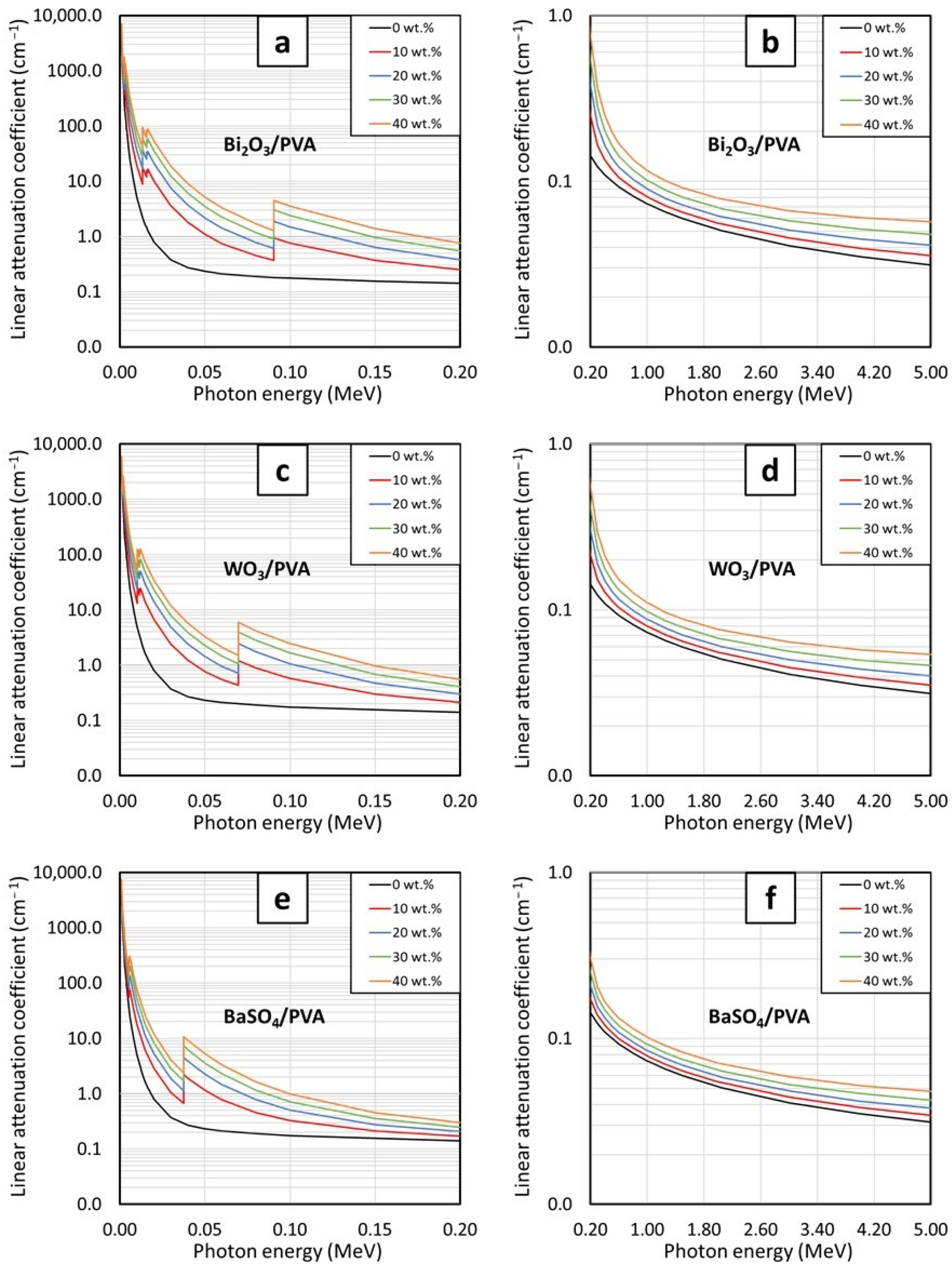
As aforementioned, this work aimed to numerically determine high-energy photon shielding properties, which included the mass attenuation coefficient (µ
m), the linear attenuation coefficient (µ), and the half-value layer (HVL), of PVA hydrogels containing either Bi
2O
3, WO
3, or BaSO
4 using an online software package, namely, XCOM [
17,
28,
29]. In order to verify results from XCOM for further investigation, the results were compared with those obtained from a Monte Carlo particle transport simulation code, namely, PHITS (Particle and Heavy Ion Transport code System) [
30,
31]. The contents of the fillers and the photon energies used for the determination were varied from 0–40 wt.% and 0.001–5 MeV, respectively. Furthermore, the least contents, which could be regarded as the recommended contents for each filler at the photon energies of 0.05, 0.08, and 0.1 MeV, were determined by comparing the values of lead equivalence (Pb
eq) of the 1-cm-thick PVA hydrogels with the required Pb
eq value of 0.5 mmPb. The outcomes of this work should not only reveal the numerical effectiveness of PVA hydrogels in photon attenuation but also promote the additional advantages of PVA hydrogels, especially the self-healing capability, in the applications of radiation protection.