Application of Sol–Gels Modified with Natural Plants Extracts as Stationary Phases in Open-Tubular Capillary Electrochromatography
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Accessories
4.2. Electrochromatographic Analyses
4.3. Preparation of Modified Capillaries for Open-Tubular Capillary Electrochromatography
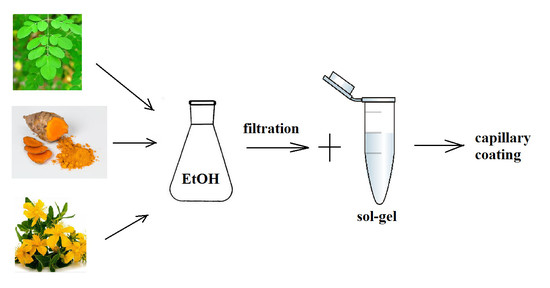
4.3.1. Preparation of the Sol–Gel Additives
4.3.2. Modification of the Inner Capillary Surface
4.3.3. Conditioning (Stabilization) of Capillaries and Electrochromatographic Separation
4.4. Analytes
4.5. Mathematical Calculations
4.6. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hu, L.-F.; Yin, S.-J.; Zhang, H.; Yang, F.-Q. Recent development of monolithic and open-tubular capillary electrochromatography (2017–2019). J. Sep. Sci. 2020, 43, 1942–1966. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Chen, Z. Advances in capillary electro-chromatography. J. Pharm. Anal. 2019, 9, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, K.; Kovařík, T.; Křenek, T.; Docheva, D.; Stich, T.; Pola, J. Recent advances and future perspectives of sol-gel derived porous active glasses: A review. RSC Adv. 2020, 10, 33782. [Google Scholar] [CrossRef]
- Lei, Q.; Guo, J.; Noureddine, A.; Wang, A.; Wuttke, S.; Brinker, C.J.; Zhu, W. Sol-gel based advanced porous silica materials for biomedical applications. Adv. Funct. Mater. 2020, 30, 1909539. [Google Scholar] [CrossRef]
- Gonçalves, M.C. Sol-gel Silica Nanoparticles in Medicine: A Natural Choice. Design, Synthesis and Products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Hitzky, E.; Darder, M.; Aranda, P.; Ariga, K. Advances in Biomimetic and Nanostructured Biohybrid materials. Adv. Mater. 2010, 22, 323–336. [Google Scholar] [CrossRef]
- Gill, I.; Ballesteros, A. Bioencapsulation within synthetic polymers (Part 1): Sol-gel encapsulated biologicals. Trends Biotechnol. 2000, 18, 282–296. [Google Scholar] [CrossRef]
- Andreani, T.; Silva, A.M.; Souto, B. Silica-based matrices: State of the art and new perspectives for therapeutic drug delivery. Biotechnol. Appl. Chem. 2015, 62, 754–764. [Google Scholar] [CrossRef]
- Gupta, R.; Chaudhury, N.K. Entrapment of biomolecules in sol-gel matrix for applications in biosensors: Problems and future prospects. Biosens. Bioelectron. 2007, 22, 2387–2399. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, N.; Meghea, A. Biocompatible Nanomaterials: Synthesis, Characterization and Applications; Kumar, S.A., Thiagarajan, S., Wang, S.-F., Eds.; Nova Science Publishers: New York, NY, USA, 2010; pp. 41–115. [Google Scholar]
- Svobodová, J.; Mikšík, I. Open-tubular capillary electrochromatographic application of a sol-gel matrix with chilli peppers, garlic, or synthetic additives. J. Sep. Sci. 2020, 43, 3691–3701. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raduly, F.M.; Raditoiu, V.; Raditoiu, A.; Purcar, V. Curcumin: Modern Applications for a Versatile Additive. Coatings 2021, 11, 519. [Google Scholar] [CrossRef]
- Mehla, J.; Gupta, P.; Pahuja, M.; Diwan, D.; Diksha, D. Indian Medicinal Herbs and Formulations for Alzheimer’s Disease, from Traditional Knowledge to Scientific Assessment. Brain Sci. 2020, 10, 964. [Google Scholar] [CrossRef] [PubMed]
- Tabeshpour, J.; Banaeeyeh, S.; Eisvand, F.; Sathyapalan, T.; Hashemzaei, M.; Sahebkar, A. Effects of Curcumin on Ion Channels and Pumps: A Review. IUBMB Life 2019, 71, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, K.; Ansari, V.A.; Singh, K.; Kushwaha, P.; Akhtar, J. Curcuma longa: Boon for Health Care System with its Biomedical Application. Int. J. Pharm. Sci. Res. (IJPSR) 2015, 6, 4168–4173. [Google Scholar]
- Zhang, X.; Chen, Q.; Wang, Y.; Peng, W.; Cai, H. Effects of curcumin on ion channels and transporters. Front. Physiol. Ren. Epithel. Physiol. 2014, 5, 94. [Google Scholar] [CrossRef] [Green Version]
- Razis, A.F.A.; Ibrahim, M.D.; Kntayya, S.B. Health benefits of Moringa Oleifera. Asian Pac. J. Cancer Prev. 2014, 15, 8571–8576. [Google Scholar] [CrossRef] [Green Version]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef]
- Saucedo-Pompa, S.; Torres-Castillo, J.A.; Castro-López, C.; Rojas, R.; Sánchez-Alejo, E.J.; Ngangyo-Heya, M.; Martínez-Ávila, G.C.G. Moringa plants: Bioactive compounds and promising applications in food products. Food Res. Int. 2018, 111, 438–450. [Google Scholar] [CrossRef]
- Chhikara, N.; Kaur, A.; Mann, S.; Garg, M.K.; Sofi, S.A.; Panghal, A. Bioactive compounds, associated health benefits and safety consideration of Moringa oleifera L.: An updated review. Nutr. Food Sci. 2021, 51, 255–277. [Google Scholar] [CrossRef]
- The Amazing Moringa Oleifera Tree. Freely Accesible. Available online: www.moringatrees.org (accessed on 20 September 2021).
- Meireles, D.; Gomez, J.; Lopes, L.; Hinzmann, M.; Machalo, J. A review of properties, nutritional and pharmaceutical applications of Moringa oleifera: Integrative approach on conventional and traditional Asian medicine. Adv. Tradit. Med. 2020, 20, 495–515. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa Oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vongsak, B.; Sithisarn, P.; Gritsanapan, W. Bioactive contents and free radical scavenging activity of Moringa oleifera leaf extract under different storage conditions. Ind. Crops Prod. 2013, 49, 419–421. [Google Scholar] [CrossRef]
- Velingkar, V.S.; Gupta, G.L.; Hegde, N.B. A current update on phytochemistry, pharmacology and herb–drug interactions of Hypericum perforatum. Phytochem. Rev. 2017, 16, 725–744. [Google Scholar] [CrossRef]
- Zirak, N.; Shafiee, M.; Soltani, G.; Mirzaei, M.; Sahebkar, A. Hypericum perforatum in the treatment of psychiatric and neurodegenerative disorders: Current evidence and potential mechanisms of action. J. Cell. Physiol. 2019, 234, 8496–8508. [Google Scholar] [CrossRef]
- Müller, W.E. Current St. John’s wort research from mode of action to clinical efficacy. Pharmacol. Res. 2003, 47, 101–109. [Google Scholar] [CrossRef]
- Russo, E.; Scicchitano, F.; Whalley, B.J.; Mazzitello, C.; Ciriaco, M.; Esposito, S.; Patanè, M.; Upton, R.; Pugliese, M.; Chimirri, S.; et al. Hypericum perforatum: Pharmacokinetic, Mechanism of Action, Tolerability, and Clinical Drug–Drug Interactions. Phytother. Res. 2014, 28, 643–655. [Google Scholar] [CrossRef]
- Saddiqe, Z.; Naeem, I.; Maimoona, A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010, 131, 511–521. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [Green Version]
- Asgarpanah, J. Phytochemistry, pharmacology and medicinal properties of Hypericum perforatum L. Afr. J. Pharm. Pharmacol. 2012, 6, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, M.; Dwivedi, L.K. Therapeutic potential of Hypericum Perforatum: A Review. IJPSR 2015, 6, 4982–4988. [Google Scholar]
- Oliveira, A.I.; Pinho, C.; Sarmento, B.; Dias, A.C.P. Neuroprotective Activity of Hypericum perforatum and Its Major Components. Front. Plant Sci. 2016, 7, 1004. [Google Scholar] [CrossRef] [Green Version]
- Galeotti, N. Hypericum perforatum (St John’s wort) beyond depression: A therapeutic perspective for pain conditions. J. Ethnopharmacol. 2017, 200, 136–146. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svobodová, J.; Mikšík, I. Application of Sol–Gels Modified with Natural Plants Extracts as Stationary Phases in Open-Tubular Capillary Electrochromatography. Gels 2022, 8, 198. https://doi.org/10.3390/gels8040198
Svobodová J, Mikšík I. Application of Sol–Gels Modified with Natural Plants Extracts as Stationary Phases in Open-Tubular Capillary Electrochromatography. Gels. 2022; 8(4):198. https://doi.org/10.3390/gels8040198
Chicago/Turabian StyleSvobodová, Jana, and Ivan Mikšík. 2022. "Application of Sol–Gels Modified with Natural Plants Extracts as Stationary Phases in Open-Tubular Capillary Electrochromatography" Gels 8, no. 4: 198. https://doi.org/10.3390/gels8040198
APA StyleSvobodová, J., & Mikšík, I. (2022). Application of Sol–Gels Modified with Natural Plants Extracts as Stationary Phases in Open-Tubular Capillary Electrochromatography. Gels, 8(4), 198. https://doi.org/10.3390/gels8040198