Aminopolycarboxylic Acids-Functionalized Chitosan-Based Composite Cryogels as Valuable Heavy Metal Ions Sorbents: Fixed-Bed Column Studies and Theoretical Analysis
Abstract
:1. Introduction
2. Results and Discussion
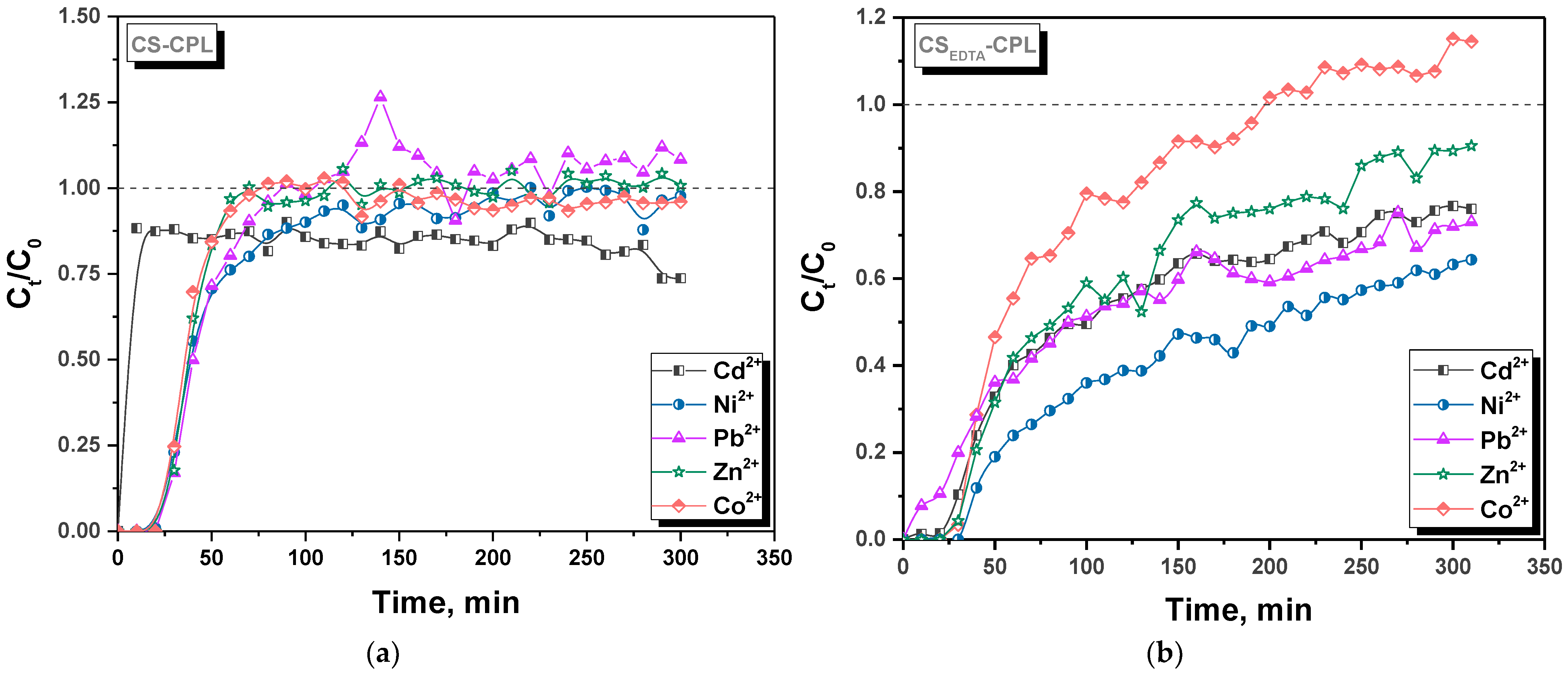
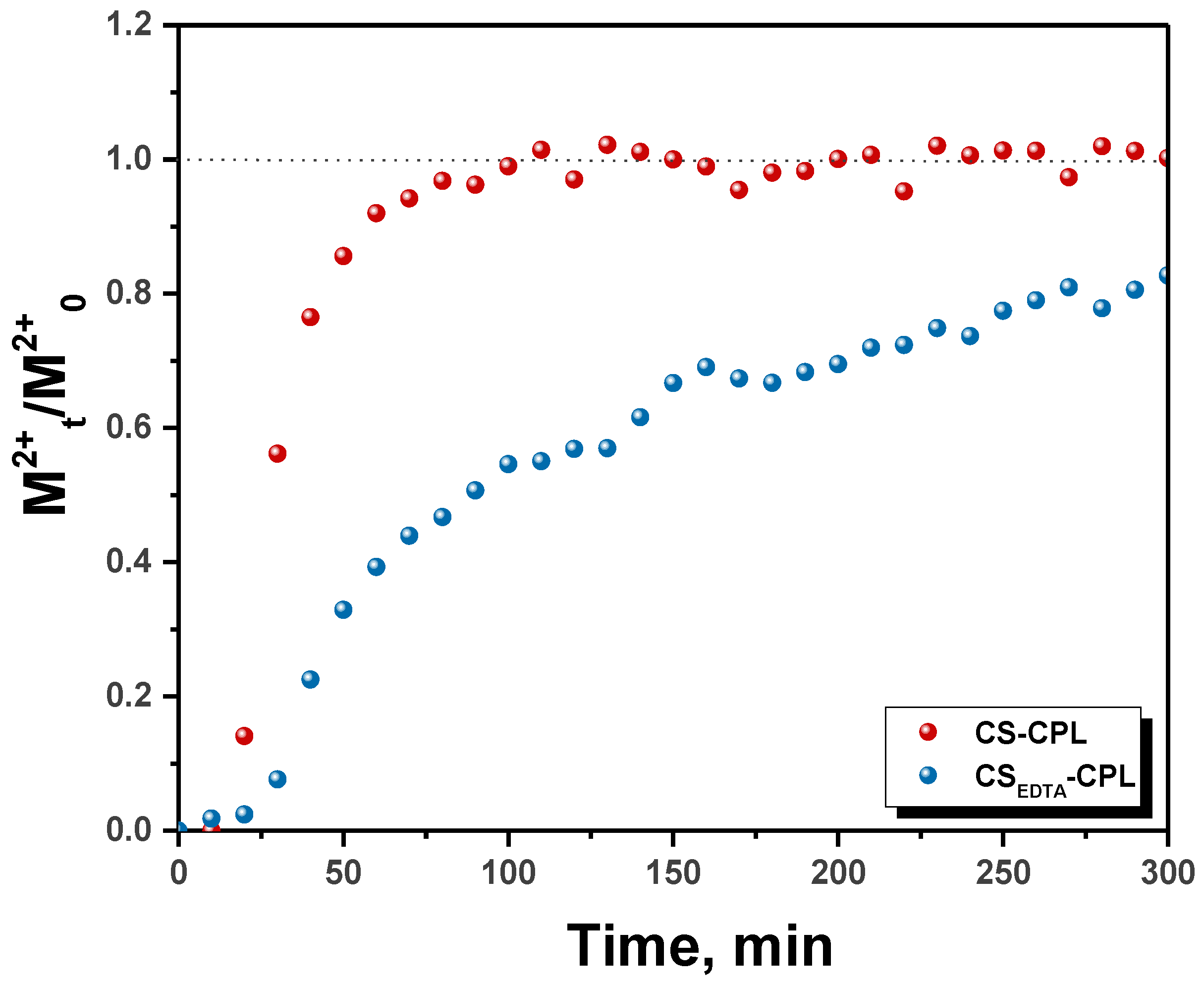
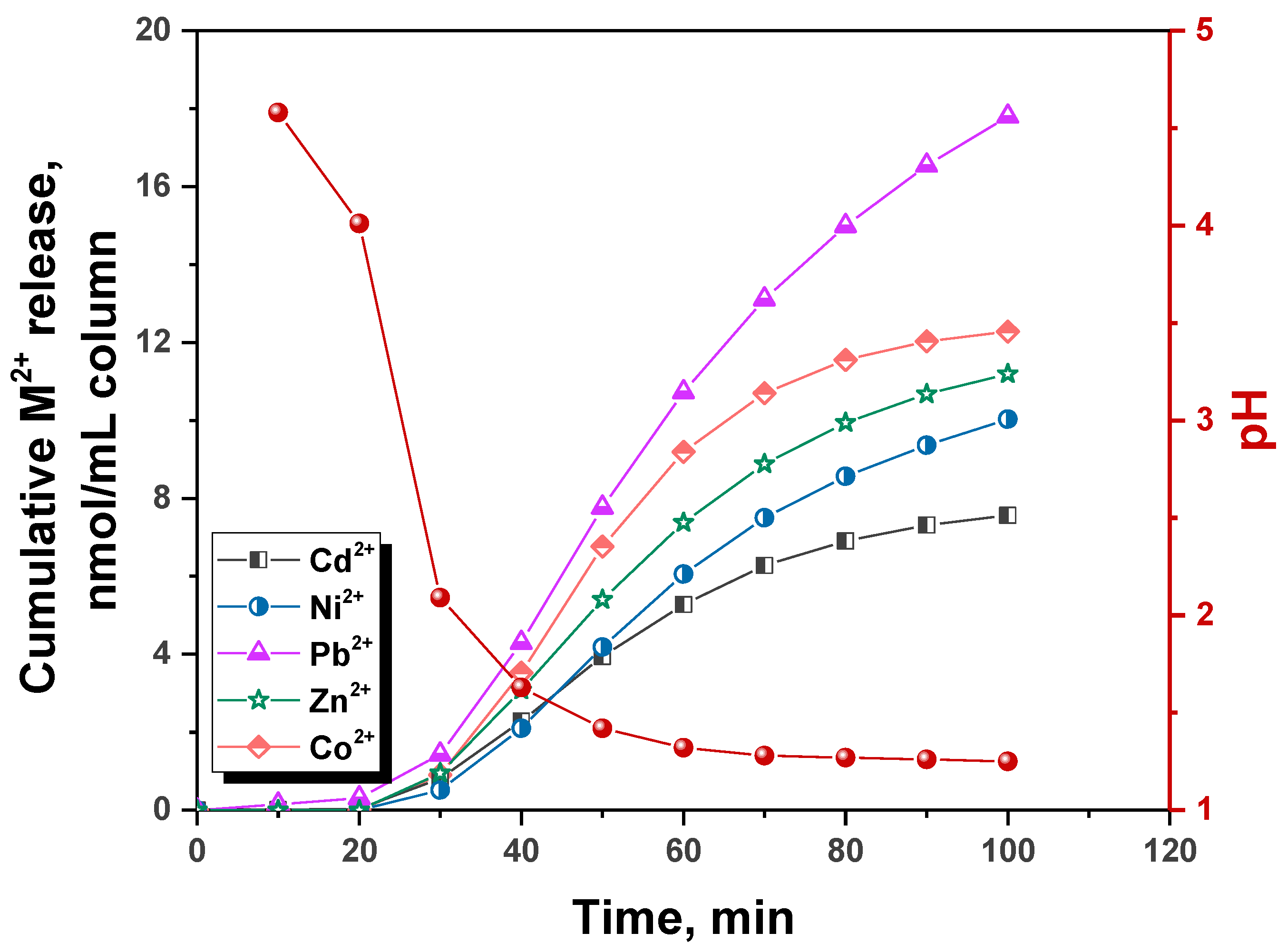
2.1. Experimental Data on HMIs Sorption in Fixed-Bed Column
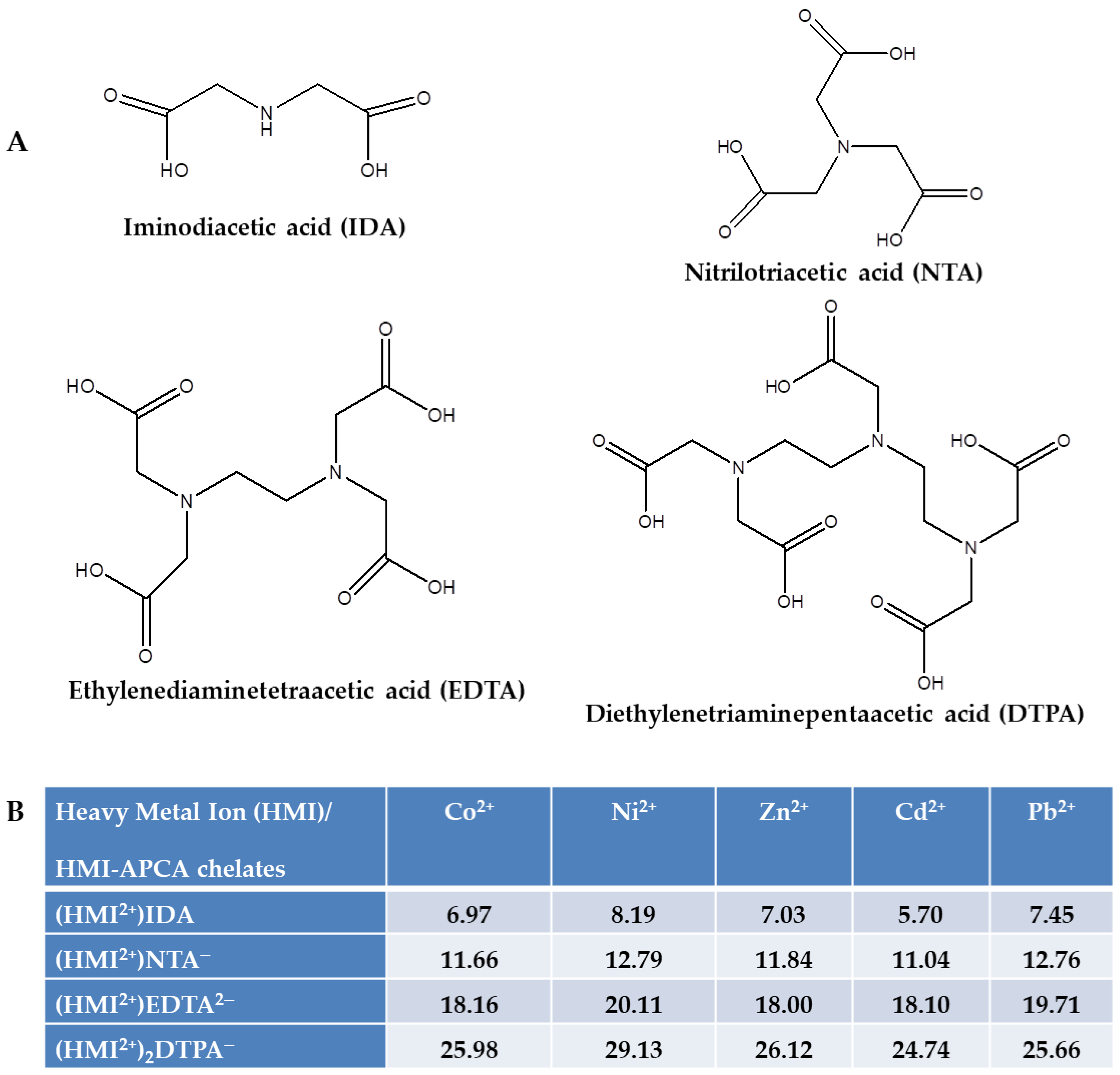
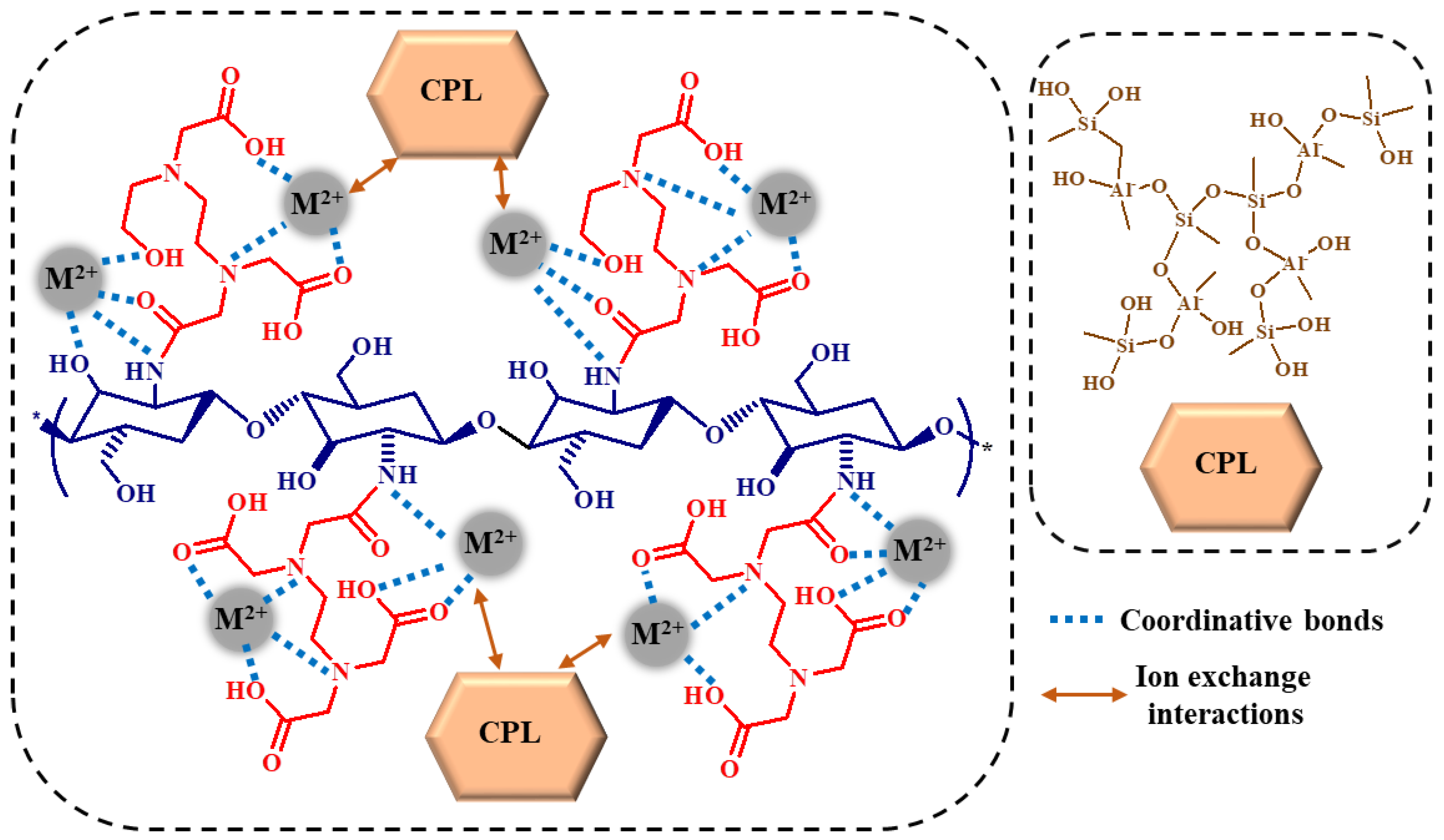
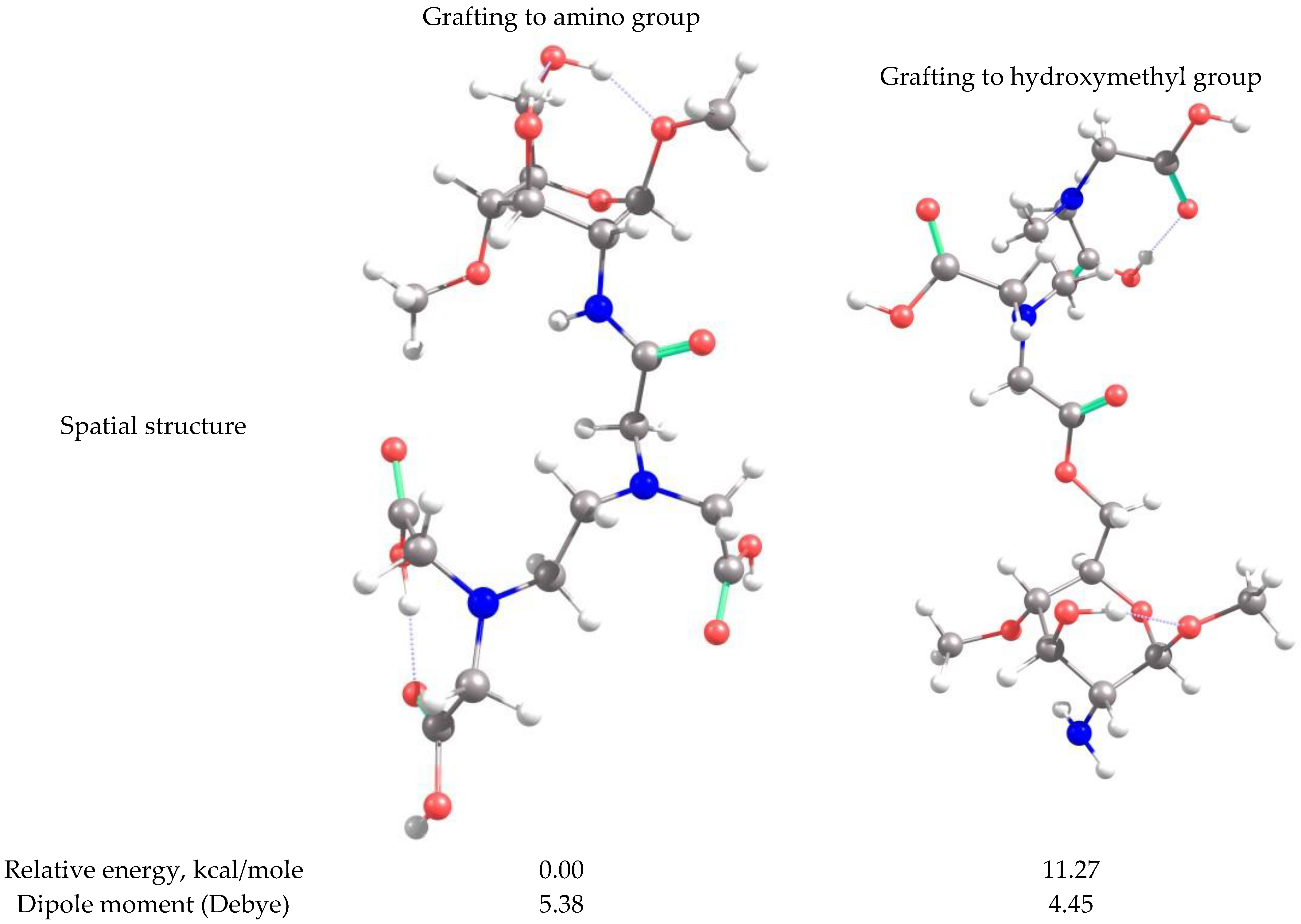
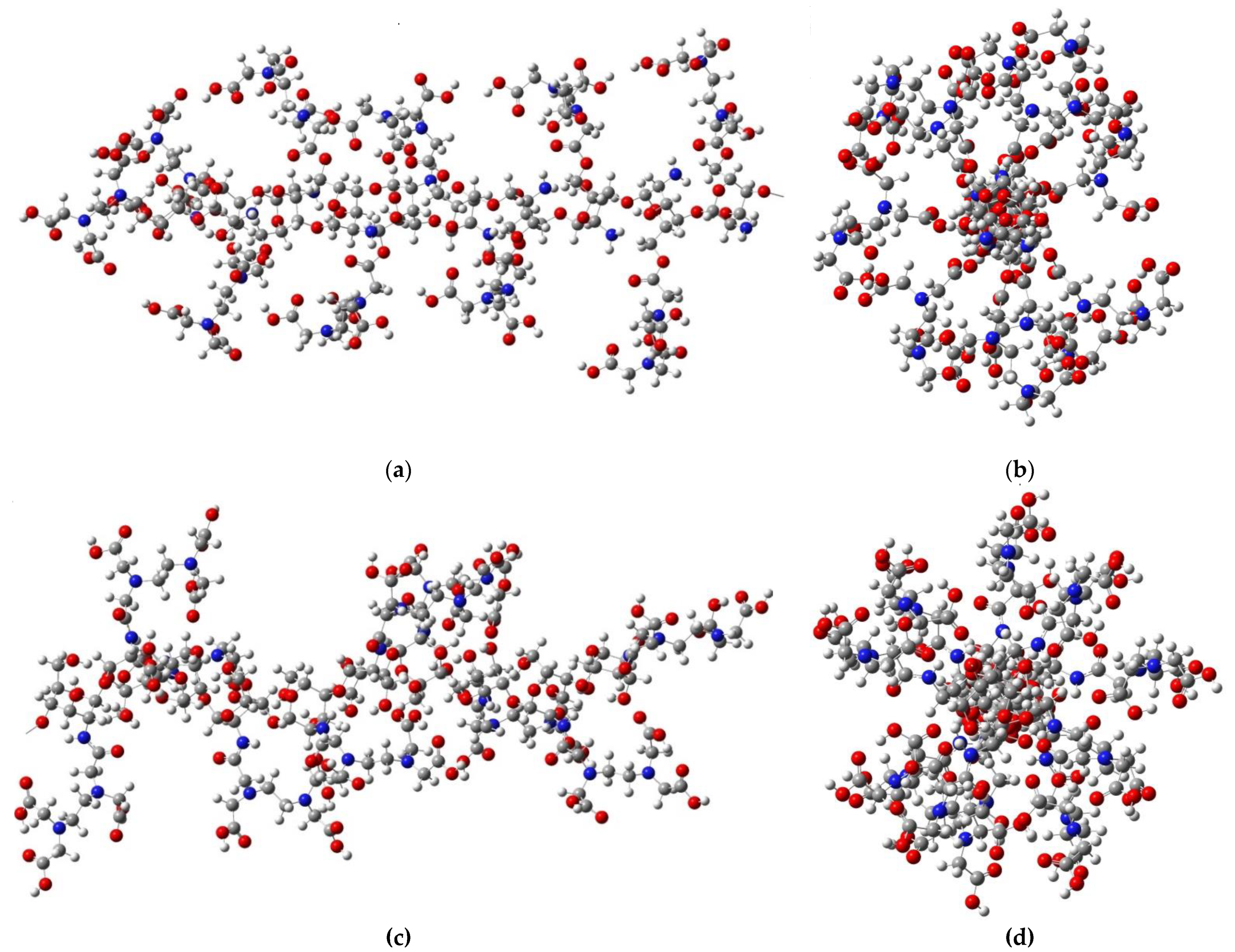
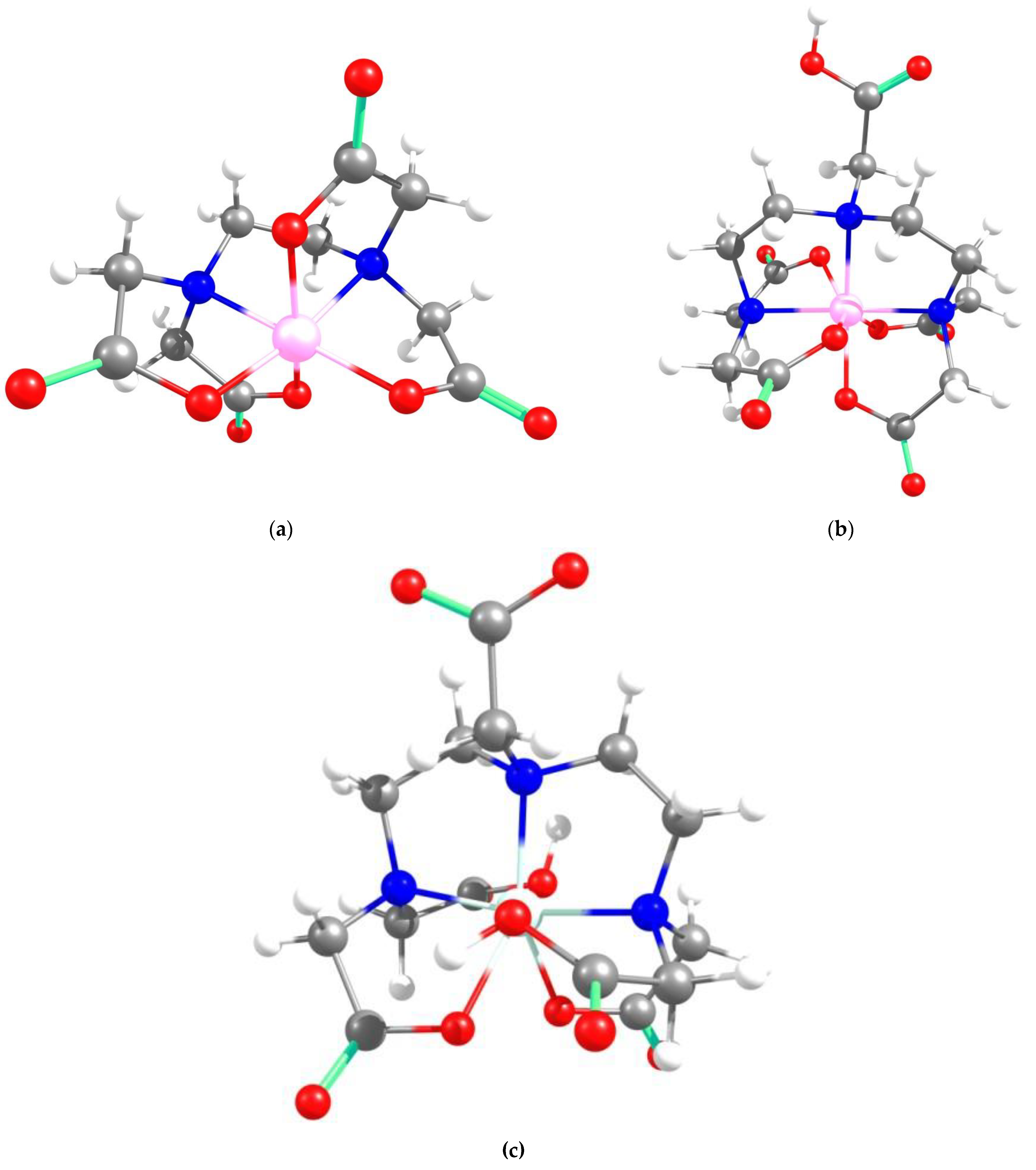
2.2. Theoretical Analysis of EDTA and DTPA Structures Attached to CS-CPL Composite Adsorbents and Their Spatial Structures within the Complex Combinations with Transition Metals
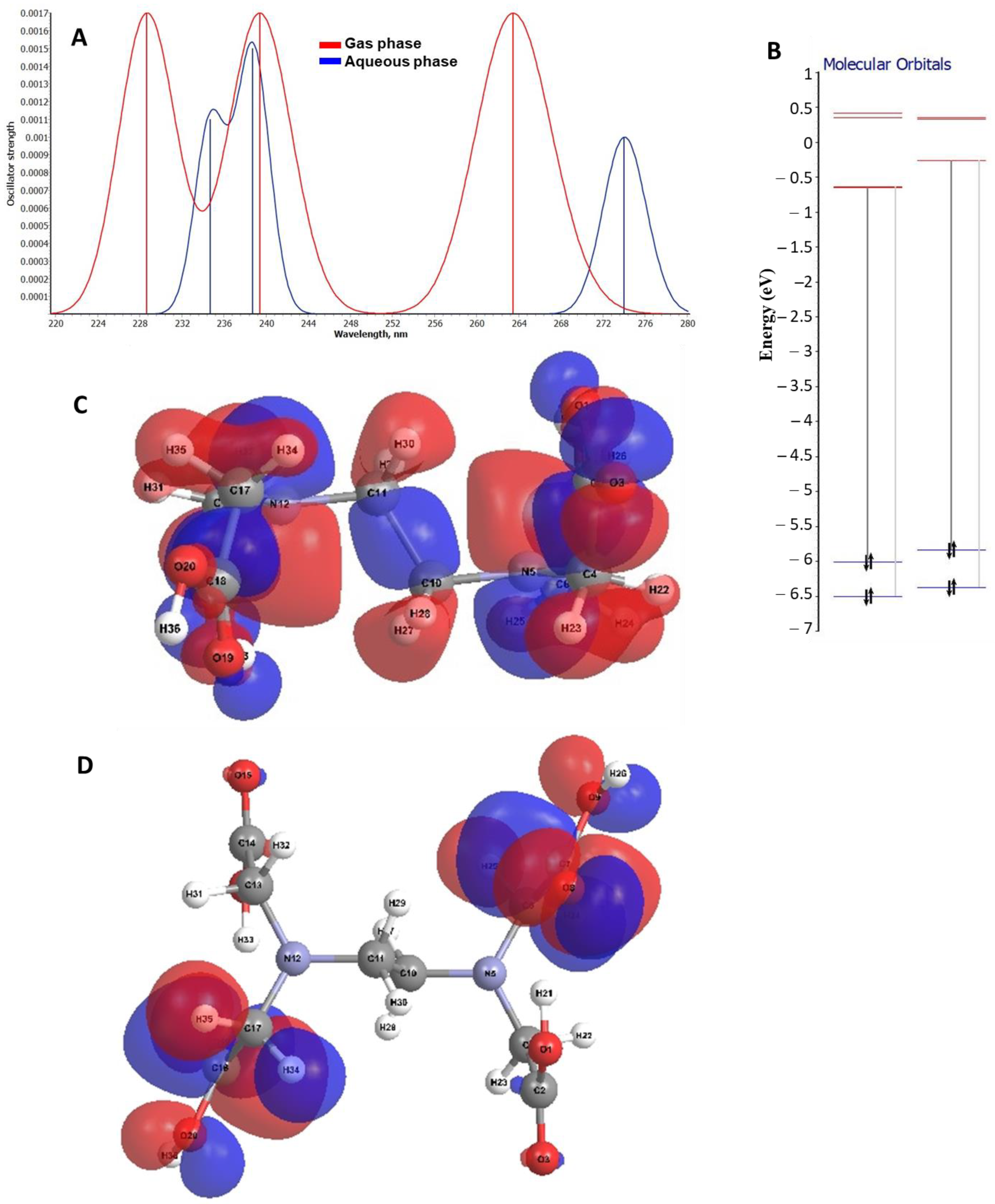
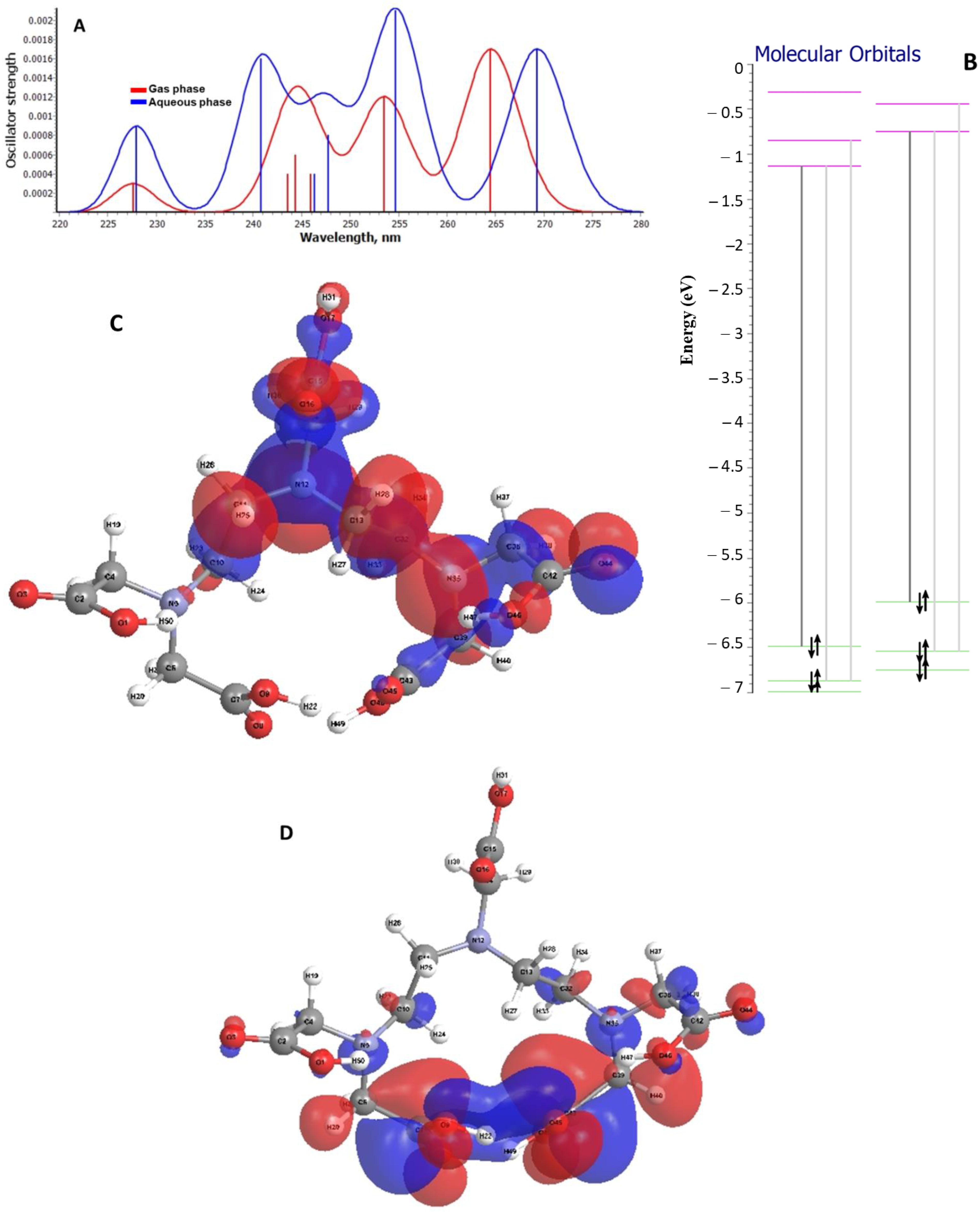
2.3. Theoretical Analysis on the Electronic Transitions of EDTA and DTPA Structures
3. Conclusions
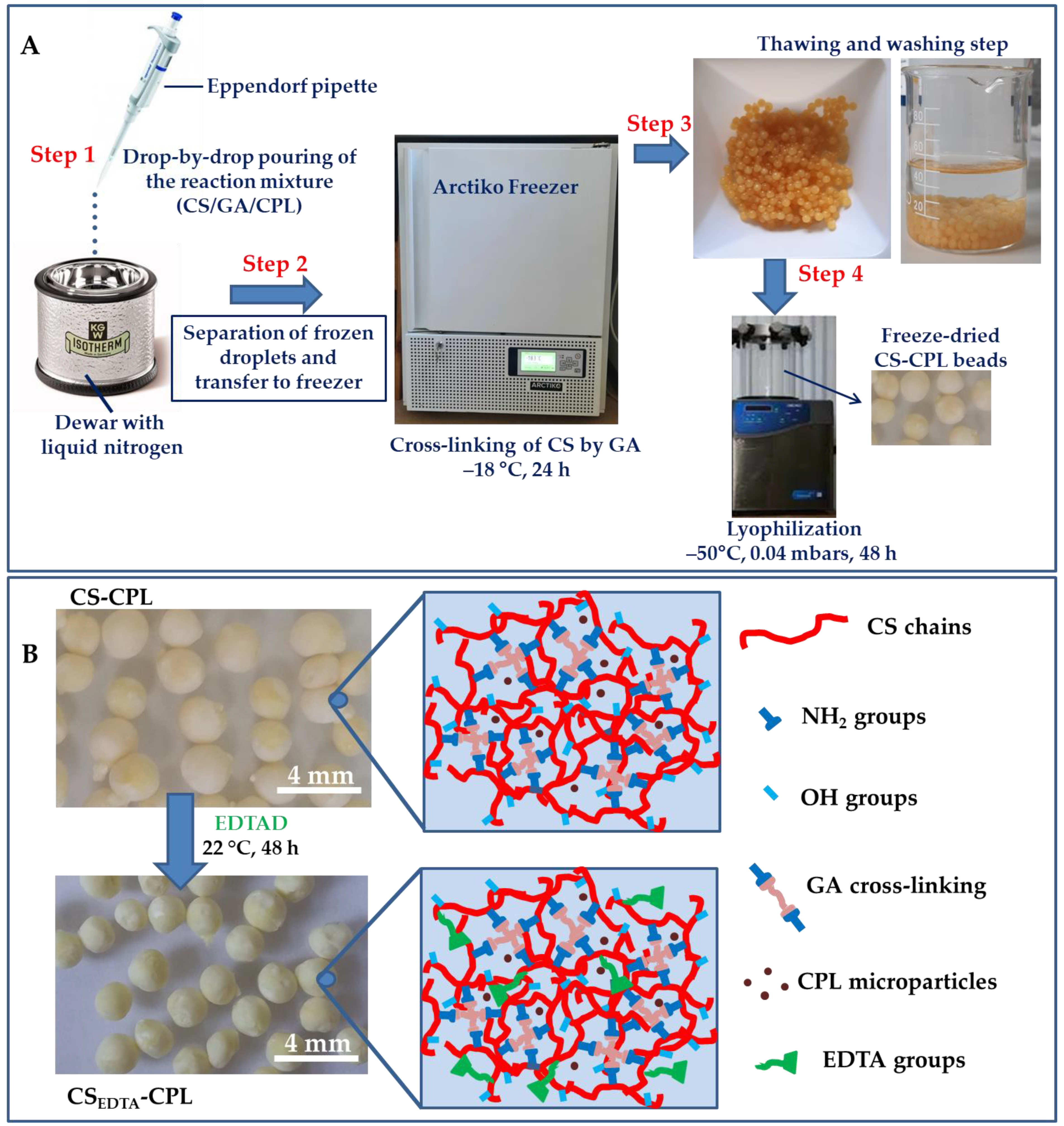
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. SEM and EDX Analysis
4.2.2. FTIR Analysis
4.2.3. Porosity Evaluation
4.2.4. Mean Pore Sizes Determination
4.2.5. Water Uptake
4.2.6. BET Surface Area
4.2.7. Fixed-Bed Column Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qi, X.; Tong, X.; Pan, W.; Zeng, Q.; You, S.; Shen, J. Recent advances in polysaccharide-based adsorbents for wastewater treatment. J. Clean. Prod. 2021, 315, 128221. [Google Scholar] [CrossRef]
- Fan, S.; Liu, Z.; Wu, Y.; Zhang, Y.; Hu, H.; Huang, Z.; Qin, Y.; Liang, J. 3D porous tubular network-structured chitosan-based beads with multifunctional groups: Highly efficient and selective removal of Cu2+. Int. J. Biol. Macromol. 2021, 171, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Prete, P.; Fiorentino, A.; Rizzo, L.; Proto, A.; Cucciniello, R. Review of aminopolycarboxylic acids–based metal complexes application to water and wastewater treatment by (photo-) Fenton process at neutral pH. Curr. Opin. Green Sustain. Chem. 2021, 28, 100451. [Google Scholar] [CrossRef]
- Ridzwan, M.H.; Yaakob, M.K.; Zabidi, Z.M.; Hamzah, A.S.; Shaameri, Z.; Rashid, F.N.A.A.; Kassim, K.; Mohammat, M.F.; Pungot, H.N.; Hamali, M.A.M.; et al. Computational insight into the quantum chemistry, interaction and adsorption energy of aminopolycarboxylic acid chelating agents towards metal cations. Comput. Theor. Chem. 2022, 1208, 113579. [Google Scholar] [CrossRef]
- Bucheli-Witschel, M.; Egli, T. Environmental fate and microbial degradation of aminopolycarboxylic acids. FEMS Microbiol. Rev. 2001, 25, 69–106. [Google Scholar] [CrossRef]
- Repo, E.; Warchoł, J.K.; Bhatnagar, A.; Mudhoo, A.; Sillanpää, M. Aminopolycarboxylic acid functionalized adsorbents for heavy metals removal from water. Water Res. 2013, 47, 4812–4832. [Google Scholar] [CrossRef]
- Dinu, M.V.; Dragan, E.S. Heavy metals adsorption on some iminodiacetate chelating resins as a function of the adsorption parameters. React. Funct. Polym. 2008, 68, 1346–1354. [Google Scholar] [CrossRef]
- Dinu, M.V.; Dragan, E.S.; Trochimczuk, A.W. Sorption of Pb(II), Cd(II) and Zn(II) by iminodiacetate chelating resins in non-competitive and competitive conditions. Desalination 2009, 249, 374–379. [Google Scholar] [CrossRef]
- Li, Y.; He, J.; Zhang, K.; Liu, T.; Hu, Y.; Chen, X.; Wang, C.; Huang, X.; Kong, L.; Liu, J. Super rapid removal of copper, cadmium and lead ions from water by NTA-silica gel. RSC Adv. 2019, 9, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Weng, S.; Li, C.; Jin, M.; Wan, D. Dense and robust aminopolycarboxylic acid-decorated porous monoliths for eliminating trace Cu(II) or Zn(II) from water. Colloids Surf. A 2020, 586, 124310. [Google Scholar] [CrossRef]
- Zhang, K.; Dai, Z.; Zhang, W.; Gao, Q.; Dai, Y.; Xia, F.; Zhang, X. EDTA-based adsorbents for the removal of metal ions in wastewater. Coord. Chem. Rev. 2021, 434, 213809. [Google Scholar] [CrossRef]
- Badsha, M.A.H.; Khan, M.; Wu, B.; Kumar, A.; Lo, I.M.C. Role of surface functional groups of hydrogels in metal adsorption: From performance to mechanism. J. Hazard. Mater. 2021, 408, 124463. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Wang, J.; Tong, X.; Zhang, S. Enhanced adsorptive removal of Cr(III) from the complex solution by NTA-modified magnetic mesoporous microspheres. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Sakairi, N. Water soluble EDTA-linked chitosan as a zwitterionic flocculant for pH sensitive removal of Cu(II) ion. RSC Adv. 2016, 6, 10385–10392. [Google Scholar] [CrossRef] [Green Version]
- Lazar, M.M.; Dinu, I.A.; Dinu, M.V. Synthesis of ethylenediminetetraacetic acid-functionalized chitosan cryogels as potential sorbents of heavy metal ions. Mater. Plast. 2021, 58, 155–166. [Google Scholar] [CrossRef]
- Nagib, S.; Inoue, K.; Yamaguchi, T.; Tamaru, T. Recovery of Ni from a large excess of al generated from spent hydrodesulfurization catalyst using picolylamine type chelating resin and complexane types of chemically modified chitosan. Hydrometallurgy 1999, 51, 73–85. [Google Scholar] [CrossRef]
- Repo, E.; Warchol, J.K.; Kurniawana, T.A.; Sillanpää, M.E.T. Adsorption of Co(II) and Ni(II) by EDTA- and/or DTPA- modified chitosan: Kinetic and equilibrium modeling. Chem. Eng. J. 2010, 161, 73–82. [Google Scholar] [CrossRef]
- Inoue, K.; Ohto, K.; Yoshizuka, K.; Yamaguchi, T.; Tanaka, T. Adsorption of lead(II) ion on complexane types of chemically modified chitosan. Bull. Chem. Soc. Jpn. 1997, 70, 2443–2447. [Google Scholar] [CrossRef]
- Ayati, A.; Tanhaei, B.; Sillanpaa, M. Lead(II)-ion removal by ethylenediaminetetraacetic acid ligand functionalized magnetic chitosan–aluminum oxide–iron oxide nanoadsorbents and microadsorbents: Equilibrium, kinetics, and thermodynamics. J. Appl. Polym. Sci. 2017, 134, 44360. [Google Scholar] [CrossRef]
- Khawar, A.; Aslam, Z.; Abbas, S.J.A. Pb (II) biosorption using DAP/EDTA-modified biopolymer (Chitosan). Chem. Eng. Commun. 2018, 205, 1555–1567. [Google Scholar] [CrossRef]
- Zheng, L.; Gao, Y.; Du, J.; Zhang, W.; Huang, Y.; Wang, L.; Zhao, Q.; Pan, X. A novel, recyclable magnetic biochar modified by chitosan–EDTA for the effective removal of Pb(II) from aqueous solution. RSC Adv. 2020, 10, 40196. [Google Scholar] [CrossRef]
- Roosen, J.; Binnemans, K. Adsorption and chromatographic separation of rare earths with EDTA- and DTPA-functionalized chitosan biopolymers. J. Mater. Chem. A 2014, 2, 1530–1540. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Repo, E.; Yin, D.; Chen, L.; Kalliola, S.; Tang, J.; Iakovleva, E.; Tam, K.C.; Sillanpää, M. One-pot synthesis of trifunctional chitosan-EDTA-β-cyclodextrin polymer for simultaneous removal of metals and organic micropollutants. Sci. Rep. 2017, 7, 15811. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Lee, I.; Hong, Y.; Kumar, V.; Kim, H. Multifunctional β-cyclodextrin- EDTA- chitosan polymer adsorbent synthesis for simultaneous removal of heavy metals and organic dyes from wastewater. Environ. Pollut. 2022, 292, 118447. [Google Scholar] [CrossRef]
- Dinu, M.V.; Humelnicu, D.; Lazar, M.M. Analysis of copper(II), cobalt(II) and iron(III) sorption in binary and ternary systems by chitosan-based composite sponges obtained by ice-segregation approach. Gels 2021, 7, 103. [Google Scholar] [CrossRef]
- Sáez, P.; Dinu, I.A.; Rodríguez, A.; Gómez, J.M.; Lazar, M.M.; Rossini, D.; Dinu, M.V. Composite cryo-beads of chitosan reinforced with natural zeolites with remarkable elasticity and switching on/off selectivity for heavy metal ions. Int. J. Biol. Macromol. 2020, 164, 2432–2449. [Google Scholar] [CrossRef]
- Dinu, M.V.; Gradinaru, A.C.; Lazar, M.M.; Dinu, I.A.; Raschip, I.E.; Ciocarlan, N.; Aprotosoaie, A.C. Physically cross-linked chitosan/dextrin cryogels entrapping Thymus vulgaris essential oil with enhanced mechanical, antioxidant and antifungal properties. Int. J. Biol. Macromol. 2021, 184, 898–908. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Q.; Gao, B.; Li, M.; Liu, C.; Qiu, Y. Removal of hexavalent chromium by biochar supported nZVI composite: Batch and fixed-bed column evaluations, mechanisms, and secondary contamination prevention. Chemosphere 2019, 217, 85–94. [Google Scholar] [CrossRef]
- Roy, D.; Neogi, S.; De, S. Adsorptive removal of heavy metals from battery industry effluent using MOF incorporated polymeric beads: A combined experimental and modeling approach. J. Hazard. Mater. 2021, 403, 123624. [Google Scholar] [CrossRef]
- Callura, J.C.; Shi, Q.; Dzombak, D.A.; Karamalidis, A.K. Selective recovery of rare earth elements with ligand-functionalized polymers in fixed-bed adsorption columns. Sep. Purif. Technol. 2021, 265, 118472. [Google Scholar] [CrossRef]
- Pathirana, C.; Ziyath, A.M.; Egodawatta, P.; Bandara, N.J.G.J.K.; Jinadasa, B.S.N.; Bandala, E.R.; Wijesiri, B.; Goonetilleke, A. Biosorption of heavy metals: Transferability between batch and column studies. Chemosphere 2022, 294, 133659. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fan, M.; Ni, J.; Peng, W.; Cao, Y.; Li, H.; Huang, Y.; Fan, G.; Zhao, Y.; Song, S. Efficient dye removal using fixed-bed process based on porous montmorillonite nanosheet/poly(acrylamide-co-acrylic acid)/sodium alginate hydrogel beads. Appl. Clay Sci. 2022, 219, 106443. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous ion exchange in a flowing system. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Appication of gas adsorption kinetics I. A theoretical model for respirator cartridge service life. Am. Ind. Hyg. Assoc. 1984, 45, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Ghiorghita, C.-A.; Dinu, M.V.; Dragan, E.S. Burst-free and sustained release of diclofenac sodium from mesoporous silica/PEI microspheres coated with carboxymethyl cellulose/chitosan layer-by-layer films. Cellulose 2022, 29, 395–412. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V.; Timpu, D. Preparation and characterization of novel composites based on chitosan and clinoptilolite with enhanced adsorption properties for Cu2+. Biores. Technol. 2010, 101, 812–817. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Dinu, M.V.; Lazar, M.M.; Dragan, E.S. Dual ionic cross-linked alginate/clinoptilolite composite microbeads with improved stability and enhanced sorption properties for methylene blue. React. Funct. Polym. 2017, 116, 31–40. [Google Scholar] [CrossRef]
- Raschip, I.E.; Dinu, M.V.; Fifere, N.; Darie-Nita, R.; Pamfil, D.; Popirda, A.; Logigan, C. Thermal, mechanical and water sorption properties of xanthan-based composite cryogels. Cellul. Chem. Technol. 2020, 54, 915–924. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Meng, J.; Liu, X.; Xu, J.; Wang, F.; Brooks, P. Zeolite-supported nanoscale zero-valent iron: New findings on simultaneous adsorption of Cd(II), Pb(II), and As(III) in aqueous solution and soil. J. Hazard. Mater. 2018, 344, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.C.; Loganathan, P.; Nguyen, T.V.; Vigneswaran, S.; Kandasamy, J. Simultaneous adsorption of Cd, Cr, Cu, Pb, and Zn by an iron-coated Australian zeolite in batch and fixed-bed column studies. Chem. Eng. J. 2015, 270, 393–404. [Google Scholar] [CrossRef]
- Šabanović, E.; Memić, M.; Sulejmanović, J.; Selović, A. Simultaneous adsorption of heavy metals from water by novel lemon-peel based biomaterial. Polish J. Chem. Technol. 2020, 22, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Kemik, Ö.F.; Ngwabebhoh, F.A.; Yildiz, U. A response surface modelling study for sorption of Cu2+, Ni2+, Zn2+ and Cd2+ using chemically modified poly(vinylpyrrolidone) and poly(vinylpyrrolidone-co-methylacrylate) hydrogels. Adsorp. Sci. Technol. 2017, 3, 263–283. [Google Scholar] [CrossRef]
- Joseph, I.V.; Tosheva, L.; Doyle, A.M. Simultaneous removal of Cd(II), Co(II), Cu(II), Pb(II), and Zn(II) ions from aqueous solutions via adsorption on FAU-type zeolites prepared from coal fly ash. J. Environ. Chem. Eng. 2020, 8, 103895. [Google Scholar] [CrossRef]
- Fato, P.F.; Li, D.W.; Zhao, L.J.; Qiu, K.; Long, Y.T. Simultaneous Removal of Multiple Heavy Metal Ions from River Water Using Ultrafine Mesoporous Magnetite Nanoparticles. ACS Omega 2019, 4, 7543–7549. [Google Scholar] [CrossRef] [Green Version]
- Humelnicu, D.; Lazar, M.M.; Ignat, M.; Dinu, I.A.; Dragan, E.S.; Dinu, M.V. Removal of heavy metal ions from multi-component aqueous solutions by eco-friendly and low-cost composite sorbents with anisotropic pores. J. Hazard. Mater 2020, 381, 120980. [Google Scholar] [CrossRef]
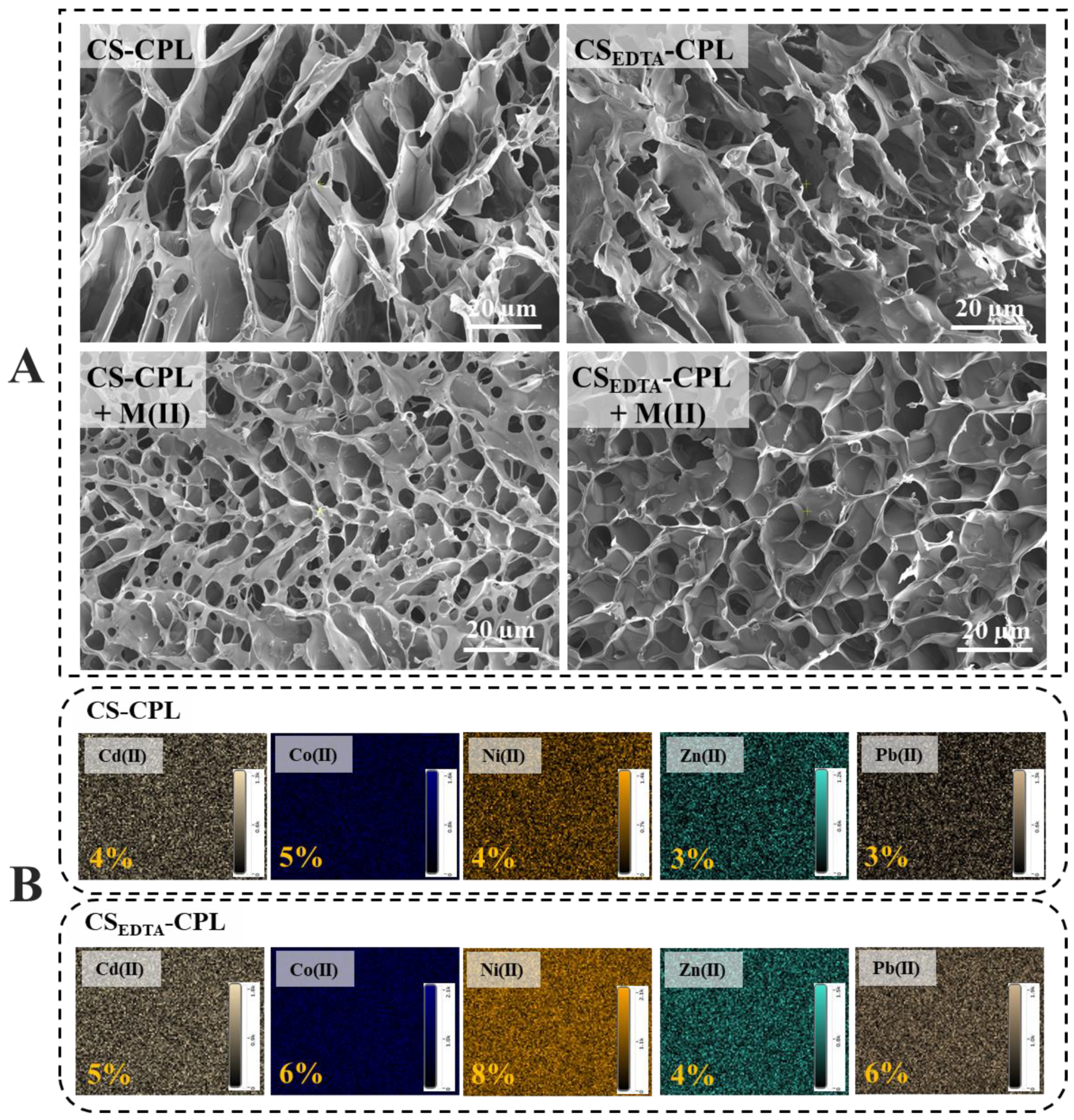


| Sample | EDX Elements Atomic Ratio, % | |||||||
|---|---|---|---|---|---|---|---|---|
| C | N | O | Al | Si | Na | K | Ca | |
| CS-CPL | 44.03 | 8.93 | 40.00 | 0.50 | 2.13 | 0.27 | 0.10 | 3.13 |
| CSEDTA-CPL | 47.97 | 9.90 | 35.50 | 0.50 | 2.00 | 0.27 | 0.17 | 2.83 |
| Sample Code | CPL, wt.% | Porosity, 1 % | Mean Pore Sizes, 2 μm | Water Uptake, 3 g/g | BETH2O Data 4 | |
|---|---|---|---|---|---|---|
| Area, m2/g | Monolayer, g/g | |||||
| CS-CPL | 20 | 78.19 ± 2.04 | 21.49 ± 5.27 | 30.27 ± 3.48 | 431.16 ± 29.31 | 0.123 ± 0.084 |
| CSEDTA-CPL | 20 | 86.57 ± 1.31 | 30.16 ± 6.18 | 45.04 ± 4.82 | 450.34 ± 30.66 | 0.128 ± 0.067 |
| Sample | m 1 (mg) | Thomas | Yoon–Nelson | ||||
|---|---|---|---|---|---|---|---|
| kTH (L/min∙mg) | q0 (mg/g) | R2 | kYN (mL/min·mg) | τ (min) | R2 | ||
| CS-CPL | 109.9 | 5.38 × 10−4 | 68.72 | 0.985 | 0.133 | 30.64 | 0.985 |
| CSEDTA-CPL | 205.8 | 4.85 × 10−5 | 145.55 | 0.862 | 0.012 | 121.51 | 0.862 |
| Conformer | Relative Energy (kcal/mole) | Spatial Structures 1 | Dipole Moment (Debye) |
|---|---|---|---|
| EDTA-1 | 0.00 |  | 0.00 |
| EDTA-2 | 6.44 |  | 6.32 |
| EDTA-3 | 6.72 | 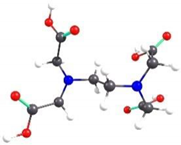 | 2.20 |
| EDTA-4 | 8.88 |  | 1.84 |
| EDTA-5 | 16.09 | 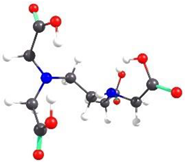 | 4.91 |
| Conformer | Relative Energy (kcal/mole) | Spatial Structures 1 | Dipole Moment (Debye) |
|---|---|---|---|
| DTPA-1 | 0.00 | 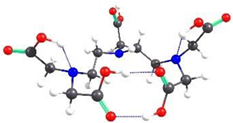 | 7.64 |
| DTPA-2 | 1.88 | 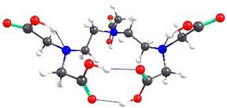 | 5.45 |
| DTPA-3 | 1.92 | 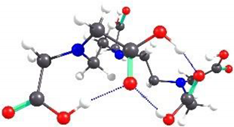 | 3.36 |
| DTPA-4 | 5.98 | 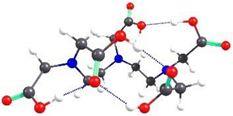 | 5.63 |
| DTPA-5 | 6.44 | 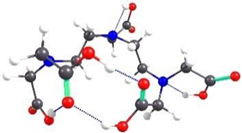 | 6.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, M.V.; Humelnicu, I.; Ghiorghita, C.A.; Humelnicu, D. Aminopolycarboxylic Acids-Functionalized Chitosan-Based Composite Cryogels as Valuable Heavy Metal Ions Sorbents: Fixed-Bed Column Studies and Theoretical Analysis. Gels 2022, 8, 221. https://doi.org/10.3390/gels8040221
Dinu MV, Humelnicu I, Ghiorghita CA, Humelnicu D. Aminopolycarboxylic Acids-Functionalized Chitosan-Based Composite Cryogels as Valuable Heavy Metal Ions Sorbents: Fixed-Bed Column Studies and Theoretical Analysis. Gels. 2022; 8(4):221. https://doi.org/10.3390/gels8040221
Chicago/Turabian StyleDinu, Maria Valentina, Ionel Humelnicu, Claudiu Augustin Ghiorghita, and Doina Humelnicu. 2022. "Aminopolycarboxylic Acids-Functionalized Chitosan-Based Composite Cryogels as Valuable Heavy Metal Ions Sorbents: Fixed-Bed Column Studies and Theoretical Analysis" Gels 8, no. 4: 221. https://doi.org/10.3390/gels8040221
APA StyleDinu, M. V., Humelnicu, I., Ghiorghita, C. A., & Humelnicu, D. (2022). Aminopolycarboxylic Acids-Functionalized Chitosan-Based Composite Cryogels as Valuable Heavy Metal Ions Sorbents: Fixed-Bed Column Studies and Theoretical Analysis. Gels, 8(4), 221. https://doi.org/10.3390/gels8040221








