One-Step Preparation of Adhesive Composite Hydrogels through Fast and Simultaneous In Situ Formation of Silver Nanoparticles and Crosslinking
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of DA-GLTs
2.2. Preparation of AgNPs-Crosslinked Composite Hydrogels
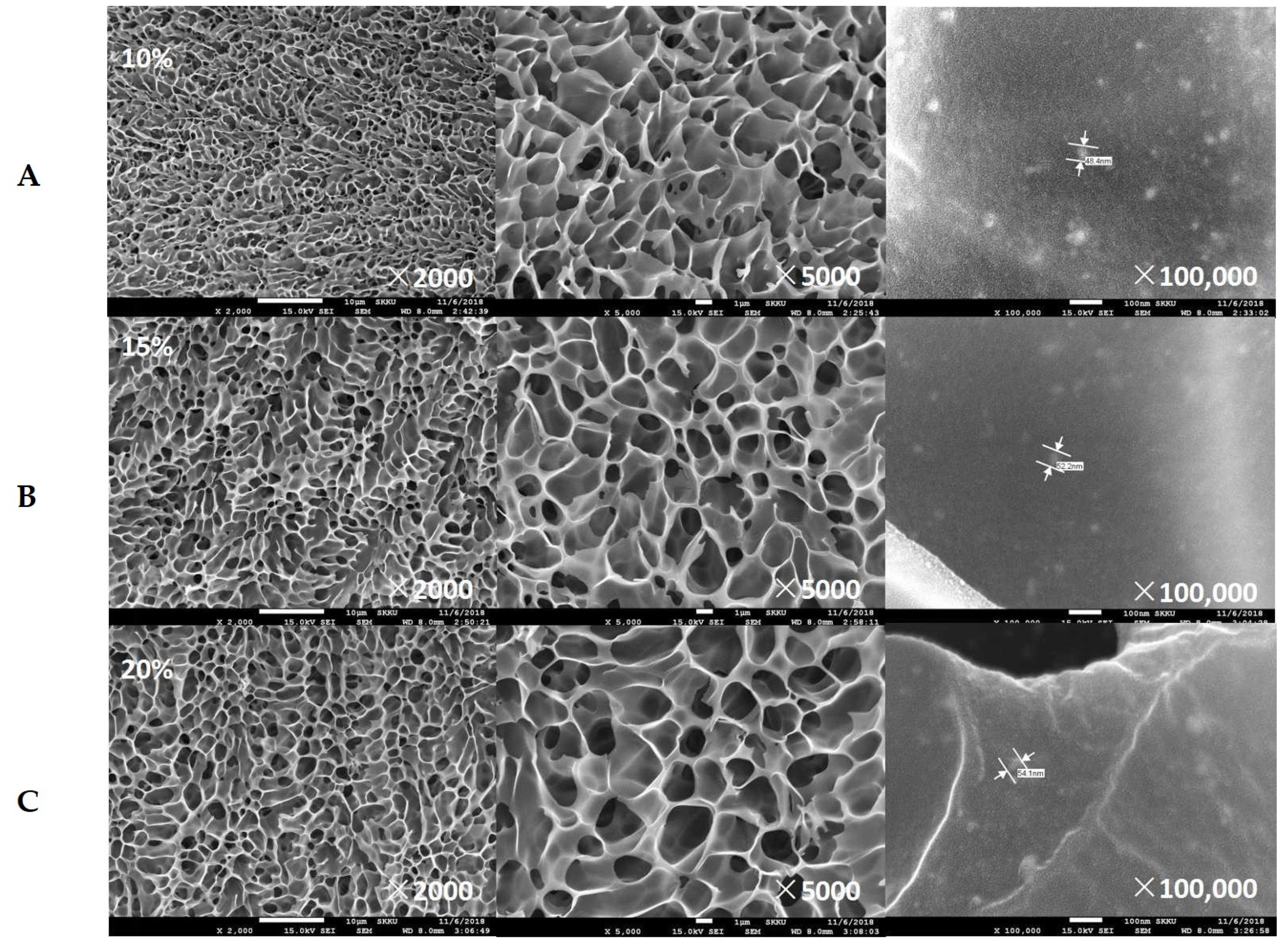
2.3. Surface Morphologies and Thermogravimetric Analysis (TGA) of Freeze-Dried Composite Hydrogels
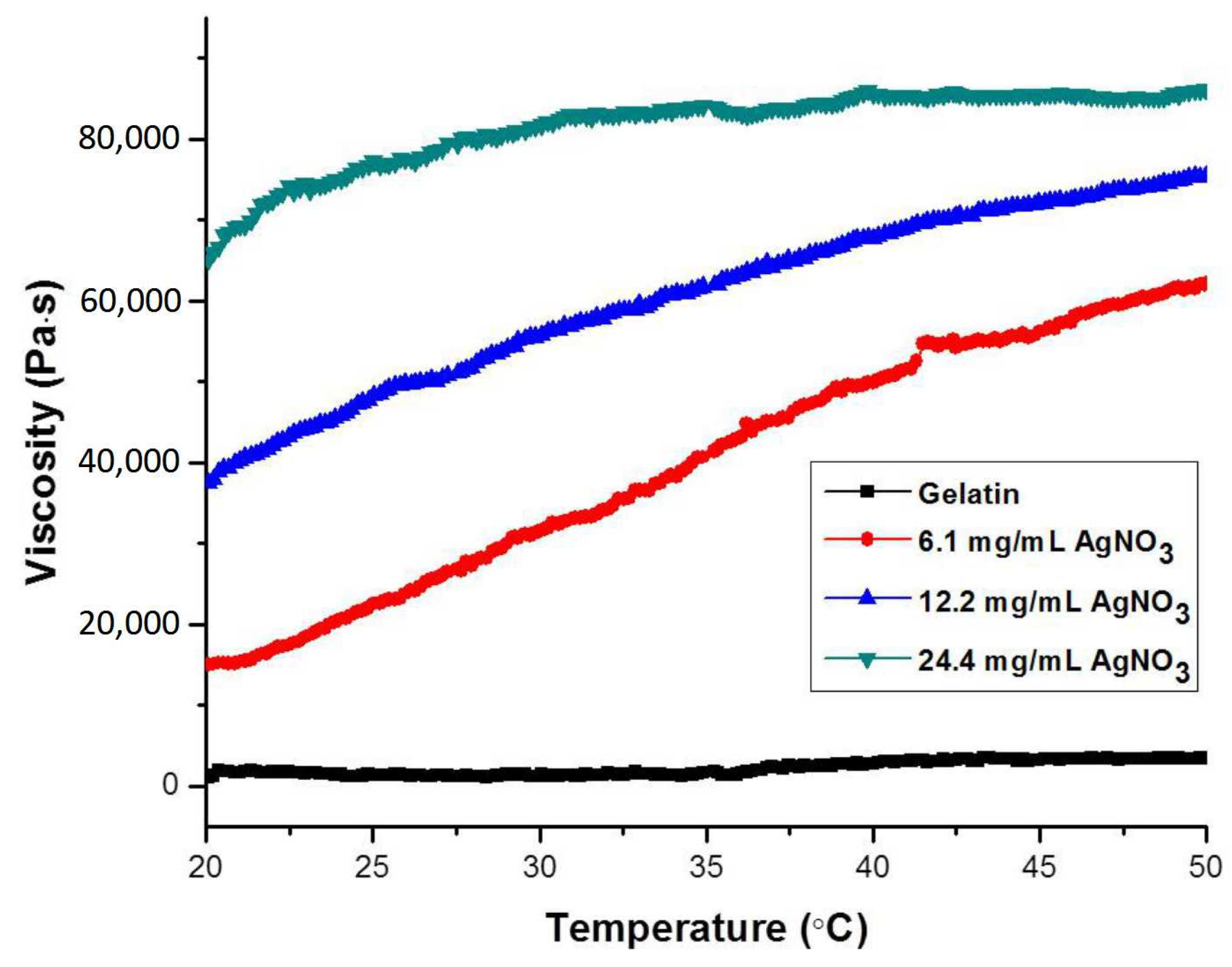
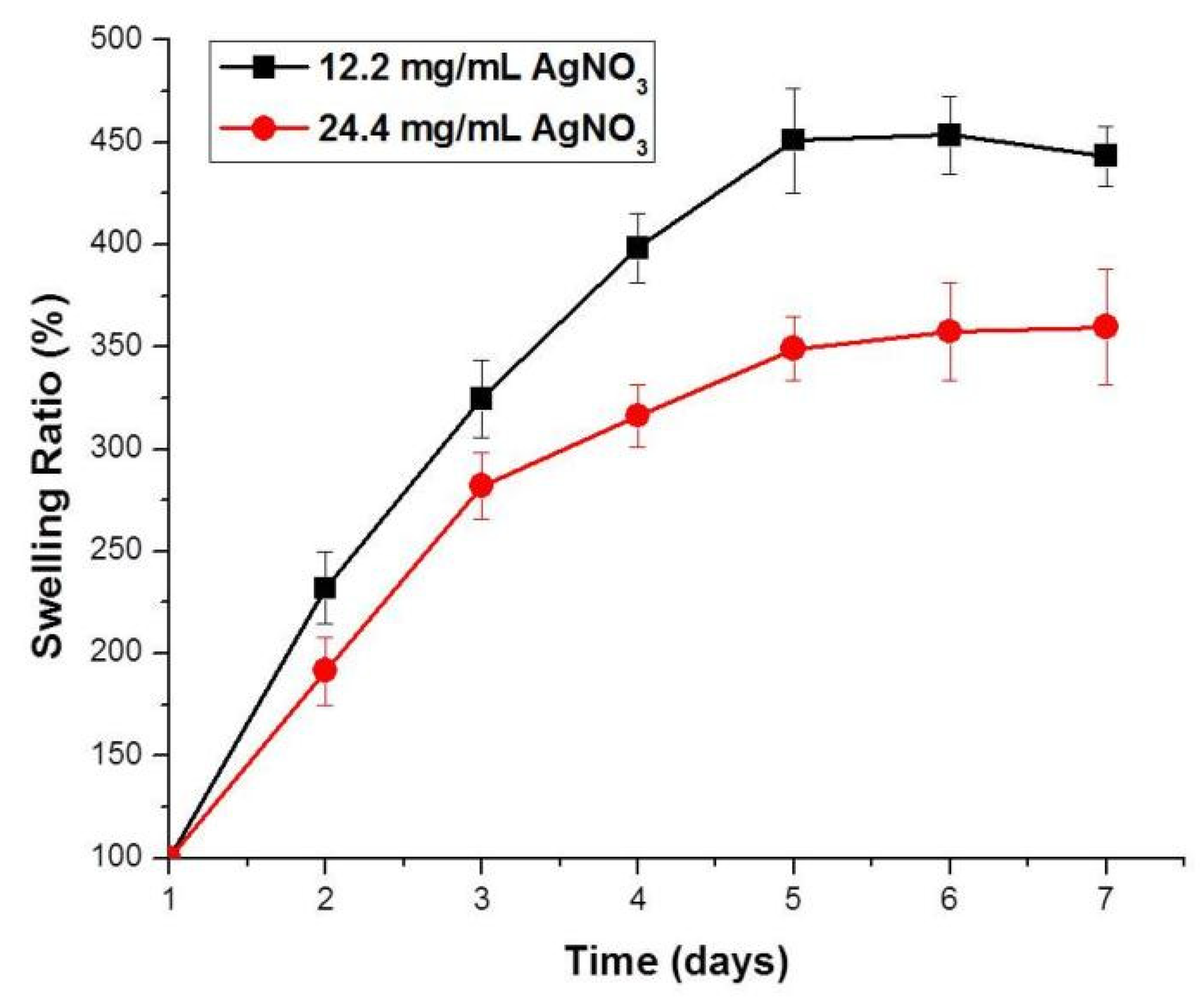
2.4. Viscosities and Swelling Behaviors of Composite Hydrogels
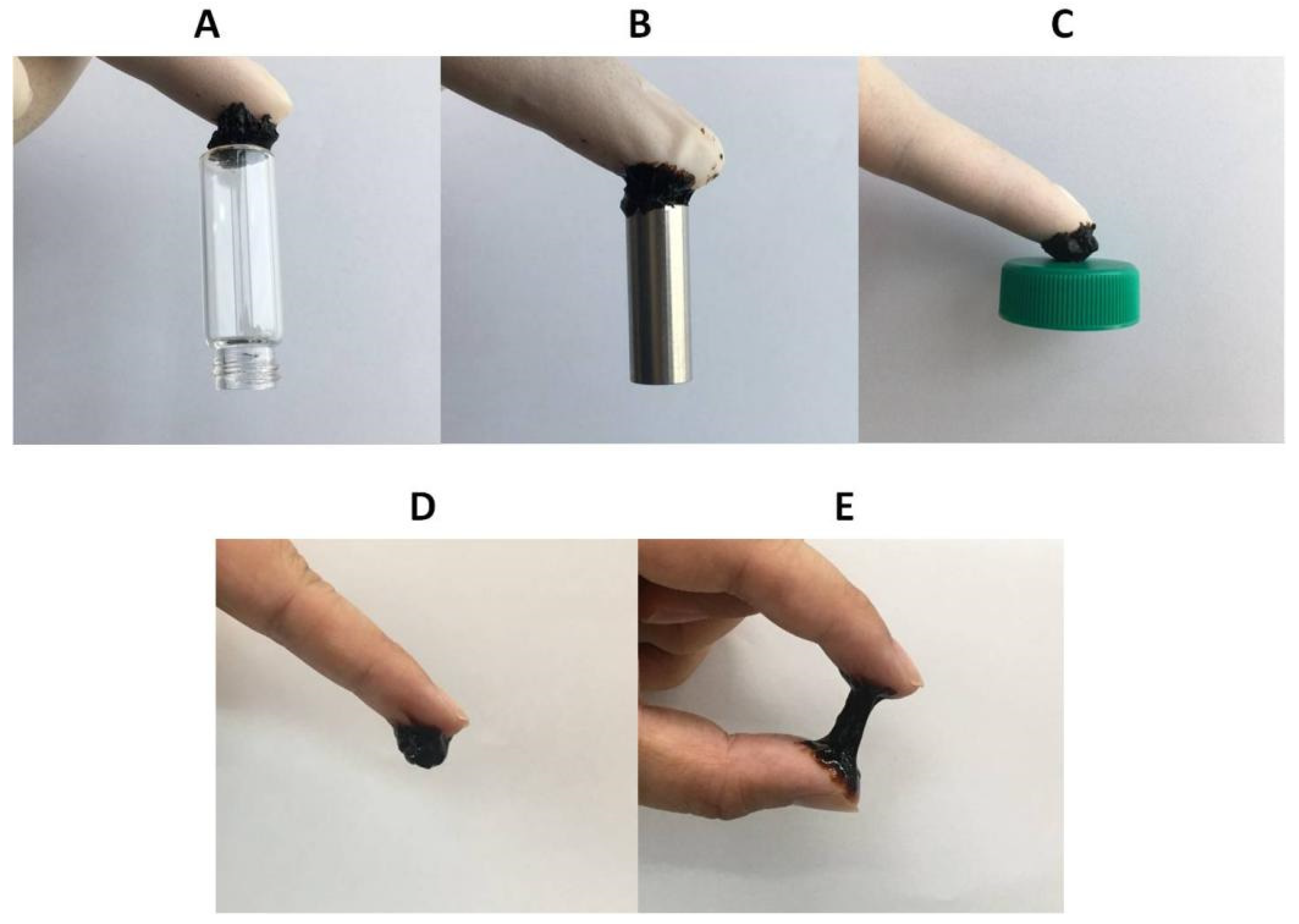
2.5. Adhesive Properties of Composite Hydrogels
2.6. Sustained Release of Model Drugs from Composite Hydrogels
3. Conclusions
4. Experimental
4.1. Materials
4.2. Synthesis of Dopamine-Conjugated Gelatin (DA-GLT)
4.3. Preparation of Gelatin/AgNPs Hydrogels
4.4. Characterizations of DA-GLTs and Composite Hydrogels
4.4.1. Characterization of DA-GLTs
4.4.2. Surface and Elemental Analysis
4.4.3. Thermogravimetric Analysis (TGA)
4.4.4. Dynamic Rheological Measurement of Hydrogels
4.4.5. Swelling Behaviors of Composite Hydrogels
4.4.6. Adhesive Properties of Composite Hydrogels
4.4.7. Drug Release Behaviors
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.; Cui, J.; Illeperuma, W.R.K.; Aizenberg, J.; Vlassak, J.J. Extremely Stretchable and Fast Self-Healing Hydrogels. Adv. Mater. 2016, 28, 4678–4683. [Google Scholar] [CrossRef] [PubMed]
- Voorhaar, L.; Hoogenboom, R. Supramolecular polymer networks: Hydrogels and bulk materials. Chem. Soc. Rev. 2016, 45, 4013–4031. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Le, T.M.D.; Duong, H.T.T.; Thambi, T.; Phan, V.G.; Jeong, J.H.; Lee, D.S. Bioinspired pH- and Temperature-Responsive Injectable Adhesive Hydrogels with Polyplexes Promotes Skin Wound Healing. Biomacromolecules 2018, 19, 3536–3548. [Google Scholar] [CrossRef]
- Yu, S.; Wang, C.; Yu, J.; Wang, J.; Lu, Y.; Zhang, Y.; Zhang, X.; Hu, Q.; Sun, W.; He, C.; et al. Injectable Bioresponsive Gel Depot for Enhanced Immune Checkpoint Blockade. Adv. Mater. 2018, 30, 1801527. [Google Scholar] [CrossRef]
- Xu, D.; Huang, J.; Zhao, D.; Ding, B.; Zhang, L.; Cai, J. High-Flexibility, High-Toughness Double-Cross-Linked Chitin Hydrogels by Sequential Chemical and Physical Cross-Linkings. Adv. Mater. 2016, 28, 5844–5849. [Google Scholar] [CrossRef]
- Gyarmati, B.; Mészár, E.Z.; Kiss, L.; Deli, M.A.; László, K.; Szilágyi, A. Supermacroporous chemically cross-linked poly(aspartic acid) hydrogels. Acta Biomater. 2015, 22, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-M.; Potta, T.; Park, K.-H.; Song, S.-C. Temperature responsive chemical crosslinkable UV pretreated hydrogel for ap-plication to injectable tissue regeneration system via differentiations of encapsulated hMSCs. Biomaterials 2017, 112, 248–256. [Google Scholar]
- Kim, M.H.; Park, H.; Nam, H.C.; Park, S.R.; Jung, J.-Y.; Park, W.H. Injectable methylcellulose hydrogel containing silver oxide nanoparticles for burn wound healing. Carbohydr. Polym. 2018, 181, 579–586. [Google Scholar] [CrossRef]
- Alarcon, E.I.; Udekwu, K.I.; Noel, C.W.; Gagnon, L.B.-P.; Taylor, P.K.; Vulesevic, B.; Simpson, M.J.; Gkotzis, S.; Islam, M.M.; Lee, C.-J.; et al. Safety and efficacy of composite collagen-silver na-noparticle hydrogels as tissue engineering scaffolds. Nanoscale 2015, 7, 18789–18798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.; McCarthy, D.T.; Deletic, A.; Zhang, X. Silver/Reduced Graphene Oxide Hydrogel as Novel Bactericidal Filter for Point-of-Use Water Disinfection. Adv. Funct. Mater. 2015, 25, 4344–4351. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Park, C.H.; Kim, C.S. In Situ Synthesis of Antimicrobial Silver Nanoparticles within Antifouling Zwitterionic Hydrogels by Catecholic Redox Chemistry for Wound Healing Application. Biomacromolecules 2016, 17, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Kumar, A.; Patil, N.B.; Viswanathan, C.; Ghosh, D. Preparation and characterization of silver nanoparticle loaded amorphous hydrogel of carboxymethylcellulose for infected wounds. Carbohydr. Polym. 2015, 130, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, M.; Farhoudian, S.; Namazi, H. One-pot synthesis of antibacterial chitosan/silver bio-nanocomposite hydrogel beads as drug delivery systems. Int. J. Biol. Macromol. 2015, 79, 37–43. [Google Scholar] [CrossRef] [PubMed]
- González-Sánchez, M.I.; Perni, S.; Tommasi, G.; Morris, N.G.; Hawkins, K.; López-Cabarcos, E.; Prokopovich, P. Silver nanopar-ticle based antibacterial methacrylate hydrogels potential for bone graft applications. Mater. Sci. Eng. C 2015, 50, 332–340. [Google Scholar] [CrossRef]
- Simon, T.; Wu, C.-S.; Liang, J.-C.; Cheng, C.; Ko, F.-H. Facile synthesis of a biocompatible silver nanoparticle derived tripeptide supramolecular hydrogel for antibacterial wound dressings. New J. Chem. 2016, 40, 2036–2043. [Google Scholar] [CrossRef]
- Jiao, T.; Guo, H.; Zhang, Q.; Peng, Q.; Tang, Y.; Yan, X.; Li, B. Reduced Graphene Oxide-Based Silver Nanoparticle-Containing Composite Hydrogel as Highly Efficient Dye Catalysts for Wastewater Treatment. Sci. Rep. 2015, 5, 11873. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Guo, T.; Yuan, X.; Zhao, Y.; Ren, L. An injectable supramolecular hydrogel hybridized with silver nanoparticles for antibacterial application. Soft Matter 2018, 14, 1227–1234. [Google Scholar] [CrossRef]
- García-Astrain, C.; Chen, C.; Burón, M.; Palomares, T.; Eceiza, A.; Fruk, L.; Corcuera, M.Á.; Gabilondo, N. Biocompatible Hydrogel Nanocomposite with Covalently Embedded Silver Nanoparticles. Biomacromolecules 2015, 16, 1301–1310. [Google Scholar] [CrossRef]
- Xie, Y.; Liao, X.; Zhang, J.; Yang, F.; Fan, Z. Novel chitosan hydrogels reinforced by silver nanoparticles with ultrahigh mechanical and high antibacterial properties for accelerating wound healing. Int. J. Biol. Macromol. 2018, 119, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-S.; Chen, K.-S.; Wu, L.-T. In situ synthesis of high swell ratio polyacrylic acid/silver nanocomposite hydrogels and their antimicrobial properties. J. Inorg. Biochem. 2016, 164, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, H.Y.; Lee, D.S. Advances in biodegradable and injectable hydrogels for biomedical applications. J. Control. Release 2021, 330, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, H.Y.; Lee, D.S. Biodegradable and Injectable Hydrogels in Biomedical Applications. Biomacromolecules 2022, 23, 609–618. [Google Scholar] [CrossRef]
- Rezaeifar, M.; Mahmoudvand, H.; Amiri, M. Formulation and evaluation of diphenhydramine gel using different gelling agents. Der Pharm. Chem. 2016, 8, 243–249. [Google Scholar]

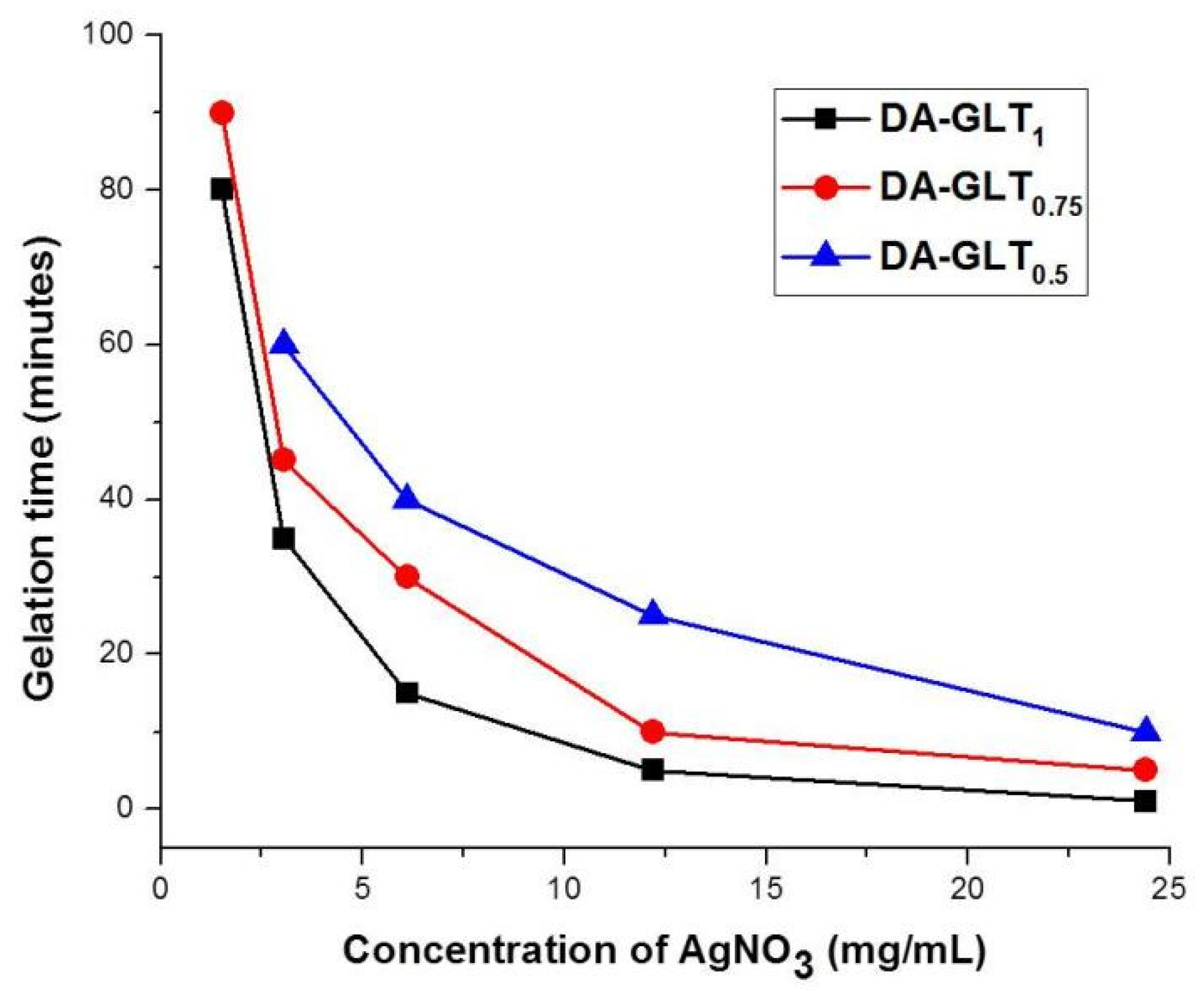

| 5 wt% (DA-GLT) | 10 wt% (DA-GLT) | 15 wt% (DA-GLT) | 20 wt% (DA-GLT) | |
|---|---|---|---|---|
| DA-GLT0.1 | No gelation | No gelation | No gelation | No gelation |
| DA-GLT0.25 | No gelation | No gelation | 10 min | 5 min |
| DA-GLT0.5 | No gelation | 10 min | 1 min | <5 s |
| DA-GLT0.75 | No gelation | 5 min | <5 s | <5 s |
| DA-GLT1 | No gelation | 1 min | <5 s | <5 s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xiao, Y.; Xi, M.; Li, G.; Jiang, Y. One-Step Preparation of Adhesive Composite Hydrogels through Fast and Simultaneous In Situ Formation of Silver Nanoparticles and Crosslinking. Gels 2022, 8, 256. https://doi.org/10.3390/gels8050256
Li Y, Xiao Y, Xi M, Li G, Jiang Y. One-Step Preparation of Adhesive Composite Hydrogels through Fast and Simultaneous In Situ Formation of Silver Nanoparticles and Crosslinking. Gels. 2022; 8(5):256. https://doi.org/10.3390/gels8050256
Chicago/Turabian StyleLi, Yi, Yunchao Xiao, Man Xi, Guibin Li, and Yang Jiang. 2022. "One-Step Preparation of Adhesive Composite Hydrogels through Fast and Simultaneous In Situ Formation of Silver Nanoparticles and Crosslinking" Gels 8, no. 5: 256. https://doi.org/10.3390/gels8050256






