Functional Hydrogels with Chondroitin Sulfate Release Properties Regulate the Angiogenesis Behaviors of Endothelial Cells
Abstract
:1. Introduction
2. Results and Discussion
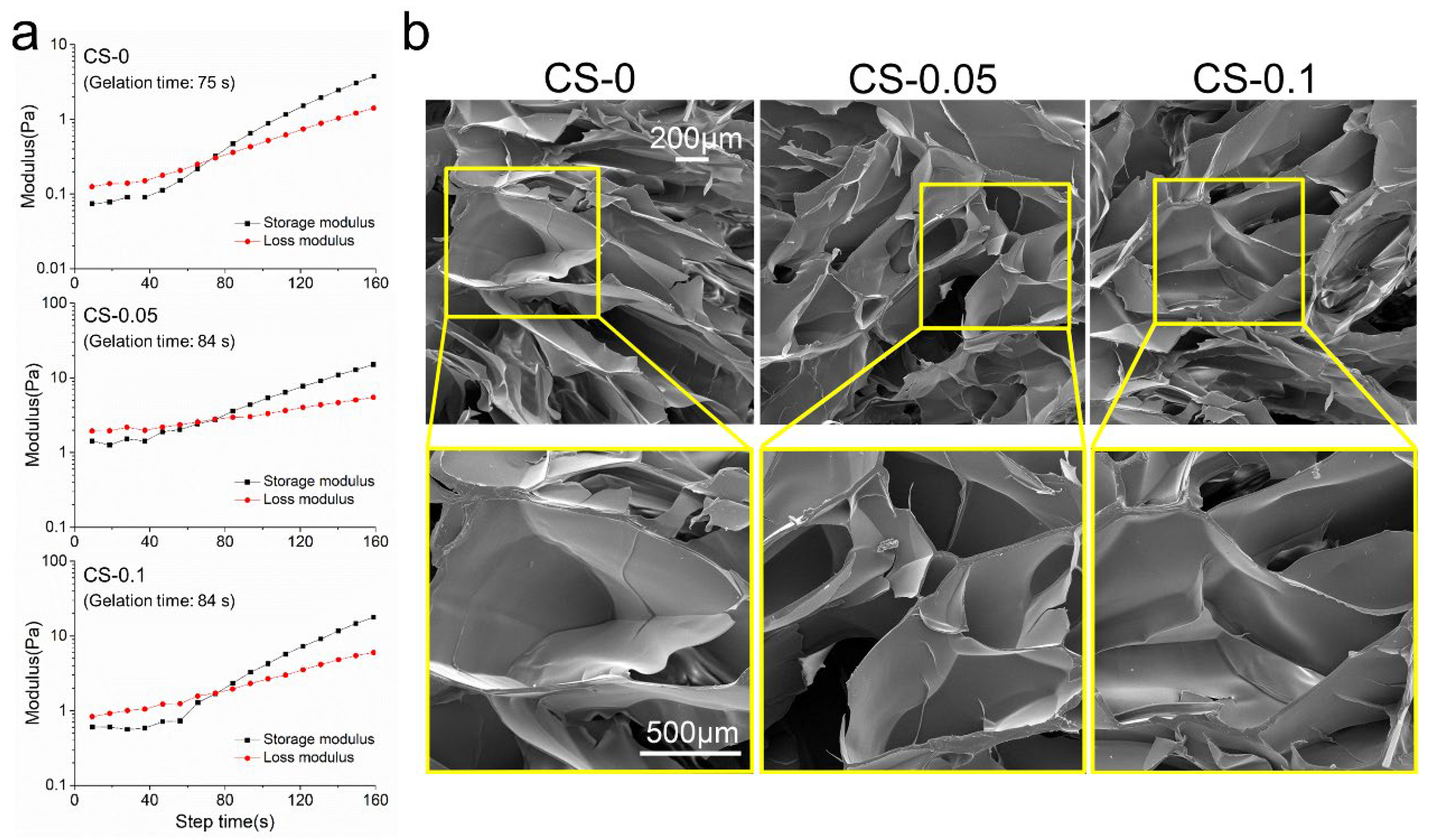
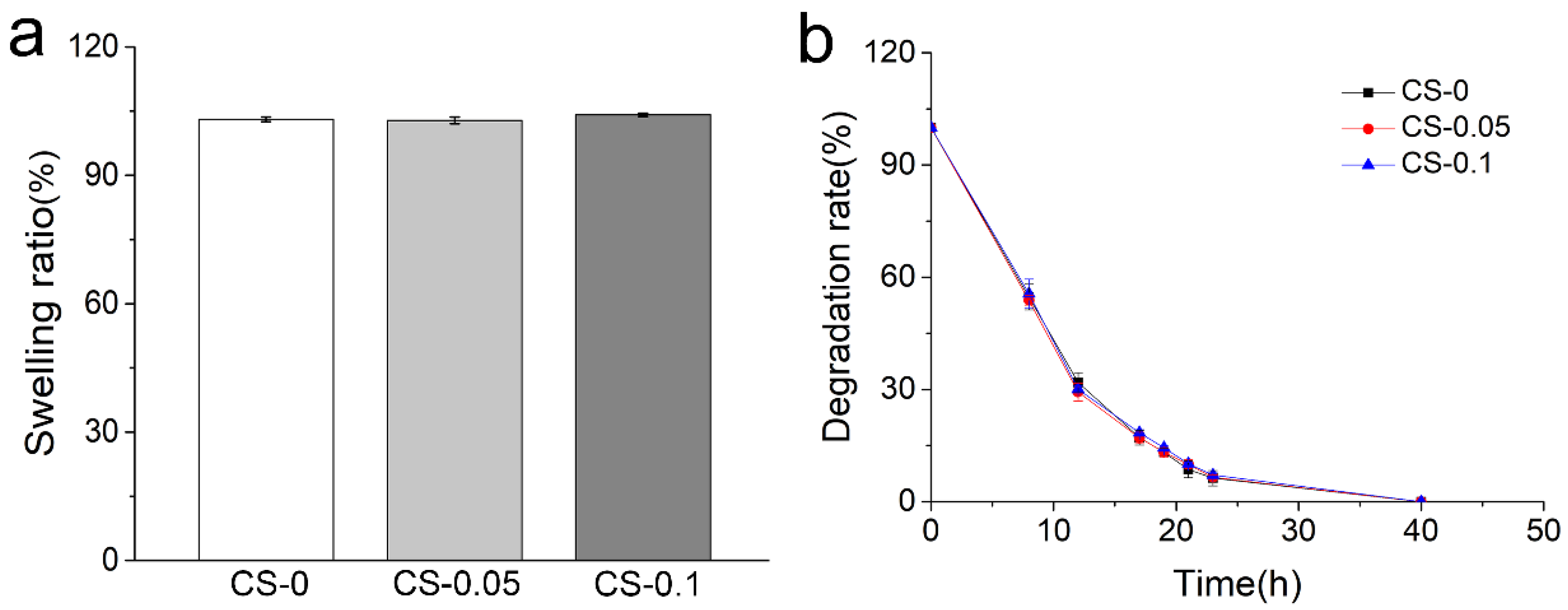
2.1. Characterization of Hydrogels
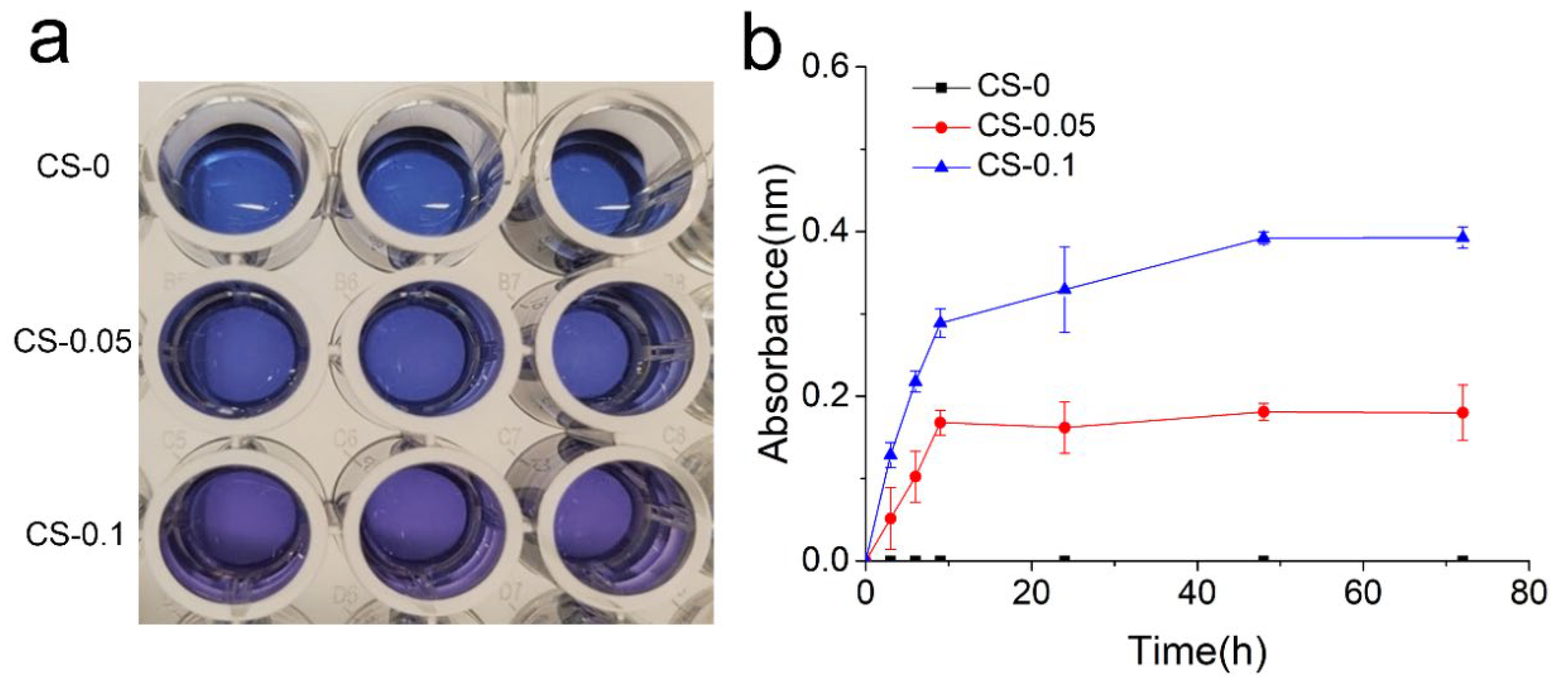
2.2. Loading and Release of CS
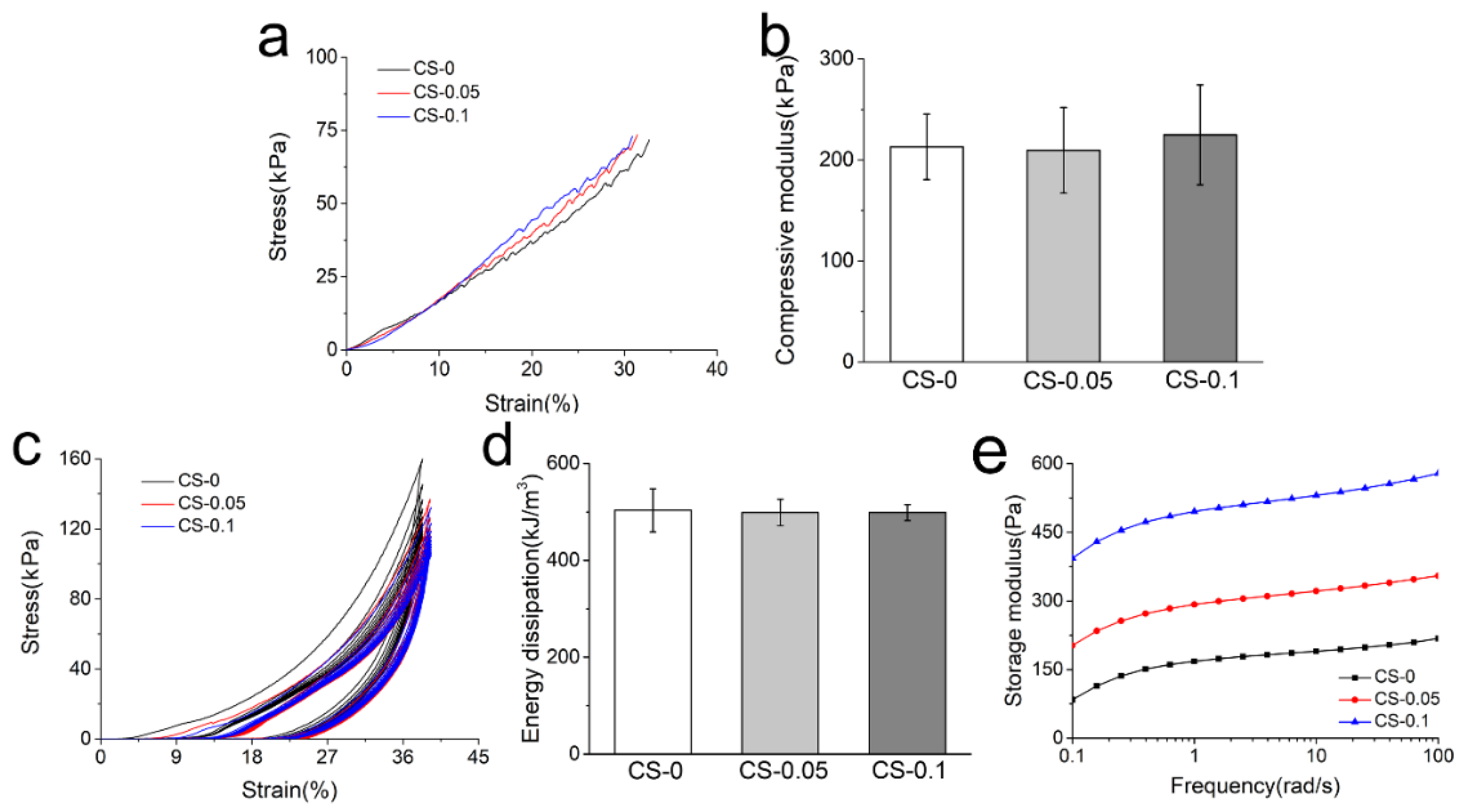
2.3. Mechanical Properties of Hydrogels
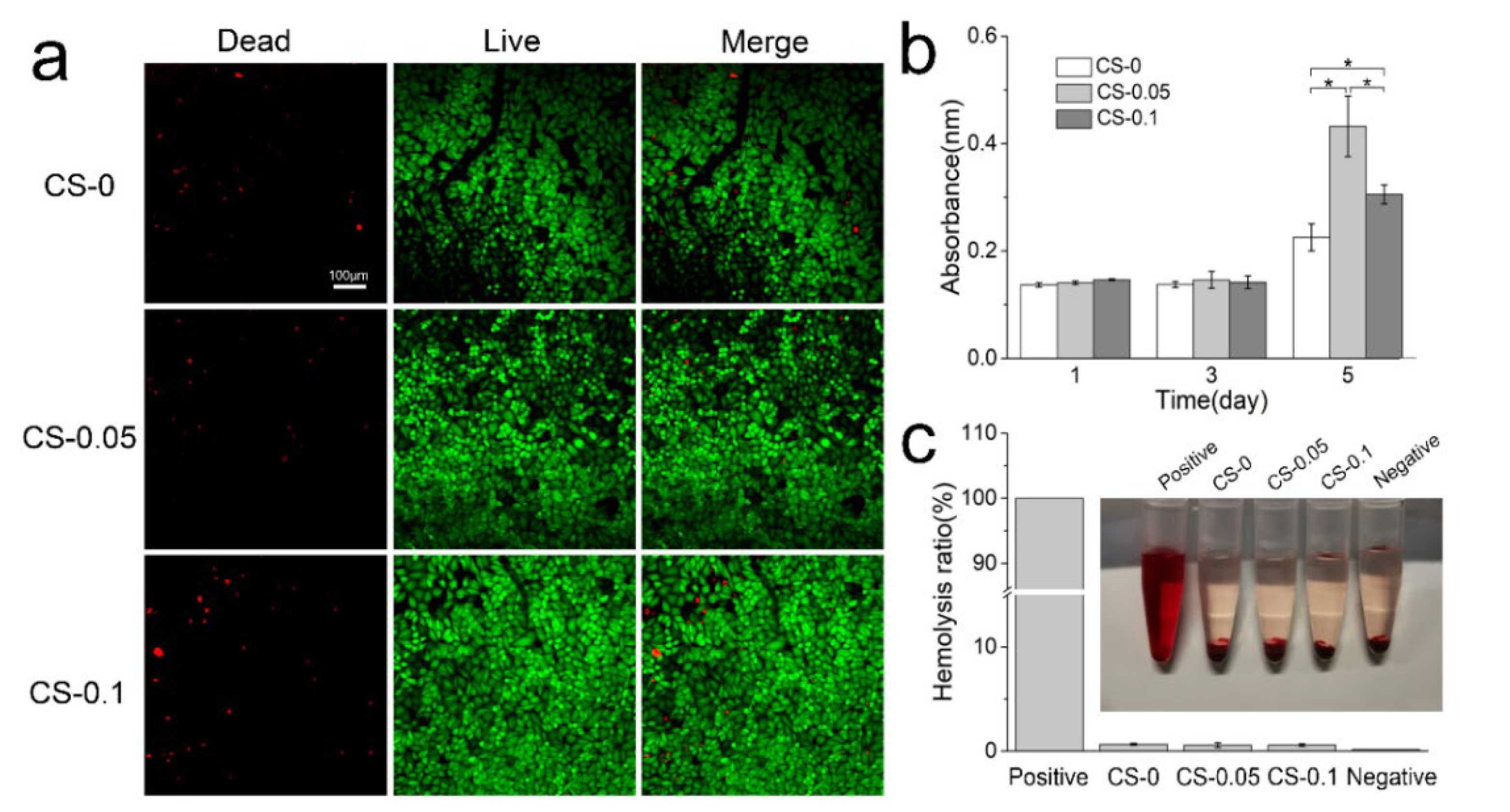
2.4. Cytocompatibility and Hemocompatibility of Hydrogels
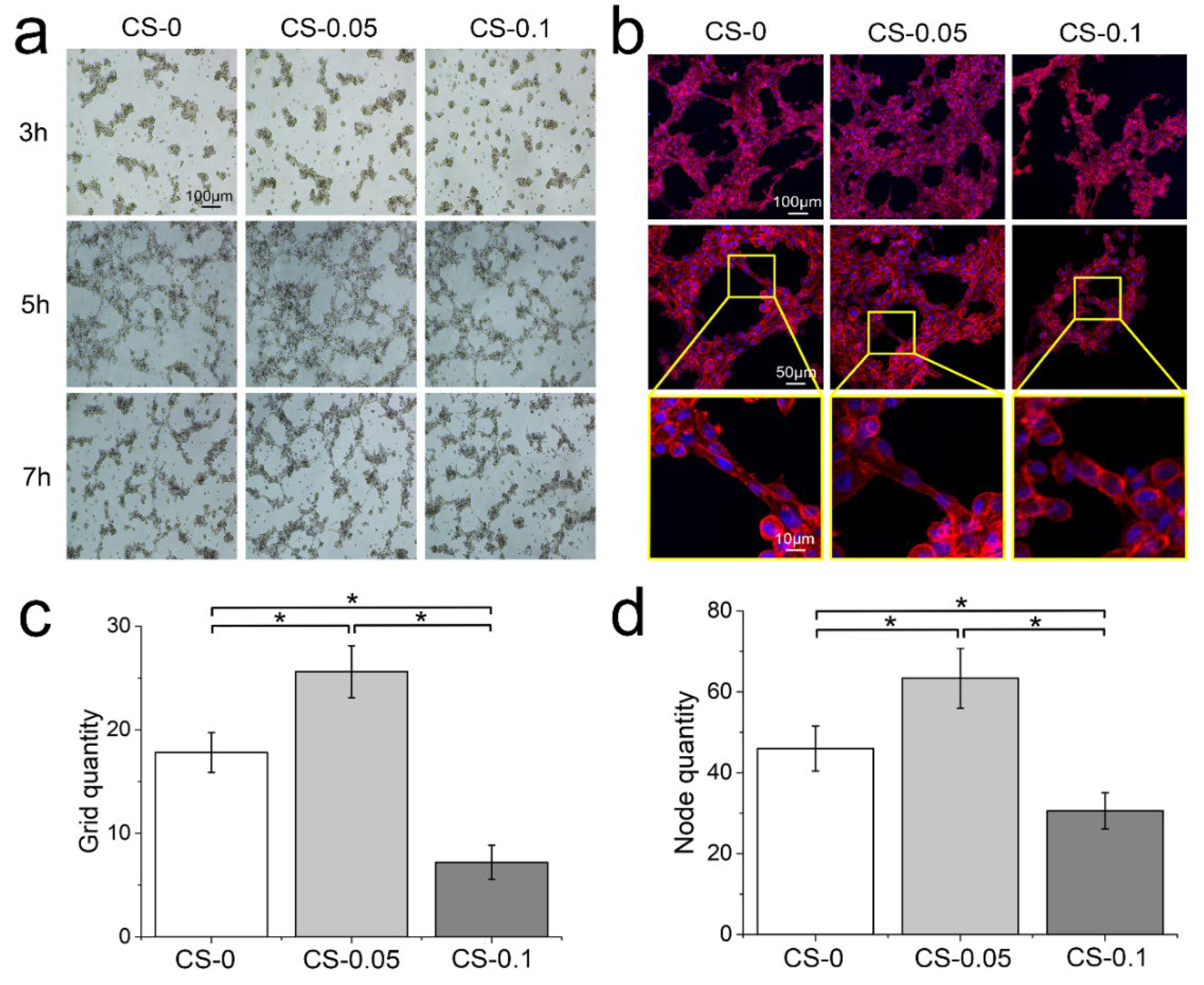
2.5. Modulation on Angiogenesis Behaviors
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Fabrication of Hydrogels
4.3. Morphology Observation
4.4. Swelling Ratio and Biodegradation Properties
4.5. X-ray Photoelectron Spectroscopy (XPS) Analysis
4.6. CS Release Behavior
4.7. Mechanical Test
4.8. Cytocompatibility and Hemocompatibility Assays
4.9. In Vitro Sprouting Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ngo, M.T.; Harley, B.A.C. Angiogenic biomaterials to promote therapeutic regeneration and investigate disease progression. Biomaterials 2020, 255, 120207. [Google Scholar] [CrossRef]
- Yu, Y.; Dai, K.; Gao, Z.; Tang, W.; Shen, T.; Yuan, Y.; Wang, J.; Liu, C. Sulfated polysaccharide directs therapeutic angiogenesis via endogenous VEGF secretion of macrophages. Sci. Adv. 2021, 7, eabd8217. [Google Scholar] [CrossRef]
- Rouwkema, J.; Khademhosseini, A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef]
- Jeong, H.-W.; Hernandez-Rodriguez, B.; Kim, J.; Kim, K.-P.; Enriquez-Gasca, R.; Yoon, J.; Adams, S.; Schoeler, H.R.; Vaquerizas, J.M.; Adams, R.H. Transcriptional regulation of endothelial cell behavior during sprouting angiogenesis. Nat. Commun. 2017, 8, e726. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, C.; Huo, H.; Ji, C.; Sun, M.; Nie, L. Injectable temperature-sensitive hydrogel with VEGF loaded microspheres for vascularization and bone regeneration of femoral head necrosis. Mater. Lett. 2018, 229, 138–141. [Google Scholar] [CrossRef]
- Stryker, Z.I.; Rajabi, M.; Davis, P.J.; Mousa, S.A. Evaluation of Angiogenesis Assays. Biomedicines 2019, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Karvinen, H.; Pasanen, E.; Rissanen, T.T.; Korpisalo, P.; Vaehaekangas, E.; Jazwa, A.; Giacca, M.; Ylae-Herttuala, S. Long-term VEGF-A expression promotes aberrant angiogenesis and fibrosis in skeletal muscle. Gene Ther. 2011, 18, 1166–1172. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.-C.; Wang, X.-F.; Xu, Y.-Y.; Qiao, Y.-H.; Guo, X.; Wang, D.-F.; Li, Q.; Turng, L.-S. Polycaprolactone nanofibers containing vascular endothelial growth factor-encapsulated gelatin particles enhance mesenchymal stem cell differentiation and angiogenesis of endothelial cells. Biomacromolecules 2018, 19, 3747–3753. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Khalili, S.; Khorasani, S.N.; Neisiany, R.E.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef] [Green Version]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef]
- Wang, H.; Heilshorn, S.C. Adaptable Hydrogel Networks with Reversible Linkages for Tissue Engineering. Adv. Mater. 2015, 27, 3717–3736. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.-H.; Khoneisser, J.; Huang, P.-C.; Xu, X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.-W.; Zhang, Y.; MacEwan, M.R.; Xia, Y. Neovascularization in Biodegradable Inverse Opal Scaffolds with Uniform and Precisely Controlled Pore Sizes. Adv. Healthc. Mater. 2013, 2, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yu, J.; Zhang, Q.; Lu, H.; Qiu, X.; Zhou, D.; Qi, Y.; Huang, Y. Dual Cross-linked HHA Hydrogel Supplies and Regulates MΦ2 for Synergistic Improvement of Immunocompromise and Impaired Angiogenesis to Enhance Diabetic Chronic Wound Healing. Biomacromolecules 2020, 21, 3795–3806. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.; Wen, H.; Luo, Y.; Huang, R.; Rong, L.; Xie, J. Recent advance in delivery system and tissue engineering applications of chondroitin sulfate. Carbohydr. Polym. 2020, 230, 115650. [Google Scholar] [CrossRef]
- Zhu, W.; Ji, Y.; Wang, Y.; He, D.; Yan, Y.; Su, N.; Zhang, C.; Xing, X.-H. Structural characterization and in vitro antioxidant activities of chondroitin sulfate purified from Andrias davidianus cartilage. Carbohydr. Polym. 2018, 196, 398–404. [Google Scholar] [CrossRef]
- de Souza Lins Borba, F.K.; Felix, G.L.Q.; Costa, E.V.L.; Silva, L.; Dias, P.F.; de Albuquerque Nogueira, R. Fractal analysis of extra-embryonic vessels of chick embryos under the effect of glucosamine and chondroitin sulfates. Microvasc. Res. 2016, 105, 114–118. [Google Scholar] [CrossRef]
- Lopez-Moya, M.; Melgar-Lesmes, P.; Kolandaivelu, K.; de la Torre Hernandez, J.M.; Edelman, E.R.; Balcells, M. Optimizing Glutaraldehyde-Fixed Tissue Heart Valves with Chondroitin Sulfate Hydrogel for Endothelialization and Shielding against Deterioration. Biomacromolecules 2018, 19, 1234–1244. [Google Scholar] [CrossRef]
- Xiong, X.; Xiao, W.; Zhou, S.; Cui, R.; Xu, H.H.K.; Qu, S. Enhanced proliferation and angiogenic phenotype of endothelial cells via negatively-charged alginate and chondroitin sulfate microsphere hydrogels. Biomed. Mater. 2021, 16, 025012. [Google Scholar] [CrossRef]
- Fox, J.M.; Kang, K.; Sherman, W.; Heroux, A.; Sastry, G.M.; Baghbanzadeh, M.; Lockett, M.R.; Whitesides, G.M. Interactions between Hofmeister Anions and the Binding Pocket of a Protein. JACS 2015, 137, 3859–3866. [Google Scholar] [CrossRef]
- Entekhabi, E.; Haghbin Nazarpak, M.; Sedighi, M.; Kazemzadeh, A. Predicting degradation rate of genipin cross-linked gelatin scaffolds with machine learning. Mater. Sci. Eng. C 2020, 107, 110362. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.Z.; Jiao, Q.C.; Ding, Y.L.; Chen, L. Study on quantitative assay of chondroitin sulfate with a spectrophotometric method of azure A. Spectrosc. Spectr. Anal. 2003, 23, 600–602. [Google Scholar]
- Li, G.; Jiang, Y.; Li, M.; Zhang, W.; Li, Q.; Tang, K. Investigation on the tunable effect of oxidized konjac glucomannan with different molecular weight on gelatin-based composite hydrogels. Int. J. Biol. Macromol. 2021, 168, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Mu, C.; Lin, W.; Ngai, T. Gelatin Effects on the Physicochemical and Hemocompatible Properties of Gelatin/PAAm/Laponite Nanocomposite Hydrogels. ACS Appl. Mater. Interfaces 2015, 7, 18732–18741. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Li, X.; Wang, S.; Yuan, L.; Ge, L.; Li, D.; Mu, C. Facile Fabrication of Biocompatible Gelatin-Based Self-Healing Hydrogels. ACS Appl. Polym. Mater. 2019, 1, 1350–1358. [Google Scholar] [CrossRef]
- Calamia, V.; Lourido, L.; Fernandez-Puente, P.; Mateos, J.; Rocha, B.; Montell, E.; Verges, J.; Ruiz-Romero, C.; Blanco, F.J. Secretome analysis of chondroitin sulfate-treated chondrocytes reveals anti-angiogenic, anti-inflammatory and anti-catabolic properties. Arthritis Res. Ther. 2012, 14, R202. [Google Scholar] [CrossRef] [Green Version]
- Le Jan, S.; Hayashi, M.; Kasza, Z.; Eriksson, I.; Bishop, J.R.; Weibrecht, I.; Heldin, J.; Holmborn, K.; Jakobsson, L.; Soderberg, O.; et al. Functional overlap between chondroitin and heparan sulfate proteoglycans during VEGF-induced sprouting angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1255–1263. [Google Scholar] [CrossRef] [Green Version]
- Del Gaudio, C.; Baiguera, S.; Boieri, M.; Mazzanti, B.; Ribatti, D.; Bianco, A.; Macchiarini, P. Induction of angiogenesis using VEGF releasing genipin-crosslinked electrospun gelatin mats. Biomaterials 2013, 34, 7754–7765. [Google Scholar] [CrossRef]
- Xu, S.; Yonese, M. Two dimensional and three dimensional interactions between bovine serum albumin and chondroitin sulfate. Polym. J. 2007, 39, 298–303. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Zhang, X.; Cai, D.; Li, J.; Mu, Q.; Zhang, W.; Zhu, S.; Jiang, Y.; Shen, W.; Zhang, S.; et al. Silk fibroin-chondroitin sulfate scaffold with immuno-inhibition property for articular cartilage repair. Acta Biomater. 2017, 63, 64–75. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, G.; Liu, J.; Li, M.; Li, Q.; Tang, K. Gelatin/oxidized konjac glucomannan composite hydrogels with high resistance to large deformation for tissue engineering applications. ACS Appl. Bio Mater. 2021, 4, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Yao, D.; Jiang, J.; Guo, X.; Gao, Y.; Li, Q.; Shen, C. Effects of aligned and random fibers with different diameter on cell behaviors. Colloids Surf. B 2018, 171, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.; Yang, K.; Fu, Y.V.; Xu, T.; Li, S.; Zhang, D.; Wang, L.-N.; Lee, C.-S. A Novel Double-Crosslinking-Double-Network Design for Injectable Hydrogels with Enhanced Tissue Adhesion and Antibacterial Capability for Wound Treatment. Adv. Funct. Mater. 2020, 30, 1904156. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Hou, J.; Xing, W.; Liu, C. Vascularization and bone regeneration in a critical sized defect using 2-N,6-O-sulfated chitosan nanoparticles incorporating BMP-2. Biomaterials 2014, 35, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; Saik, J.E.; Poche, R.A.; Leslie-Barbick, J.E.; Lee, S.-H.; Smith, A.A.; Dickinson, M.E.; West, J.L. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials 2010, 31, 3840–3847. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, Q.; Jiang, Y.; Wang, X. Functional Hydrogels with Chondroitin Sulfate Release Properties Regulate the Angiogenesis Behaviors of Endothelial Cells. Gels 2022, 8, 261. https://doi.org/10.3390/gels8050261
Wang H, Li Q, Jiang Y, Wang X. Functional Hydrogels with Chondroitin Sulfate Release Properties Regulate the Angiogenesis Behaviors of Endothelial Cells. Gels. 2022; 8(5):261. https://doi.org/10.3390/gels8050261
Chicago/Turabian StyleWang, Haonan, Qian Li, Yongchao Jiang, and Xiaofeng Wang. 2022. "Functional Hydrogels with Chondroitin Sulfate Release Properties Regulate the Angiogenesis Behaviors of Endothelial Cells" Gels 8, no. 5: 261. https://doi.org/10.3390/gels8050261
APA StyleWang, H., Li, Q., Jiang, Y., & Wang, X. (2022). Functional Hydrogels with Chondroitin Sulfate Release Properties Regulate the Angiogenesis Behaviors of Endothelial Cells. Gels, 8(5), 261. https://doi.org/10.3390/gels8050261






