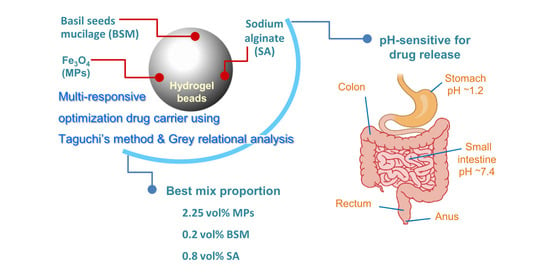
Multi-Responsive Optimization of Novel pH-Sensitive Hydrogel Beads Based on Basil Seed Mucilage, Alginate, and Magnetic Particles
Abstract
:1. Introduction
2. Results and Discussion
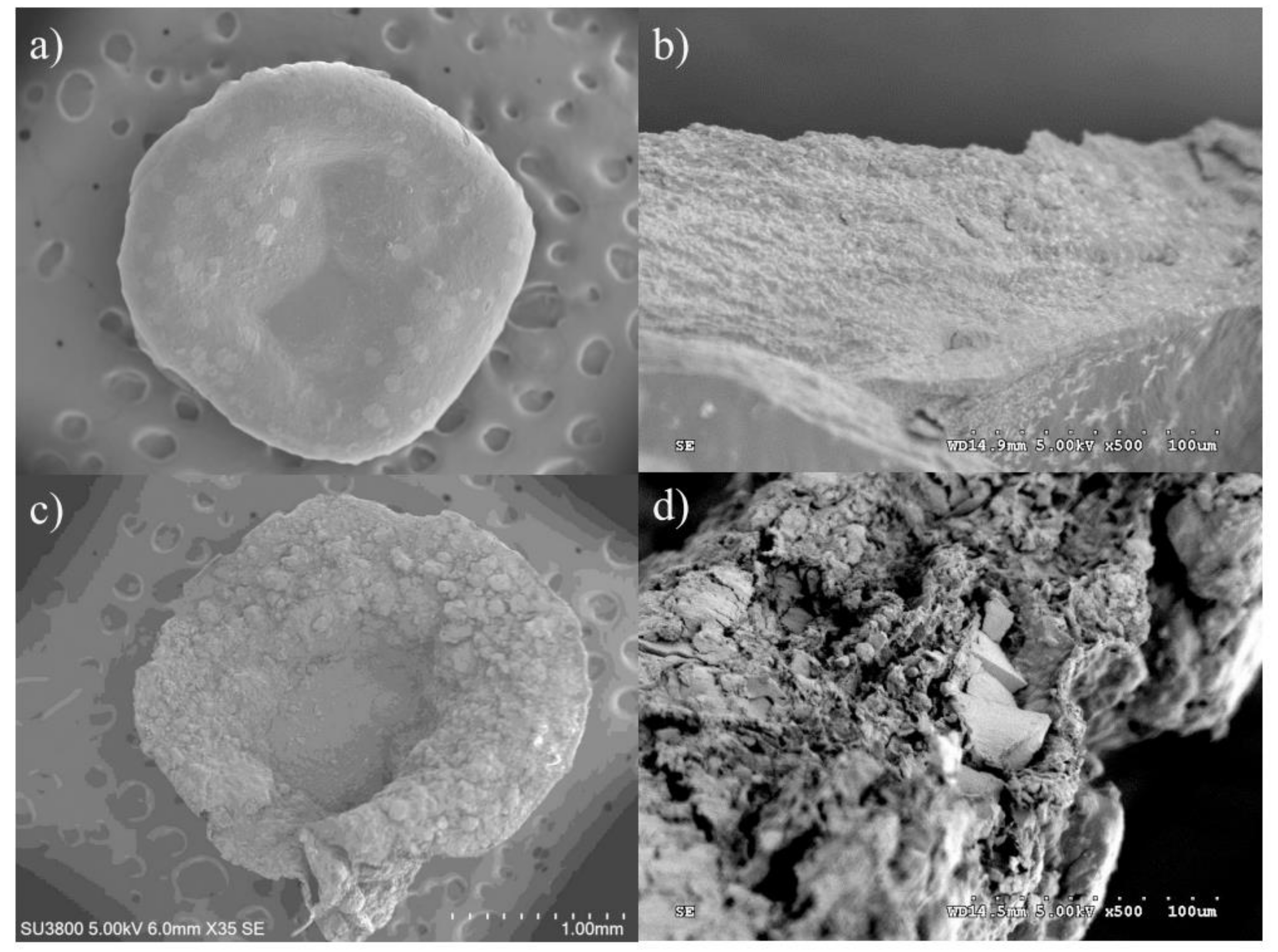
2.1. Morphology of the BSM/SA/MPs Hydrogel Beads
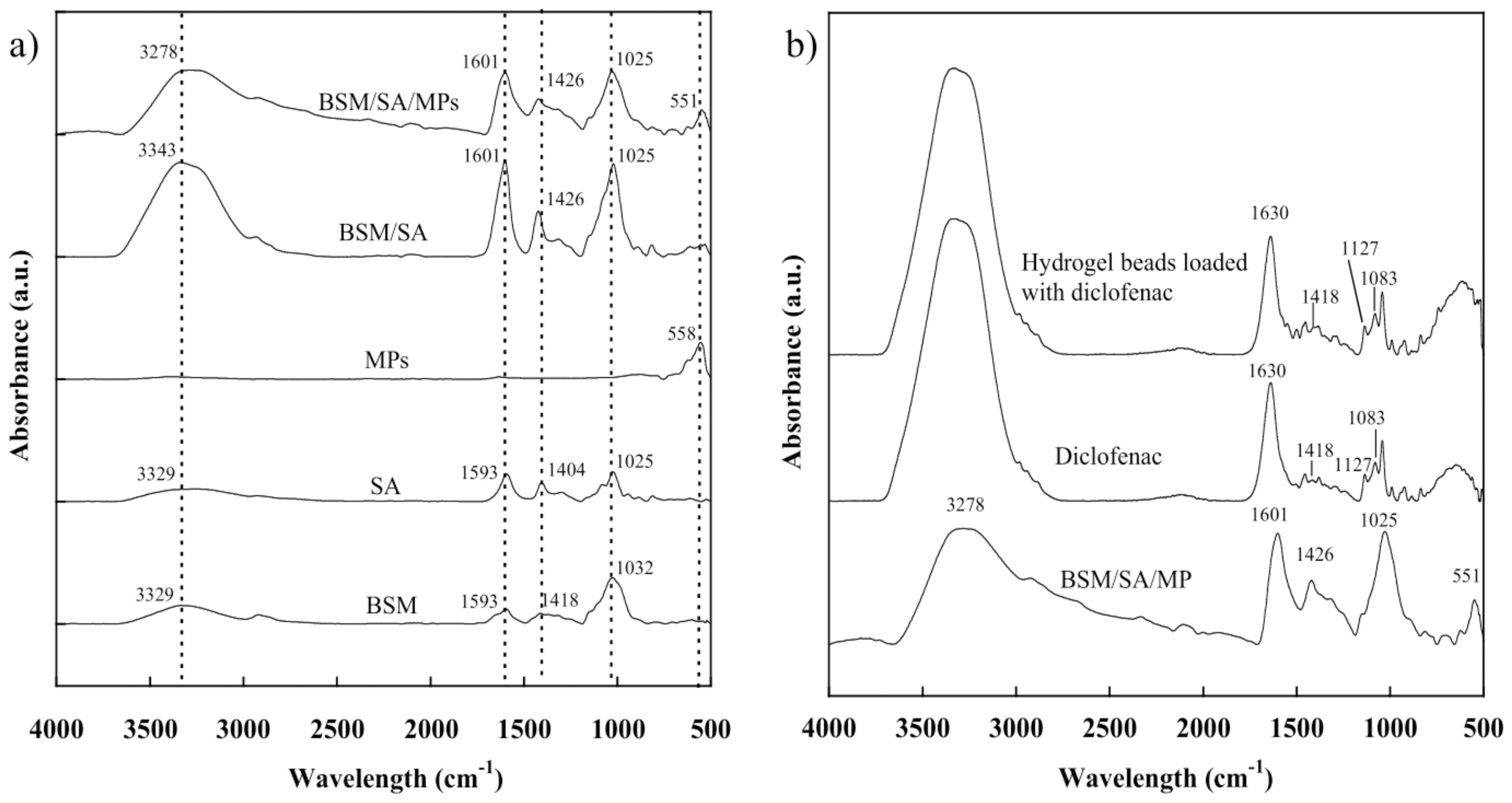
2.2. Chemical Functionality of the BSM/SA/MPs Hydrogel Beads
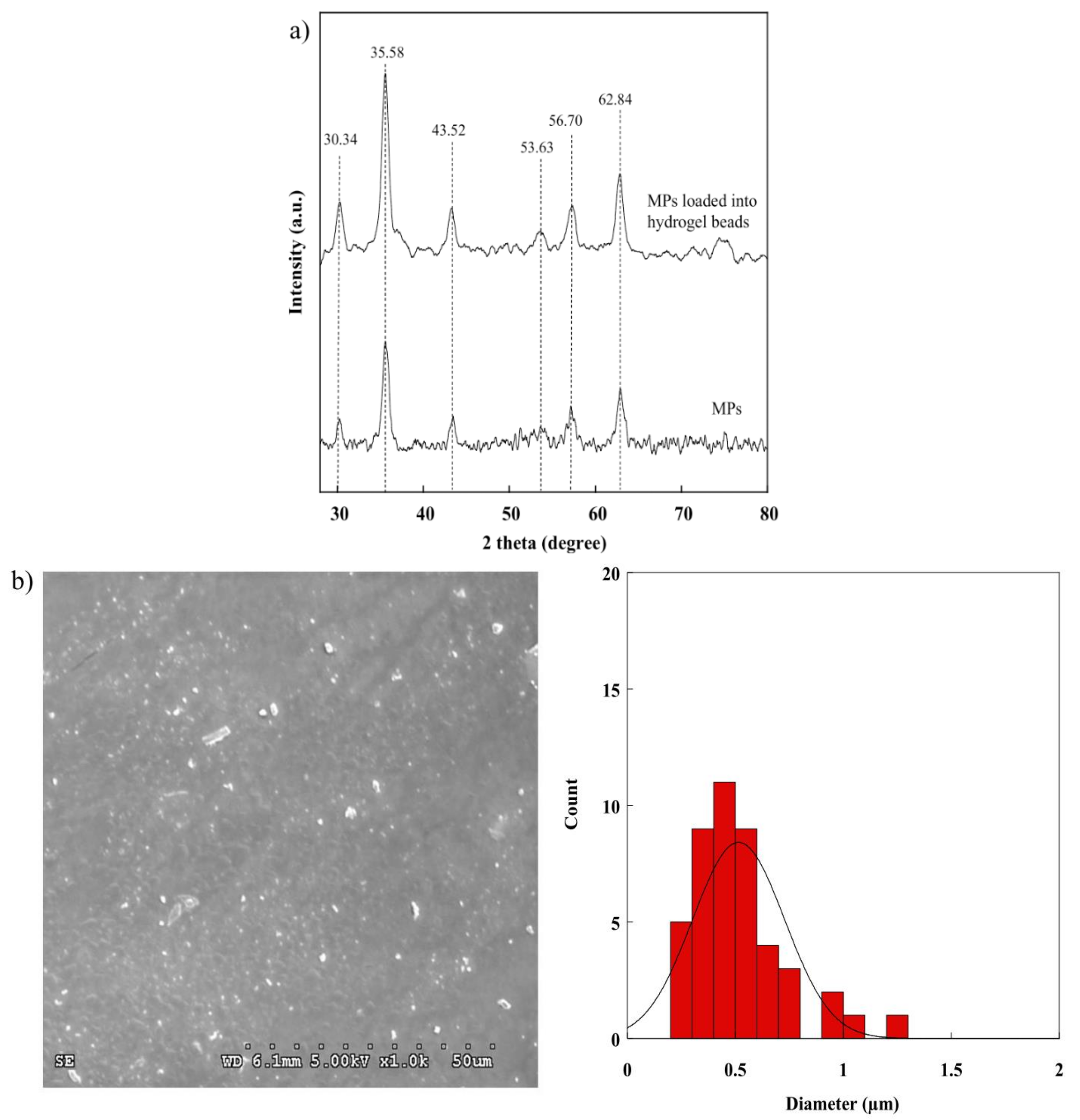
2.3. XRD Pattern of the BSM/SA/MPs Hydrogel Beads
2.4. Swelling Study of the BSM/SA/MPs Hydrogel Beads
2.5. Drug Loading of the BSM/SA/MPs Hydrogel Beads
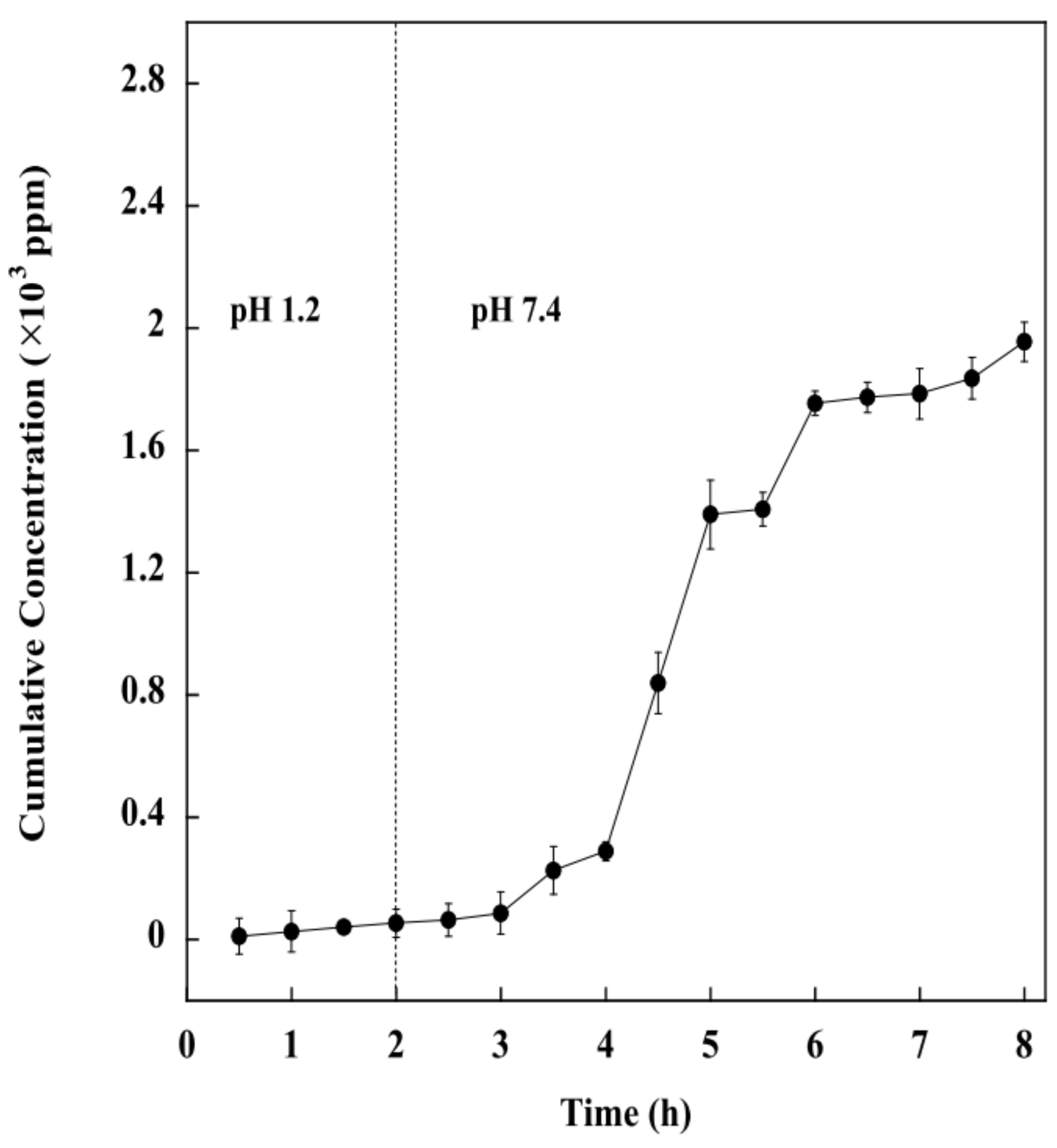
2.6. In Vitro Drug Release of the BSM/SA/MPs Hydrogel Beads
2.7. Release of Antioxidants from Hydrogel Beads
2.8. Optimization of the Hydrogel Bead Compositions Using Grey Relational Analysis
2.9. Effect of pH on Drug Release of the BSM/SA/MPs Hydrogel Beads
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of BSM
4.3. Preparation of MPs
4.4. Hydrogel Bead Preparation
4.5. Characterizations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yari, K.; Akbari, I.; Yazdi, S.A.V. Development and evaluation of sodium alginate-basil seeds mucilage beads as a suitable carrier for controlled release of metformin. Int. J. Biol. Macromol. 2020, 159, 1–10. [Google Scholar] [CrossRef]
- Rayegan, A.; Allafchian, A.; Abdolhosseini Sarsari, I.; Kameli, P. Synthesis and characterization of basil seed mucilage coated Fe(3)O(4) magnetic nanoparticles as a drug carrier for the controlled delivery of cephalexin. Int. J. Biol. Macromol. 2018, 113, 317–328. [Google Scholar] [CrossRef]
- Singh, A.K.; Nutan, B.; Raval, I.H.; Jewrajka, S.K. Self-assembly of partiall alkylated dextran-graft-poly[(2-dimethylamino)ethyl methacrylate] copolymer facilitating hydrophobic/hydrophilic drug delivery and improving conetwork hydrogel properties. Biomacromoleculus 2018, 19, 1142–1153. [Google Scholar] [CrossRef]
- Nutan, B.; Chandel, A.K.; Bhanlani, D.V.; Jewrajka, S.K. Synthesis and tailoring the degradation of multi-responsive amphiphilic conetwork gels and hydrogels fo poly(β-amino ester) and poly(amido amine). Polymer 2017, 111, 265–274. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Bera, A.; Nutan, B.; Jewrajka, S.K. Reactive compatibilizer mediated precise synthesis and application of stimuli responsive polysaccharides-pollycaprolactone amphiphilic co-network gels. Polymer 2016, 99, 470–479. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Nabid, M.R. Synthesis of chemically cross-linked hydrogel films based on basil seed (Ocimum basilicum L.) mucilage for wound dressing drug delivery applications. Int. J. Biol. Macromol. 2020, 163, 336–347. [Google Scholar] [CrossRef]
- Khampieng, T.; Wongkittithavorn, S.; Chaiarwut, S.; Ekabutr, P.; Pavasant, P.; Supaphol, P. Silver nanoparticles-based hydrogel: Characterization of material parameters for pressure ulcer dressing applications. J. Drug Deliv. Sci. Technol. 2018, 44, 91–100. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, S.-H.; Kim, I.-S.; Kim, K.; Kwon, S.K.; Hwang, N.S. Gelatin-based micro-hydrogel carrying genetically engineered human endothelial cells for neovascularization. Acta Biomater. 2019, 95, 285–296. [Google Scholar] [CrossRef]
- Javanbakht, V.; Shafiei, R. Preparation and performance of alginate/basil seed mucilage biocomposite for removal of eriochrome black T dye from aqueous solution. Int. J. Biol. Macromol. 2020, 152, 990–1001. [Google Scholar] [CrossRef]
- Tosif, M.M.; Najda, A.; Bains, A.; Kaushik, R.; Dhull, S.B.; Chawla, P.; Walasek-Janusz, M. A Comprehensive Review on Plant-Derived Mucilage: Characterization, Functional Properties, Applications, and Its Utilization for Nanocarrier Fabrication. Polymer 2021, 13, 1066. [Google Scholar] [CrossRef]
- Akbari, I.; Ghoreishi, S.M.; Habibi, N. Generation and precipitation of paclitaxel nanoparticles in basil seed mucilage via combination of supercritical gas antisolvent and phase inversion techniques. J. Supercrit. Fluids 2014, 94, 182–188. [Google Scholar] [CrossRef]
- Maqsood, H.; Uroos, M.; Muazzam, R.; Naz, S.; Muhammad, N. Extraction of basil seed mucilage using ionic liquid and preparation of AuNps/mucilage nanocomposite for catalytic degradation of dye. Int. J. Biol. Macromol. 2020, 164, 1847–1857. [Google Scholar] [CrossRef]
- Wang, H.; Gong, X.; Guo, X.; Liu, C.; Fan, Y.-Y.; Zhang, J.; Niu, B.; Li, W. Characterization, release, and antioxidant activity of curcumin-loaded sodium alginate/ZnO hydrogel beads. Int. J. Biol. Macromol. 2019, 121, 1118–1125. [Google Scholar] [CrossRef]
- Hua, S.; Ma, H.; Li, X.; Yang, H.; Wang, A. pH-sensitive sodium alginate/poly(vinyl alcohol) hydrogel beads prepared by combined Ca2+ crosslinking and freeze-thawing cycles for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2010, 46, 517–523. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Irani, M.; Esmaeilkhanian, A.; Bazli, L.; Asl, M.S.; Jang, H.W.; Kim, S.Y.; Ramakrishna, S.; Shokouhimehr, M.; Varma, R.S. Polymer incorporated magnetic nanoparticles: Applications for magnetoresponsive targeted drug delivery. Mater. Sci. Eng. B 2021, 272, 115358. [Google Scholar] [CrossRef]
- Aisida, S.O.; Akpa, P.A.; Ahmad, I.; Zhao, T.; Maaza, M.; Ezema, F.I. Bio-inspired encapsulation and functionalization of iron oxide nanoparticles for biomedical applications. Eur. Polym. J. 2020, 122, 109371. [Google Scholar] [CrossRef]
- Dacrory, S.; Moussa, M.; Turky, G.; Kamel, S. In situ synthesis of Fe3O4@ cyanoethyl cellulose composite as antimicrobial and semiconducting film. Carbohydr. Polym. 2020, 236, 116032. [Google Scholar] [CrossRef]
- Supramaniam, J.; Adnan, R.; Mohd Kaus, N.H.; Bushra, R. Magnetic nanocellulose alginate hydrogel beads as potential drug delivery system. Int. J. Biol. Macromol. 2018, 118, 640–648. [Google Scholar] [CrossRef]
- Mohammadi, R.; Saboury, A.; Javanbakht, S.; Foroutan, R.; Shaabani, A. Carboxymethylcellulose/polyacrylic acid/starch-modified Fe3O4 interpenetrating magnetic nanocomposite hydrogel beads as pH-sensitive carrier for oral anticancer drug delivery system. Eur. Polym. J. 2021, 153, 110500. [Google Scholar] [CrossRef]
- Tavares Luiz, M.; Santos Rosa Viegas, J.; Palma Abriata, J.; Viegas, F.; Testa Moura de Carvalho Vicentini, F.; Lopes Badra Bentley, M.V.; Chorilli, M.; Maldonado Marchetti, J.; Tapia-Blácido, D.R. Design of experiments (DoE) to develop and to optimize nanoparticles as drug delivery systems. Eur. J. Pharm. Biopharm. 2021, 165, 127–148. [Google Scholar] [CrossRef]
- Janaum, N.; Butsiri, T.; Kasemsiri, P.; Souvanh, M.; Pongsa, U.; Theerakulpisut, S.; Hiziroglu, S.; Okhawilai, M. Multi Response Optimization of Bioactive Starch Foam Composite Using Taguchi’s Method and Grey Relational Analysis. J. Polym. Environ. 2020, 28, 1513–1525. [Google Scholar] [CrossRef]
- Ounkaew, A.; Kasemsiri, P.; Pongsa, U.; Hiziroglu, S.; Pasuwan, P.; Boonlai, Y.; Theerakulpisut, S. Multiple Response Optimization of Poly(vinyl alcohol)/Starch Based Bioactive Composite Films for Antimicrobial Packaging Applications. J. Polym. Environ. 2022, 30, 1787–1802. [Google Scholar] [CrossRef]
- Shafiee, S.; Ahangar, H.A.; Saffar, A. Taguchi method optimization for synthesis of Fe3O4 @chitosan/Tragacanth Gum nanocomposite as a drug delivery system. Carbohydr. Polym. 2019, 222, 114982. [Google Scholar] [CrossRef]
- Tantiwatcharothai, S.; Prachayawarakorn, J. Characterization of an antibacterial wound dressing from basil seed (Ocimum basilicum L.) mucilage-ZnO nanocomposite. Int. J. Biol. Macromol. 2019, 135, 133–140. [Google Scholar] [CrossRef]
- Glukhova, S.A.; Molchanov, V.S.; Chesnokov, Y.M.; Lokshin, B.V.; Kharitonova, E.P.; Philippova, O.E. Green nanocomposite gels based on binary network of sodium alginate and percolating halloysite clay nanotubes for 3D printing. Carbohydr. Polym. 2022, 282, 119106. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Javanbakht, S.; Nia, S.B.; Namazi, H. Carboxymethyl cellulose/mesoporous magnetic graphene oxide as a safe and sustained ibuprofen delivery bio-system: Synthesis, characterization, and study of drug release kinetic. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124662. [Google Scholar] [CrossRef]
- Amini-Fazl, M.S.; Mohammadi, R.; Kheiri, K. 5-Fluorouracil loaded chitosan/polyacrylic acid/Fe3O4 magnetic nanocomposite hydrogel as a potential anticancer drug delivery system. Int. J. Biol. Macromol. 2019, 132, 506–513. [Google Scholar] [CrossRef]
- Kurd, F.; Fathi, M.; Shekarchizadeh, H. Basil seed mucilage as a new source for electrospinning: Production and physicochemical characterization. Int. J. Biol. Macromol. 2017, 95, 689–695. [Google Scholar] [CrossRef]
- Ounkaew, A.; Janaum, N.; Kasemsiri, P.; Okhawilai, M.; Hiziroglu, S.; Chindaprasirt, P. Synergistic effect of starch/polyvinyl alcohol/citric acid films decorated with in-situ green-synthesized nano silver on bioactive packaging films. J. Environ. Chem. Eng. 2021, 9, 106793. [Google Scholar] [CrossRef]
- Ray, S.; Banerjee, S.; Maiti, S.; Laha, B.; Barik, S.; Sa, B.; Bhattacharyya, U.K. Novel interpenetrating network microspheres of xanthan gum–poly(vinyl alcohol) for the delivery of diclofenac sodium to the intestine—In vitro and in vivo evaluation. Drug Deliv. 2010, 17, 508–519. [Google Scholar] [CrossRef] [Green Version]
- Bi, L.; Chen, Z.; Li, L.; Kang, J.; Zhao, S.; Wang, B.; Yan, P.; Li, Y.; Zhang, X.; Shen, J. Selective adsorption and enhanced photodegradation of diclofenac in water by molecularly imprinted TiO2. J. Hazard. Mater. 2021, 407, 124759. [Google Scholar] [CrossRef]
- Pan, X.; Cheng, S.; Su, T.; Zuo, G.; Zhao, W.; Qi, X.; Wei, W.; Dong, W. Fenton-like catalyst Fe3O4@polydopamine-MnO2 for enhancing removal of methylene blue in wastewater. Colloids Surf. B Biointerfaces 2019, 181, 226–233. [Google Scholar] [CrossRef]
- Javanbakht, S.; Shadi, M.; Mohammadian, R.; Shaabani, A.; Ghorbani, M.; Rabiee, G.; Amini, M.M. Preparation of Fe3O4@SiO2@Tannic acid double core-shell magnetic nanoparticles via the Ugi multicomponent reaction strategy as a pH-responsive co-delivery of doxorubicin and methotrexate. Mater. Chem. Phys. 2020, 247, 122857. [Google Scholar] [CrossRef]
- Yang, L.; Tian, J.; Meng, J.; Zhao, R.; Li, C.; Ma, J.; Jin, T. Modification and Characterization of Fe3O4 Nanoparticles for Use in Adsorption of Alkaloids. Molecules 2018, 23, 562. [Google Scholar] [CrossRef] [Green Version]
- Tantiwatcharothai, S.; Prachayawarakorn, J. Property improvement of antibacterial wound dressing from basil seed (O. basilicum L.) mucilage- ZnO nanocomposite by borax crosslinking. Carbohydr. Polym. 2020, 227, 115360. [Google Scholar] [CrossRef]
- Reddy, S.G.; Pandit, A.S. Biodegradable sodium alginate and lignosulphonic acid blends: Characterization and swelling studies. Polimeros 2013, 23, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Mahdavinia, G.R.; Rahmani, Z.; Karami, S.; Pourjavadi, A. Magnetic/pH-sensitive κ-carrageenan/sodium alginate hydrogel nanocomposite beads: Preparation, swelling behavior, and drug delivery. J. Biomater. Sci. Polym. Ed. 2014, 25, 1891–1906. [Google Scholar] [CrossRef]
- Chen, S.-C.; Wu, Y.-C.; Mi, F.-L.; Lin, Y.-H.; Yu, L.-C.; Sung, H.-W. A novel pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J. Control. Release 2004, 96, 285–300. [Google Scholar] [CrossRef]
- Ecke, A.; Westphalen, T.; Hornung, J.; Voetz, M.; Schneider, R.J. A rapid magnetic bead-based immunoassay for sensitive determination of diclofenac. Anal. Bioanal. Chem. 2022, 414, 1563–1573. [Google Scholar] [CrossRef]
- Cong, Z.; Shi, Y.; Wang, Y.; Wang, Y.; Niu, J.; Chen, N.; Xue, H. A novel controlled drug delivery system based on alginate hydrogel/chitosan micelle composites. Int. J. Biol. Macromol. 2018, 107, 855–864. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, Y.; Lang, M. Micelles/sodium-alginate composite gel beads: A new matrix for oral drug delivery of indomethacin. Carbohydr. Polym. 2012, 87, 790–798. [Google Scholar] [CrossRef]
- Yin, Z.-C.; Wang, Y.-L.; Wang, K. A pH-responsive composite hydrogel beads based on agar and alginate for oral drug delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 12–18. [Google Scholar] [CrossRef]
- Xie, C.-X.; Tian, T.-C.; Yu, S.-T.; Li, L. pH-sensitive hydrogel based on carboxymethyl chitosan/sodium alginate and its application for drug delivery. J. Appl. Polym. Sci. 2019, 136, 46911. [Google Scholar] [CrossRef]
- Naderi, Z.; Azizian, J.; Moniri, E.; Farhadyar, N. Synthesis and Characterization of Carboxymethyl Cellulose/β-Cyclodextrin/Chitosan Hydrogels and Investigating the Effect of Magnetic Nanoparticles (Fe3O4) on a Novel Carrier for a Controlled Release of Methotrexate as Drug Delivery. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1339–1351. [Google Scholar] [CrossRef]
- Omer, A.M.; Ahmed, M.S.; El-Subruiti, G.M.; Khalifa, R.E.; Eltaweil, A.S. pH-Sensitive Alginate/Carboxymethyl Chitosan/Aminated Chitosan Microcapsules for Efficient Encapsulation and Delivery of Diclofenac Sodium. Pharmer 2021, 13, 338. [Google Scholar] [CrossRef]
- Guo, T.; Pei, Y.; Tang, K.; He, X.; Huang, J.; Wang, F. Mechanical and drug release properties of alginate beads reinforced with cellulose. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Calderón Bravo, H.; Vera Céspedes, N.; Zura-Bravo, L.; Muñoz, L.A. Basil Seeds as a Novel Food, Source of Nutrients and Functional Ingredients with Beneficial Properties: A Review. Foods 2021, 10, 1467. [Google Scholar] [CrossRef]
- Pandya, V.J.; Rathod, P.P. Optimization of Mechanical Properties of Green Composites by Gray Relational Analysis. Mater. Today Proc. 2020, 27, 19–22. [Google Scholar] [CrossRef]
- Tan, L.S.; Tan, H.L.; Deekonda, K.; Wong, Y.Y.; Muniyandy, S.; Hashim, K.; Pushpamalar, J. Fabrication of radiation cross-linked diclofenac sodium loaded carboxymethyl sago pulp/chitosan hydrogel for enteric and sustained drug delivery. Carbohydr. Polym. Technol. Appl. 2021, 2, 100084. [Google Scholar] [CrossRef]
- Suhail, M.; Khan, A.; Rosenholm, J.M.; Minhas, M.U.; Wu, P.C. Fabrication and characterization of diclofenac sodium loaded hydrogels of sodium alginate as sustained release carrier. Gels 2021, 7, 10. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Luan, Y.; Liu, W.; Chen, T.; Liu, P.; Liu, Z. Preparation of pH-responsive cellulose nanofibril/sodium alginate based hydrogels for drug release. J. Appl. Polym. Sci. 2022, 139, 51647. [Google Scholar] [CrossRef]
- Sadri, M.; Mohammadi, A.; Hosseini, H. Drug release rate and kinetic investigation of composite polymeric nanofibers. Nanomed. Res. J. 2016, 1, 112–121. [Google Scholar]
- Nawaz, S.; Khan, S.; Farooq, U.; Haider, M.S.; Ranjha, N.M.; Rasul, A.; Nawaz, A.; Arshad, N.; Hameed, R. Biocompatible hydrogels for the controlled delivery of anti-hypertensive agent: Development, characterization and in vitro evaluation. Des. Monomers Polym. 2018, 21, 18–32. [Google Scholar] [CrossRef] [Green Version]
- Suhail, M.; Vu, Q.L.; Wu, P.-C. Formulation, Characterization, and In Vitro Drug Release Study of β-Cyclodextrin-Based Smart Hydrogels. Gels 2022, 8, 207. [Google Scholar] [CrossRef]
- Anwar, H.; Ahmad, M.; Minhas, M.U.; Rehmani, S. Alginate-polyvinyl alcohol based interpenetrating polymer network for prolonged drug therapy, Optimization and in-vitro characterization. Carbohydr. Polym. 2017, 166, 183–194. [Google Scholar] [CrossRef]
- Khan, M.A.; Azad, A.K.; Safdar, M.; Nawaz, A.; Akhlaq, M.; Paul, P.; Hossain, M.K.; Rahman, M.H.; Baty, R.S.; El-kott, A.F.; et al. Synthesis and Characterization of Acrylamide/Acrylic Acid Co-Polymers and Glutaraldehyde Crosslinked pH-Sensitive Hydrogels. Gels 2022, 8, 47. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Dharmalingam, K.; Anandalakshmi, R. Fabrication, characterization and drug loading efficiency of citric acid crosslinked NaCMC-HPMC hydrogel films for wound healing drug delivery applications. Int. J. Biol. Macromol. 2019, 134, 815–829. [Google Scholar] [CrossRef]
- Kasemsiri, P.; Dulsang, N.; Pongsa, U.; Hiziroglu, S.; Chindaprasirt, P. Optimization of Biodegradable Foam Composites from Cassava Starch, Oil Palm Fiber, Chitosan and Palm Oil Using Taguchi Method and Grey Relational Analysis. J. Polym. Environ. 2017, 25, 378–390. [Google Scholar] [CrossRef]
- Arifeen, W.U.; Kim, M.; Choi, J.; Yoo, K.; Kurniawan, R.; Ko, T.J. Optimization of porosity and tensile strength of electrospun polyacrylonitrile nanofibrous membranes. Mater. Chem. Phys. 2019, 229, 310–318. [Google Scholar] [CrossRef]


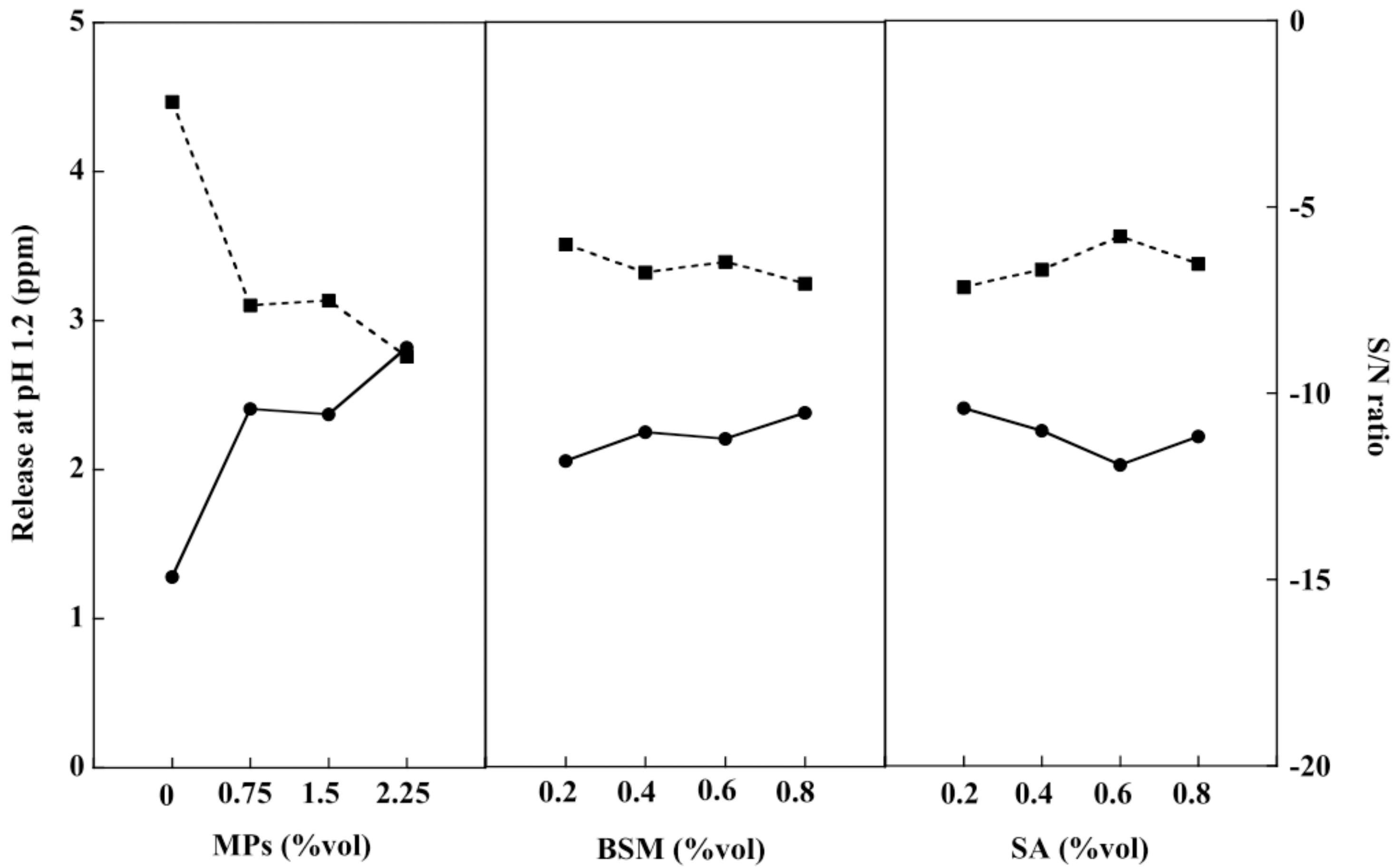
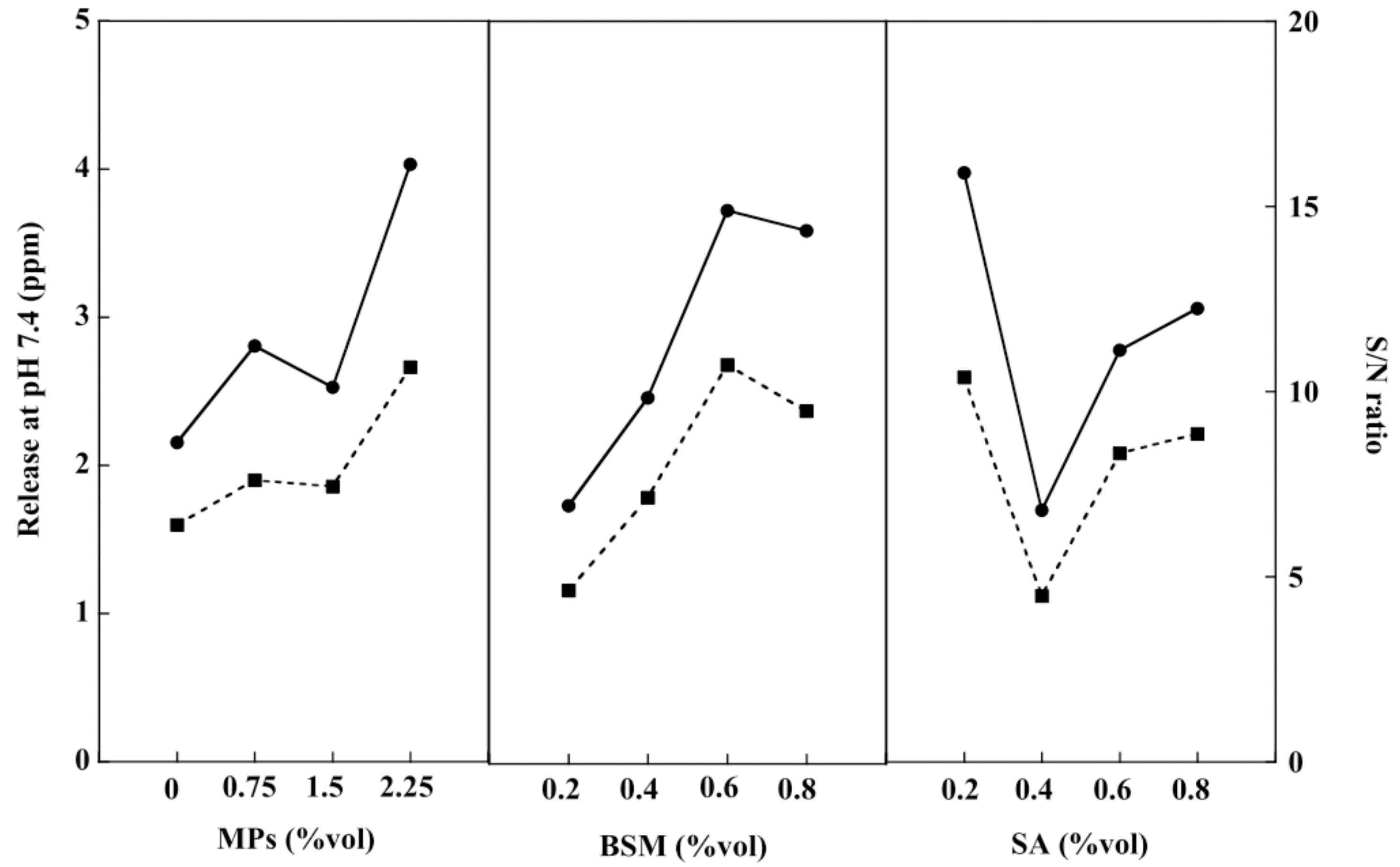
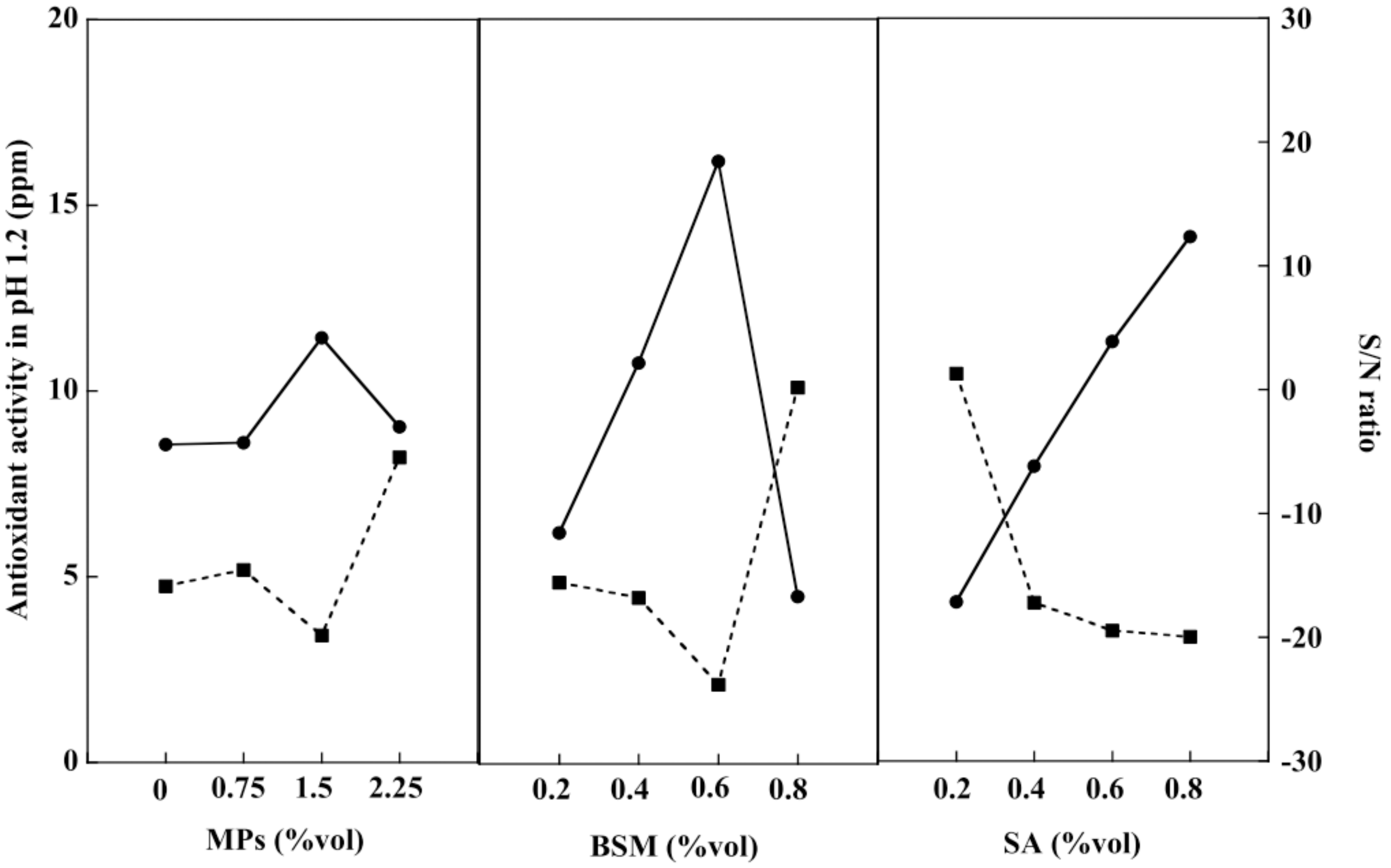
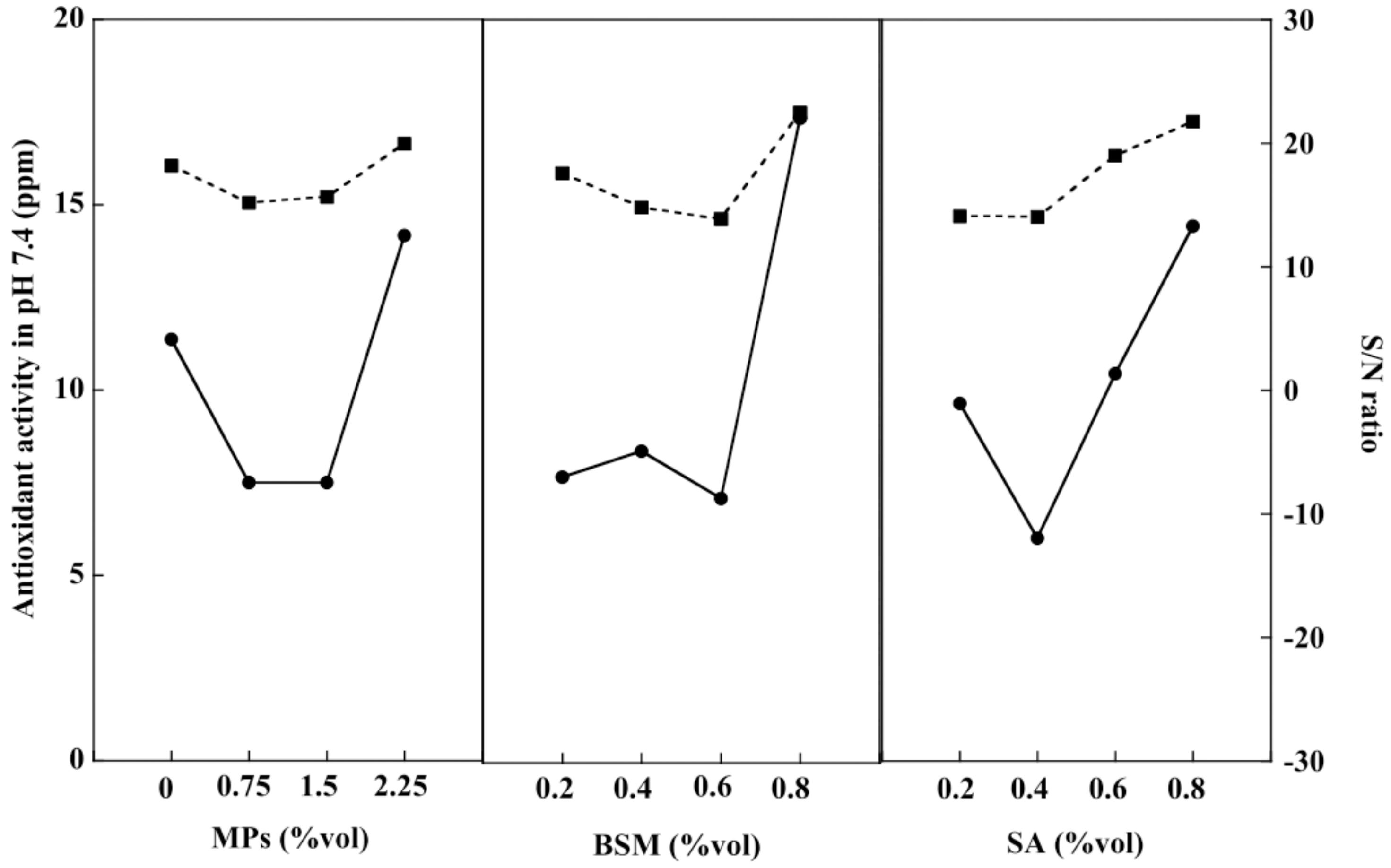
| Factor | DF a | SS b | MS c | F-Value | p-Value | Contribution (%) |
|---|---|---|---|---|---|---|
| Swelling | ||||||
| MPs | 3 | 87,231.00 | 29,077 | 5.97 | 0.031 | 15.33 |
| BSM | 3 | 387,831.00 | 129,277 | 26.56 | 0.001 | 68.17 |
| SA | 3 | 64,681.00 | 21,560 | 4.43 | 0.058 | 11.37 |
| Error | 6 | 29,200.00 | 4867 | - | - | 5.13 |
| Total | 15 | 568,944.00 | - | - | - | 100.00 |
| Drug loading | ||||||
| MPs | 3 | 0.0014 | 0.0005 | 0.29 | 0.830 | 3.31 |
| BSM | 3 | 0.0051 | 0.0017 | 1.07 | 0.429 | 12.14 |
| SA | 3 | 0.0259 | 0.0086 | 5.47 | 0.038 | 61.91 |
| Error | 6 | 0.0095 | 0.0016 | - | - | 22.64 |
| Total | 15 | 0.0418 | - | - | - | 100.00 |
| Release rate at pH 1.2 | ||||||
| MPs | 3 | 5.2066 | 1.7355 | 49.41 | 0.000 | 87.95 |
| BSM | 3 | 0.2119 | 0.0706 | 2.01 | 0.214 | 3.58 |
| SA | 3 | 0.2906 | 0.0969 | 2.76 | 0.134 | 4.91 |
| Error | 6 | 0.2108 | 0.0351 | - | - | 3.56 |
| Total | 15 | 5.9199 | - | - | - | 100.00 |
| Release rate at pH 7.4 | ||||||
| MPs | 3 | 7.9350 | 2.6450 | 0.86 | 0.513 | 16.58 |
| BSM | 3 | 10.8010 | 3.6000 | 1.16 | 0.398 | 22.56 |
| SA | 3 | 10.5900 | 3.5300 | 1.14 | 0.405 | 22.12 |
| Error | 6 | 18.5460 | 3.0910 | - | - | 38.74 |
| Total | 15 | 47.8720 | - | - | - | 100.00 |
| Antioxidant activity at pH 1.2 | ||||||
| MPs | 3 | 22.4500 | 7.4850 | 0.20 | 0.894 | 2.83 |
| BSM | 3 | 328.2000 | 109.4010 | 2.89 | 0.125 | 41.31 |
| SA | 3 | 216.6000 | 72.2010 | 1.91 | 0.230 | 27.26 |
| Error | 6 | 227.2300 | 37.8710 | - | - | 28.60 |
| Total | 15 | 794.4800 | - | - | - | 100.00 |
| Antioxidant activity at pH 7.4 | ||||||
| MPs | 3 | 126.9000 | 42.3000 | 0.510 | 0.690 | 12.10 |
| BSM | 3 | 280.3000 | 93.4200 | 1.12 | 0.411 | 26.73 |
| SA | 3 | 143.0000 | 47.6700 | 0.57 | 0.653 | 13.63 |
| Error | 6 | 498.7000 | 83.1100 | - | - | 47.55 |
| Total | 15 | 1048.8000 | - | - | - | 100.00 |
| Predicted | Experiment | |
|---|---|---|
| Grey relational grade | 0.720 | 0.584 |
| Models | Parameters | PBS at pH 7.4 |
|---|---|---|
| Zero order | k0 | 387.400 ± 0.092 |
| R2 | 0.957 ± 0.076 | |
| First order | k1 | 1.106 ± 0.081 |
| R2 | 0.783 ± 0.109 | |
| Higuchi | kH | 0.495 ± 0.095 |
| R2 | 0.875 ± 0.103 | |
| Kormeyer-Peppas | kKP | 0.215 ± 0.087 |
| n | 0.207 ± 0.098 | |
| R2 | 0.989 ± 0.097 |
| Parameter | Level 1 | Level 2 | Level 3 | Level 4 |
|---|---|---|---|---|
| BSM (%vol) | 0.2 | 0.4 | 0.6 | 0.8 |
| SA (%vol) | 0.2 | 0.4 | 0.6 | 0.8 |
| MP (%vol) | 0.0 | 0.75 | 1.50 | 2.25 |
| No. | Composition (%vol) | ||
|---|---|---|---|
| BSM | SA | MPs | |
| 1 | 0.2 | 0.2 | 0.0 |
| 2 | 0.4 | 0.4 | 0.0 |
| 3 | 0.6 | 0.6 | 0.0 |
| 4 | 0.8 | 0.8 | 0.0 |
| 5 | 0.4 | 0.2 | 0.75 |
| 6 | 0.2 | 0.4 | 0.75 |
| 7 | 0.8 | 0.6 | 0.75 |
| 8 | 0.6 | 0.8 | 0.75 |
| 9 | 0.6 | 0.2 | 1.50 |
| 10 | 0.8 | 0.4 | 1.50 |
| 11 | 0.2 | 0.6 | 1.50 |
| 12 | 0.4 | 0.8 | 1.50 |
| 13 | 0.8 | 0.2 | 2.25 |
| 14 | 0.6 | 0.4 | 2.25 |
| 15 | 0.4 | 0.6 | 2.25 |
| 16 | 0.2 | 0.8 | 2.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srikhao, N.; Chirochrapas, K.; Kwansanei, N.; Kasemsiri, P.; Ounkaew, A.; Okhawilai, M.; Likitaporn, C.; Theerakulpisut, S.; Uyama, H. Multi-Responsive Optimization of Novel pH-Sensitive Hydrogel Beads Based on Basil Seed Mucilage, Alginate, and Magnetic Particles. Gels 2022, 8, 274. https://doi.org/10.3390/gels8050274
Srikhao N, Chirochrapas K, Kwansanei N, Kasemsiri P, Ounkaew A, Okhawilai M, Likitaporn C, Theerakulpisut S, Uyama H. Multi-Responsive Optimization of Novel pH-Sensitive Hydrogel Beads Based on Basil Seed Mucilage, Alginate, and Magnetic Particles. Gels. 2022; 8(5):274. https://doi.org/10.3390/gels8050274
Chicago/Turabian StyleSrikhao, Natwat, Korrapat Chirochrapas, Nessaraporn Kwansanei, Pornnapa Kasemsiri, Artjima Ounkaew, Manunya Okhawilai, Chutiwat Likitaporn, Somnuk Theerakulpisut, and Hiroshi Uyama. 2022. "Multi-Responsive Optimization of Novel pH-Sensitive Hydrogel Beads Based on Basil Seed Mucilage, Alginate, and Magnetic Particles" Gels 8, no. 5: 274. https://doi.org/10.3390/gels8050274