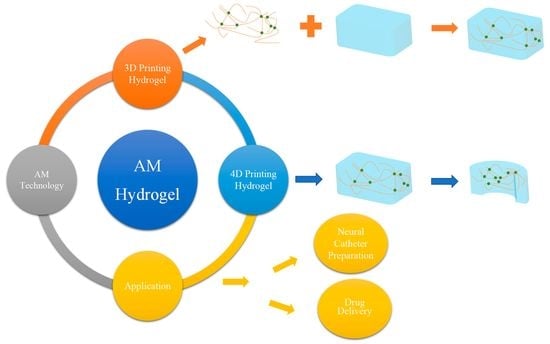
Application and Prospects of Hydrogel Additive Manufacturing
Abstract
:1. Introduction
2. Additive Manufacturing Technologies for Hydrogels
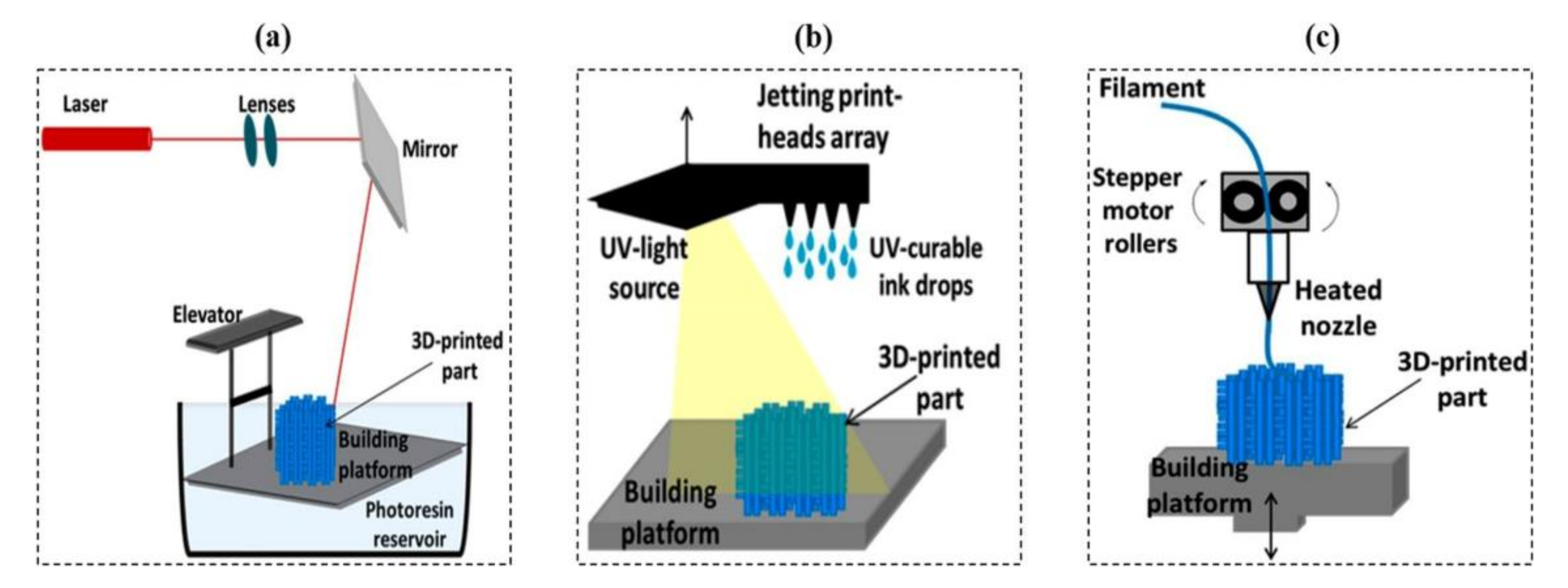
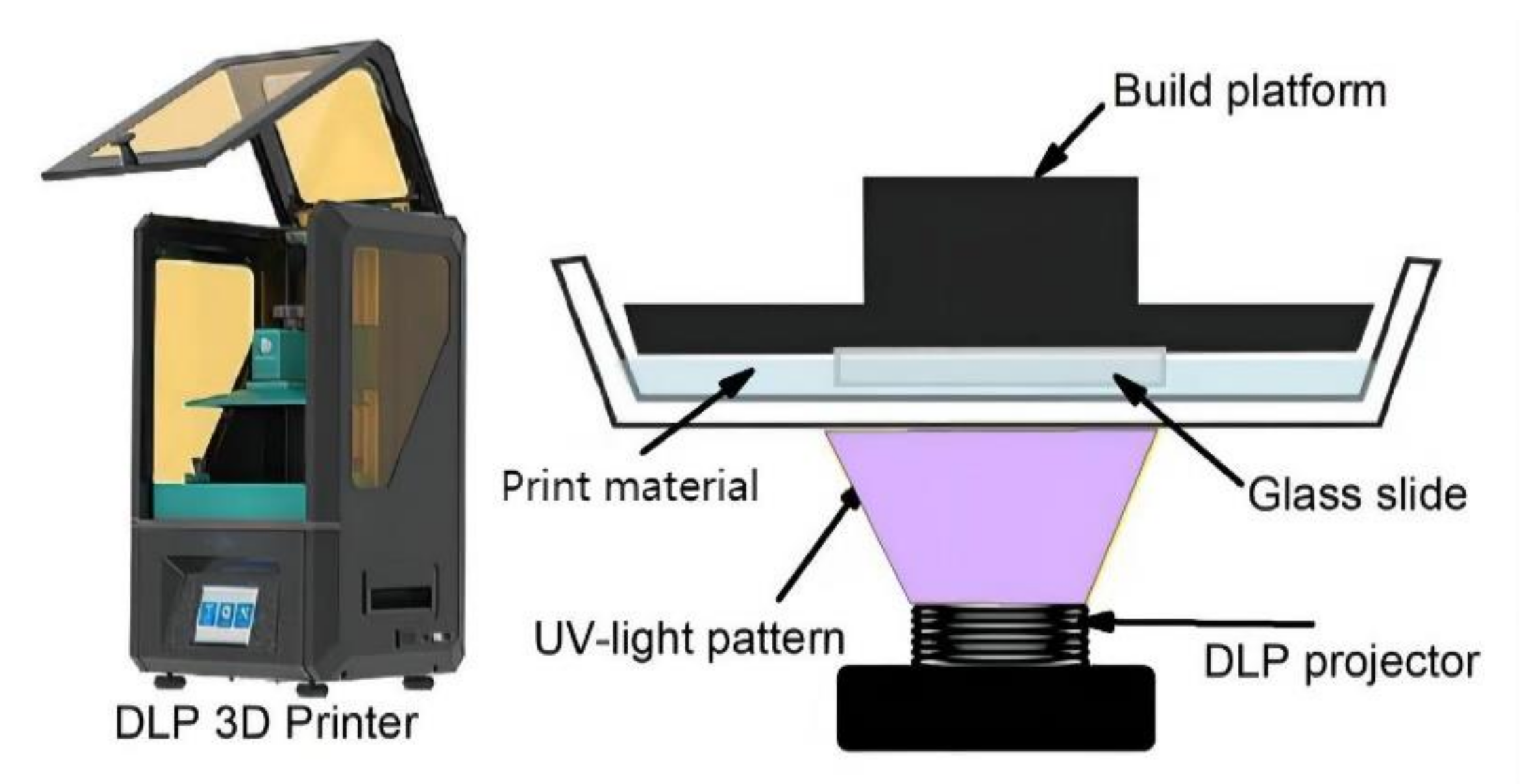
2.1. 3D Printing
| Process Category | Examples of Technique | Type of Raw Materials | Form of Raw Materials | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Material extrusion |
|
|
|
|
|
| Material jetting |
|
|
|
|
|
| Vat polymerization |
|
|
|
|
|
| Powder bed fusion |
|
|
|
|
|
| Binder jetting |
|
|
|
|
|
| Sheet lamination |
|
|
|
|
|
| Directed energy deposition |
|
|
|
|
|
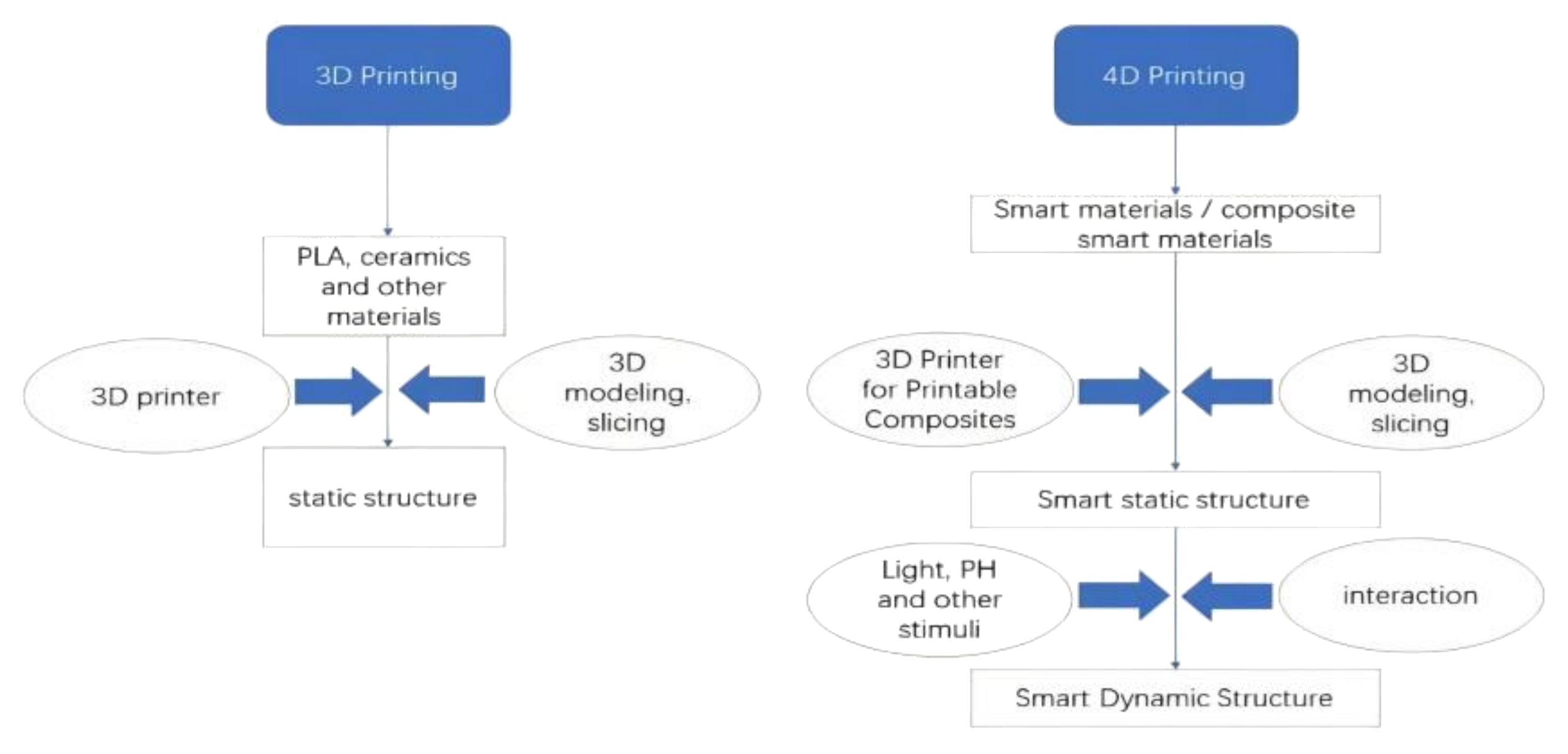
2.2. 4D Printing
2.3. Comparative Analysis of 3D Hydrogel Printing, 4D Hydrogel Printing, and SMP-Based 4D Printing
3. Smart Hydrogels
3.1. Stimulation C;assification of Stimuli-Responsive Hydrogels
3.1.1. Temperature
3.1.2. Magnetic Hydrogels
3.1.3. pH Value
3.1.4. Electricity
3.1.5. Light
3.2. Corresponding Advantages and Disadvantages of Each Type of Stimulus
3.3. Non-Responsive Hydrogel Deswelling/Swelling Deformation
3.4. Reversibility of the Different Kinds of Smart Hydrogels
4. Application of Hydrogel Additive Manufacturing
4.1. Additive Manufacturing Combined with Hydrogels in the Direction of Nerve Catheter Preparation
4.1.1. Design Concept and Characteristics of 3D-Printed Neural Catheters
4.1.2. Preparation and Application of 3D-printed Nerve Conduits
4.2. Drug Delivery
4.3. Research Potential of Smart Hydrogels
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lewis, J.A.; Ahn, B.Y. Device fabrication: Three-dimensional printed electronics. Nature 2015, 518, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Wei, T.S.; Ahn, B.Y.; Jung, Y.S.; Shen, J.D.; Jennifer, A.L. 3D printing of interdigitated Li-ion microbattery architectures. Adv. Mater. 2013, 25, 4539–4543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Mannoor, M.S.; Jiang, Z.; James, T.; Kong, Y.L.; Malatesta, K.A.; Soboyejo, W.O.; Naveen, V.; David H., G.; Michael, C.M. 3D printed bionic ears. Nano Lett. 2013, 13, 2634–2639. [Google Scholar] [CrossRef] [Green Version]
- Jonusauskas, L.; Gailevicius, D.; Mikoliunaite, L.; Sakalauskas, D.; Šakirzanovas, S.; Juodkazis, S.; Malinauskas, M. Optically Clear and Resilient Free-Form micro-Optics 3D-Printed via Ultrafast Laser Lithography. Materials 2017, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Anzalone, N.C.; Faria, R.P.; Pearce, J.M. Open-source 3D-printable optics equipment. PLoS ONE 2013, 8, e59840. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.C.; Chang, K.P.; Wu, P.H.; Lin, C.C.; Chuang, C.S.; Tsau, F.H. 3D Printing Optical Engine for Controlling Material Microstructure. Phys. Procedia 2016, 83, 847–853. [Google Scholar] [CrossRef] [Green Version]
- Tibbits, S. 4D printing: Multi-material shape change. Archit. Des. 2014, 84, 116–121. [Google Scholar] [CrossRef]
- Huang, W.M.; Yang, B.; An, L.; Li, C.; Chan, Y.S. Water-driven programmable polyurethane shape memory polymer: Demonstration and mechanism. Appl. Phys. Lett. 2005, 86, 114105. [Google Scholar] [CrossRef]
- Rose, A.; Zhu, Z.; Madigan, C.F.; Swager, T.M.; Bulović, V. Sensitivity gains in chemosensing by lasing action in organic polymers. Nature 2005, 434, 876–879. [Google Scholar] [CrossRef]
- Bakarich, S.E.; Gorkin, R.; Panhuis, M.I.H.; Spinks, G.M. 4D Printing with Mechanically Robust, Thermally Actuating Hydrogels. Macromol. Rapid Commun. 2015, 36, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Liu, Y.; Lv, H.; Wang, X.; Leng, J.; Du, S. Fiber reinforced shape-memory polymer composite and its application in a deployable hinge. Smart Mater. Struct. 2009, 18, 024002. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lv, H.; Lan, X.; Leng, J.; Du, S. Review of electro-active shape-memory polymer composite. Compos. Sci. Technol. 2009, 69, 2064–2068. [Google Scholar] [CrossRef]
- Mohr, R.; Kratz, K.; Weigel, T.; Lucka-Gabor, M.; Moneke, M.; Lendlein, A. Initiation of shapememory effect by inductive heating of magnetic nanoparticles in thermoplastic polymers. Proc. Natl. Acad. Sci. USA 2006, 103, 3540–3545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadgorny, M.; Xiao, Z.; Chen, C.; Connal, L.A. Three-Dimensional Printing of pH-Responsive and Functional Polymers on an Affordable Desktop Printer. ACS Appl. Mater. Interfaces 2016, 8, 28946–28954. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Li, G. A review of stimuli-responsive shape memory polymer composites. Polymer 2013, 54, 2199–2221. [Google Scholar] [CrossRef] [Green Version]
- Truby, R.L.; Lewis, J.A. Printing soft matter in three dimensions. Nature 2016, 540, 371–378. [Google Scholar] [CrossRef]
- Wu, H.; Li, D.; Tang, Y.; Sun, B.; Xu, D. Rapid fabrication of alumina-based ceramic cores for gas turbine blades by stereolithography and gelcasting. J. Mater. Processing Technol. 2009, 209, 5886–5891. [Google Scholar] [CrossRef]
- ISO/ASTM52900; Additive Manufacturing—General Principles—Terminology. F42 Committee; ASTM International: West Conshohocken, PA, USA, 2015.
- Invernizzi, M.; Turri, S.; Levi, M.; Suriano, R. 4D printed thermally activated self-healing and shape memory polycaprolactone-based polymers. Eur. Polym. J. 2018, 101, 169–176. [Google Scholar] [CrossRef]
- Ge, L.; Dong, L.; Wang, D.; Ge, Q.; Gu, G. A digital light processing 3D printer for fast and high-precision fabrication of soft pneumatic actuators. Sens. Actuators A Phys. 2018, 273, 285–292. [Google Scholar] [CrossRef]
- Lewis, J.A. Direct Ink Writing of 3D Functional Materials. Adv. Funct. Mater. 2006, 16, 2193–2204. [Google Scholar] [CrossRef]
- Joshi, S.; Rawat, K.; Karunakaran, C.; Rajamohan, V.; Mathew, A.T.; Koziol, K.; Thakur, V.K.; Balan, A.S. 4D printing of materials for the future: Opportunities and challenges. Appl. Mater. Today 2020, 18, 100490. [Google Scholar] [CrossRef]
- Gao, B.; Yang, Q.; Zhao, X.; Jin, G.; Ma, Y.; Xu, F. 4D Bioprinting for Biomedical Applications. Trends Biotechnol. 2016, 34, 746–756. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, F.; Liu, Y.; Leng, J. 4D printed shape memory polymers and their structures for biomedical applications. Sci. China Technol. Sci. 2020, 63, 545–560. [Google Scholar] [CrossRef] [Green Version]
- Kuang, X.; Chen, K.; Dunn, C.K.; Wu, J.; Li, V.C.; Qi, H.J. 3D Printing of Highly Stretchable, Shape-Memory, and Self-Healing Elastomer toward Novel 4D Printing. ACS Appl. Mater. Interfaces 2018, 10, 7381–7388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.L.; Cheng, X.D.; Zhang, Y.J.; Chen, M.; Chen, H.; Yang, Y.; Fang, D. Weather-Manipulated Smart Broadband Electromagnetic Metamaterials. ACS Appl. Mater. Interfaces 2018, 10, 40815–40823. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhang, Y.; Wang, M.; Liang, Y.; Ren, L.; Ren, L. Recent progress in 4D printing of stimuli-responsive polymeric materials. Sci. China Technol. Sci. 2019, 63, 532–544. [Google Scholar] [CrossRef]
- Khoo, Z.X.; Teoh, J.E.M.; Liu, Y.; Chua, C.K.; Yang, S.; An, J.; Yeong, W.Y. 3D printing of smart materials: A review on recent progresses in 4D printing. Virtual Phys. Prototyp. 2015, 10, 103–122. [Google Scholar] [CrossRef]
- Sun, L.; Huang, W.M.; Ding, Z.; Zhao, Y.; Wang, C.C.; Purnawali, H.; Tang, C. Stimulus-responsive shape memory materials: A review. Mater. Des. 2012, 33, 577–640. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A.J.S. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Tobushi, H.; Hara, H.; Yamada, E.; Hayashi, S. Thermomechanical properties in a thin film of shape memory polymer of polyurethane series. Smart Mater. Struct. 1996, 5, 483. [Google Scholar] [CrossRef]
- Liu, Y.; Han, C.; Tan, H.; Du, X. Thermal, mechanical and shape memory properties of shape memory epoxy resin. Mater. Sci. Eng. A 2010, 527, 2510–2514. [Google Scholar] [CrossRef]
- Brenken, B.; Barocio, E.; Favaloro, A.; Kunc, V.; Pipes, R.B. Fused filament fabrication of fiber-reinforced polymers: A review. Addit. Manuf. 2018, 21, 1–16. [Google Scholar] [CrossRef]
- Yuan, C.; Kowsari, K.; Panjwani, S.; Chen, Z.; Wang, D.; Zhang, B.; Ge, Q. Ultrafast three-dimensional printing of optically smooth microlens arrays by oscillation-assisted digital light processing. ACS Appl. Mater. Interfaces 2019, 11, 40662–40668. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Habib, F.N.; Iovenitti, P.; Masood, S.H.; Nikzad, M. Fabrication of polymeric lattice structures for optimum energy absorption using Multi Jet Fusion technology. Mater. Des. 2018, 155, 86–98. [Google Scholar] [CrossRef]
- Terrani, K.A.; Jolly, B.C.; Trammell, M.P.; Vasudevamurthy, G.; Schappel, D.; Ade, B.; Nelson, A.T. Architecture and properties of TCR fuel form. J. Nuclear Mater. 2021, 547, 152781. [Google Scholar] [CrossRef]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels 2022, 8, 179. [Google Scholar] [CrossRef]
- Liu, Y.J.; Li, S.J.; Zhang, L.C.; Hao, Y.L.; Sercombe, T.B. Early plastic deformation behaviour and energy absorption in porous β-type biomedical titanium produced by selective laser melting. Scripta Mater. 2018, 153, 99–103. [Google Scholar] [CrossRef]
- Farid, M.I.; Wu, W.; Liu, X.; Wang, P. Additive manufacturing landscape and materials perspective in 4D printing. Int. J. Adv. Manuf. Technol. 2021, 115, 2973–2988. [Google Scholar] [CrossRef]
- ASTM International. Standard Terminology for Additive Manufacturing Technologies; ASTM International: West Conshohocken, PA, USA, 2012; pp. 1–9. [Google Scholar]
- Gonzalez-Ocegueda, P.A.; Herrera-Saucedo, A.; Quiñones-Galvan, J.G.; Valero-Luna, C.; Alvarado-Perea, L.; Canizales-Davalos, L.; Bañuelos-Frias, A. DLP fabrication of TiO2 nanoparticle thin films. Mater. Lett. 2021, 296, 129873. [Google Scholar] [CrossRef]
- Sirrine, J.M.; Meenakshisundaram, V.; Moon, N.G.; Scott, P.J.; Mondschein, R.J.; Weiseman, T.F.; Long, T.E. Functional siloxanes with photo-activated, simultaneous chain extension and crosslinking for lithography-based 3D printing. Polymer 2018, 152, 25–34. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Q.; Yao, Y.; Liu, L.; Liu, Y.; Leng, J. Direct-Write Fabrication of 4D Active Shape-Changing Structures Based on a Shape Memory Polymer and Its Nanocomposite. ACS Appl. Mater. Interfaces 2017, 9, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; DeSimone, J.M. Continuous liquid interface production of 3D objects. Science 2015, 347, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.E.; Bhattacharya, I.; Heidari, H.; Shusteff, M.; Spadaccini, C.M.; Taylor, H.K. Volumetric additive manufacturing via tomographic reconstruction. Science 2019, 363, 1075–1079. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, J.; Mu, X.; Chen, H.; Qi, H.J.; Fang, D. Origami by frontal photopolymerization. Science Adv. 2017, 3, e1602326. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.E.; Moreau, J.E.; Berman, A.M.; McSherry, H.J.; Coburn, J.M.; Schmidt, D.F.; Kaplan, D.L. Shape memory silk protein sponges for minimally invasive tissue regeneration. Adv. Healthcare Mater. 2017, 6, 1600762. [Google Scholar] [CrossRef]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef]
- Kotikian, A.; Truby, R.L.; Boley, J.W.; White, T.J.; Lewis, J. 3D printing of liquid crystal elastomeric actuators with spatially programed nematic order. Adv. Mater. 2018, 30, 1706164. [Google Scholar] [CrossRef]
- Mutlu, R.; Alici, G.; Panhuis, M.; Spinks, G.M. 3D printed flexure hinges for soft monolithic prosthetic fingers. Soft Robotics 2016, 3, 120–133. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Burbine, S.; Kodihalli Shivaprakash, N.; Mead, J. 3D-printable PP/SEBS thermoplastic elastomeric blends, preparation and properties. Polymers 2019, 11, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abang Ismawi Hassim, D.H.; Nik Ismail, N.I.; Sarkawi, S.S.; Ngeow, Y.W.; Ibrahim, S.; Yong, K.C. The feasibility of using ethylene-vinyl acetate/natural rubber (EVA/NR)-based thermoplastic elastomer as filament material in fused deposition modelling (FDM)-3D printing application. J. Rubber Res. 2021, 24, 659–668. [Google Scholar] [CrossRef]
- Sánchez, D.M.; de la Mata, M.; Delgado, F.J.; Casal, V.; Molina, S.I. Development of carbon fiber acrylonitrile styrene acrylate composite for large format additive manufacturing. Mater. Des. 2020, 191, 108577. [Google Scholar] [CrossRef]
- El Magri, A.; Salah Eddine, O.; Vaudreuil, S. Effects of printing parameters on the tensile behavior of 3D-printed acrylonitrile styrene acrylate (ASA) material in Z direction. Polymer Eng. Sci. 2022, 62, 848–860. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites, A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Zhong, W.; Li, F.; Zhang, Z.; Song, L.; Li, Z. Short fiber reinforced composites for fused deposition modeling. Mater. Sci. Eng. A 2001, 301, 125–130. [Google Scholar] [CrossRef]
- Weng, Z.; Wang, J.; Senthil, T.; Wu, L. Mechanical and thermal properties of ABS/montmorillonite nanocomposites for fused deposition modeling 3D printing. Mater. Des. 2016, 102, 276–283. [Google Scholar] [CrossRef]
- Compton, B.G.; Lewis, J.A. 3D-printing of lightweight cellular composites. Adv. Mater. 2014, 26, 5930–5935. [Google Scholar] [CrossRef]
- Hector Sandoval, J.; Wicker, R.B. Functionalizing stereolithography resins, effects of dispersed multi-walled carbon nanotubes on physical properties. Rapid Prototyp. J. 2006, 12, 292–303. [Google Scholar] [CrossRef]
- Krivec, M.; Roshanghias, A.; Abram, A.; Binder, A. Exploiting the combination of 3D polymer printing and inkjet Agnanoparticle printing for advanced packaging. Microelectron. Eng. 2017, 176, 1–5. [Google Scholar] [CrossRef]
- Shao, H.; He, Y.; Fu, J.; He, D.; Yang, X.; Xie, J.; Yao, C.; Ye, J.; Xu, S.; Gou, Z. 3D printing magnesium-doped wollastonite/β-TCP bioceramics scaffolds with high strength and adjustable degradation. J. Eur. Ceram. Soc. 2016, 36, 1495–1503. [Google Scholar] [CrossRef] [Green Version]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Wu, J.J.; Huang, L.M.; Zhao, Q.; Xie, T. 4D printing, history and recent progress. Chinese J. Polym. Sci. 2018, 36, 563–575. [Google Scholar] [CrossRef]
- Choi, J.; Kwon, O.C.; Jo, W.; Lee, H.J.; Moon, M.W. 4D printing technology, a review. 3D Print. Addit. Manuf. 2015, 2, 159–167. [Google Scholar] [CrossRef]
- Du, F.P.; Ye, E.Z.; Yang, W.; Shen, T.H.; Tang, C.Y.; Xie, X.L.; Law, W.C. Electroactive shape memory polymer based on optimized multi-walled carbon nanotubes/polyvinyl alcohol nanocomposites. Compos. Part B 2015, 68, 170–175. [Google Scholar] [CrossRef]
- Akbari, S.; Sakhaei, A.H.; Kowsari, K.; Yang, B.; Serjouei, A.; Yuanfang, Z.; Ge, Q. Enhanced Multimaterial 4D Printing with Active Hinges. Smart Mater. Struct. 2018, 27, 065027. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Lan, X.; Leng, J.; Wu, A.S.; Bryson, T.M.; Chou, T.W. Shape memory behavior and recovery force of 4D printed textile functional composites. Compos. Sci. Technol. 2018, 160, 224–230. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Zhang, F.; Leng, J.; Pei, S.; Wang, L.; Chou, T.W. Microstructural design for enhanced shape memory behavior of 4D printed composites based on carbon nanotube/polylactic acid filament. Compos. Sci. Technol. 2019, 181, 107692. [Google Scholar] [CrossRef]
- Mosiewicz, K.A.; Kolb, L.; Van Der Vlies, A.J.; Martino, M.M.; Lienemann, P.S.; Hubbell, J.A.; Lutolf, M.P. In situ cell manipulation through enzymatic hydrogel photopatterning. Nat. Mater. 2013, 12, 1072–1078. [Google Scholar] [CrossRef]
- Sepantafar, M.; Maheronnaghsh, R.; Mohammadi, H.; Radmanesh, F.; Hasani-Sadrabadi, M.M.; Ebrahimi, M.; Baharvand, H. Engineered hydrogels in cancer therapy and diagnosis. Trends Biotechnol. 2017, 35, 1074–1087. [Google Scholar] [CrossRef]
- Huang, J.; Xiong, J.; Wang, D.; Zhang, J.; Yang, L.; Sun, S.; Liang, Y. 3D bioprinting of hydrogels for cartilage tissue engineering. Gels 2021, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Neerooa, B.N.H.M.; Ooi, L.T.; Shameli, K.; Dahlan, N.A.; Islam, J.M.; Pushpamalar, J.; Teow, S.Y. Development of polymer-assisted nanoparticles and nanogels for cancer therapy, An update. Gels 2021, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Samchenko, Y.; Ulberg, Z.; Korotych, O. Multipurpose smart hydrogel systems. Adv. Colloid Interface Sci. 2011, 168, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Dewi, R.E.; Heilshorn, S.C. Injectable hydrogel with in situ double network formation enhance retention of transplanted stem cells. Adv. Funct. Mater. 2015, 25, 1344–1351. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Le, X.; Tang, X.; He, J.; Xiao, P.; Zheng, J.; Chen, T. A multiresponsive anisotropic hydrogel with macroscopic 3D complex deformations. Adv. Funct. Mater. 2016, 26, 8670–8676. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Ma, J.; Vancso, G.J. Thermoresponsive semi-IPN hydrogel microfibers from continuous fluidic processing with high elasticity and fast actuation. ACS Appl. Mater. Interfaces 2017, 9, 901–908. [Google Scholar] [CrossRef]
- Xiao Jun, W.L.C. Research progress of stimulus-responsive hydrogels for tissue engineering. J. Nanjing Tech. Univer. 2020, 42, 690–699. [Google Scholar]
- Laronda, M.M.; Rutz, A.L.; Xiao, S.; Whelan, K.A.; Duncan, F.E.; Roth, E.W.; Shah, R.N. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun. 2017, 8, 15261. [Google Scholar] [CrossRef]
- Su, M.; Xu, T.; Lai, Z.; Huang, C.; Liu, J.; Wu, X. Double-modal locomotion and application of soft cruciform thin-film microrobot. IEEE Robot. Autom. Lett. 2020, 5, 806–812. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels, theory, modern advances, and applications. Mat. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Heilshorn, S.C. Adaptable hydrogel networks with reversible linkages for tissue engineering. Adv. Mater. 2015, 27, 3717–3736. [Google Scholar] [CrossRef] [PubMed]
- De, S.K.; Aluru, N.R. A chemo-electro-mechanical mathematical model for simulation of pH sensitive hydrogels. Mech. Mater. 2004, 36, 395–410. [Google Scholar] [CrossRef]
- Firestone, B.; Siegel, R.A. Kinetics and mechanisms of water sorption in hydrophobic, ionizable copolymer gels. J. Appl. Polym. Sci. 1991, 43, 901–914. [Google Scholar] [CrossRef]
- Jian, Y.; Wu, B.; Le, X.; Liang, Y.; Zhang, Y.; Zhang, D.; Chen, T. Antifreezing and stretchable organohydrogels as soft actuators. Research 2019, 2019, 2384347. [Google Scholar] [CrossRef] [Green Version]
- Halacheva, S.S.; Freemont, T.J.; Saunders, B. pH-responsive physical gels from poly (meth) acrylic acid-containing crosslinked particles, the relationship between structure and mechanical properties. J. Mater. Chem. B 2013, 1, 4065–4078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, S.; Xin, C.; Zheng, Z.S. The Electric-Field-Sensitive Hydrogels. Prog. Chem. 2007, 19, 1393–1399. [Google Scholar]
- Han, D.; Farino, C.; Yang, C.; Scott, T.; Browe, D.; Choi, W.; Lee, H. Soft robotic manipulation and locomotion with a 3D printed electroactive hydrogel. ACS Appl. Mater. Interfaces 2018, 10, 17512–17518. [Google Scholar] [CrossRef]
- Palza, H.; Zapata, P.A.; Angulo-Pineda, C. Electroactive smart polymers for biomedical applications. Materials 2019, 12, 277. [Google Scholar] [CrossRef] [Green Version]
- Tam, R.Y.; Smith, L.J.; Shoichet, M.S. Engineering cellular microenvironments with photo- and enzymatically responsive hydrogels, toward biomimetic 3D cell culture models. Acc. Chem. Res. 2017, 50, 703–713. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.; Hutmacher, D.W. 25th anniversary article, engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Kuksenok, O.; Balazs, A.C. Stimuli-responsive behavior of composites integrating thermo-responsive gels with photo-responsive fibers. Mater. Horizons 2016, 3, 53–62. [Google Scholar] [CrossRef]
- Wang, Z.J.; Li, C.Y.; Zhao, X.Y.; Wu, Z.L.; Zheng, Q. Thermo- and photo-responsive composite hydrogels with programmed deformations. J. Mater. Chem. B 2019, 7, 1674–1678. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pi, Q.; Maharjan, S.; Yan, X.; Liu, X.; Singh, B.; van Genderen, A.M.; Zhang, Y.S. Digitally tunable microfluidic bioprinting of multilayered cannular tissues. Adv. Mater. 2018, 30, 1706913. [Google Scholar] [CrossRef]
- De Cicco, F.; Reverchon, E.; Adami, R.; Auriemma, G.; Russo, P.; Calabrese, E.C.; Del Gaudio, P. In situ forming antibacterial dextran blend hydrogel for wound dressing, SAA technology vs. spray drying. Carbohydr. Polym. 2014, 101, 1216–1224. [Google Scholar] [CrossRef]
- Rasool, N.; Yasin, T.; Heng, J.Y.; Akhter, Z. Synthesis and characterization of novel pH-, ionic strength and temperature-sensitive hydrogel for insulin delivery. Polymer 2010, 51, 1687–1693. [Google Scholar] [CrossRef]
- Deng, Y.; Dixon, J.B.; White, G.N. Bonding mechanisms and conformation of poly (ethylene oxide)-based surfactants in interlayer of smectite. Colloid Polymer Sci. 2006, 284, 347–356. [Google Scholar] [CrossRef]
- Chen, Z. 3D printing of multi-functional, multi-material hydrogels and their mechanical models. Adv. Funct. Mater. 2019, 29, 11900971. [Google Scholar]
- Van Gemert, G.M.L.; Peeters, J.W.; Sontjens, S.H.; Janssen, H.M.; Bosman, A.W. Self-healing supramolecular polymers in action. Macromol. Chem. Phys. 2012, 213, 234–242. [Google Scholar] [CrossRef]
- Qi, X.; Yao, X.; Deng, S.; Zhou, T.; Fu, Q. Water- induced shape memory effect of graphene oxide reinforced polyvinyl alcohol nanocomposites. J. Mater. Chem. A 2014, 2, 2240–2249. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, D.; Sheiko, S.S. Gooling -triggered shapeshifting hydrogels with multi-shapememory performance. advancedMaterials 2018, 30, 1707461. [Google Scholar]
- Trenor, S.R.; Shultz, A.R.; Love, B.J.; Long, T.E. Coumarins in polymers, from light harvesting to photo-cross-linkable tissue scaffolds. Chem. Rev. 2004, 104, 3059–3077. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; He, M.; Deng, X.Y.; Du, L.; Fan, C.J.; Yang, K.K.; Wang, Y.Z. Design of poly(L-lactide)-poly(ethylene glycol)copolymer with light-induced shape-memory effect triggered by pendant anthracene groups. ACS Appl. Mater. Interfaces 2016, 8, 9431–9439. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Niu, R.; Tang, C.; Xia, J.; Ji, F.; Dong, D.; Yao, F. A dual-crosslinked strategy to construct physical hydrogels with high strength, toughness, good mechanical recoverability, and shape-memory ability. Macromol. Mater. Eng. 2018, 303, 1700396. [Google Scholar] [CrossRef]
- Peng, K.; Yu, H.; Yang, H.; Hao, X.; Yasin, A.; Zhang, X. A mechanically robust hydrogel with thermally induced plasticity and a shape memory effect. Soft Matter. 2017, 13, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Le, X.; Zhang, J.; Huang, Y.; Chen, T. Supramolecular shape memory hydrogels, a new bridge between stimuli-responsive polymers and supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 1284–1294. [Google Scholar] [CrossRef]
- Lu, L.; Tian, T.; Wu, S.; Xiang, T.; Zhou, S. A pH-induced self-healable shape memory hydrogel with metal-coordination cross-links. Polym. Chem. 2019, 10, 1920–1929. [Google Scholar] [CrossRef]
- Norkus, T.; Norkus, M.; Ramanauskas, T.J. Donor, recipient and nerve grafts in brachial plexus reconstruction, anatomical and technical features for facilitating the exposure. Surg. Radiol. Anat. 2005, 27, 524–530. [Google Scholar] [CrossRef]
- Ray, W.Z.; Mackinnon, S.E. Management of nerve gaps, autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp. Neurol. 2010, 223, 77. [Google Scholar] [CrossRef] [Green Version]
- Midha, R. Emerging techniques for nerve repair, nerve transfers and nerve guidance tubes. Clin. Neurosurg. 2006, 53, 185–190. [Google Scholar]
- Joung, D.; Lavoie, N.S.; Guo, S.Z.; Park, S.H.; Parr, A.M.; McAlpine, M.C. 3D printed neural regeneration devices. Adv. Funct. Mater. 2020, 30, 1906237. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Cui, Y.; Zhang, M.; Zhao, D.; Liu, G.; Ding, J. Engineered three-dimensional scaffolds for enhanced bone regeneration in osteonecrosis. Bioactive Mater. 2020, 5, 584–601. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Nectow, A.R.; Marra, K.G.; Kaplan, D.L. Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng. Part B Rev. 2012, 18, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthusamy, S.; Kannan, S.; Lee, M.; Sanjairaj, V.; Lu, W.F.; Fuh, J.Y.; Cao, T. 3D bioprinting and microscale organization of vascularized tissue constructs using collagen- based bioink. Biotechnol. Bioeng. 2021, 118, 3150–3163. [Google Scholar] [CrossRef]
- Johnson, B.N.; Lancaster, K.Z.; Zhen, G.; He, J.; Gupta, M.K.; Kong, Y.L.; McAlpine, M.C. 3D printed anatomical nerve regeneration pathways. Adv. Funct. Mater. 2015, 25, 6205–6217. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, J.; Zou, J.; Yang, X.; Guo, H.; Tian, H.; Chen, X. Electroactive composite scaffold with locally expressed osteoinductive factor for synergistic bone repair upon electrical stimulation. Biomaterials 2020, 230, 119617. [Google Scholar] [CrossRef]
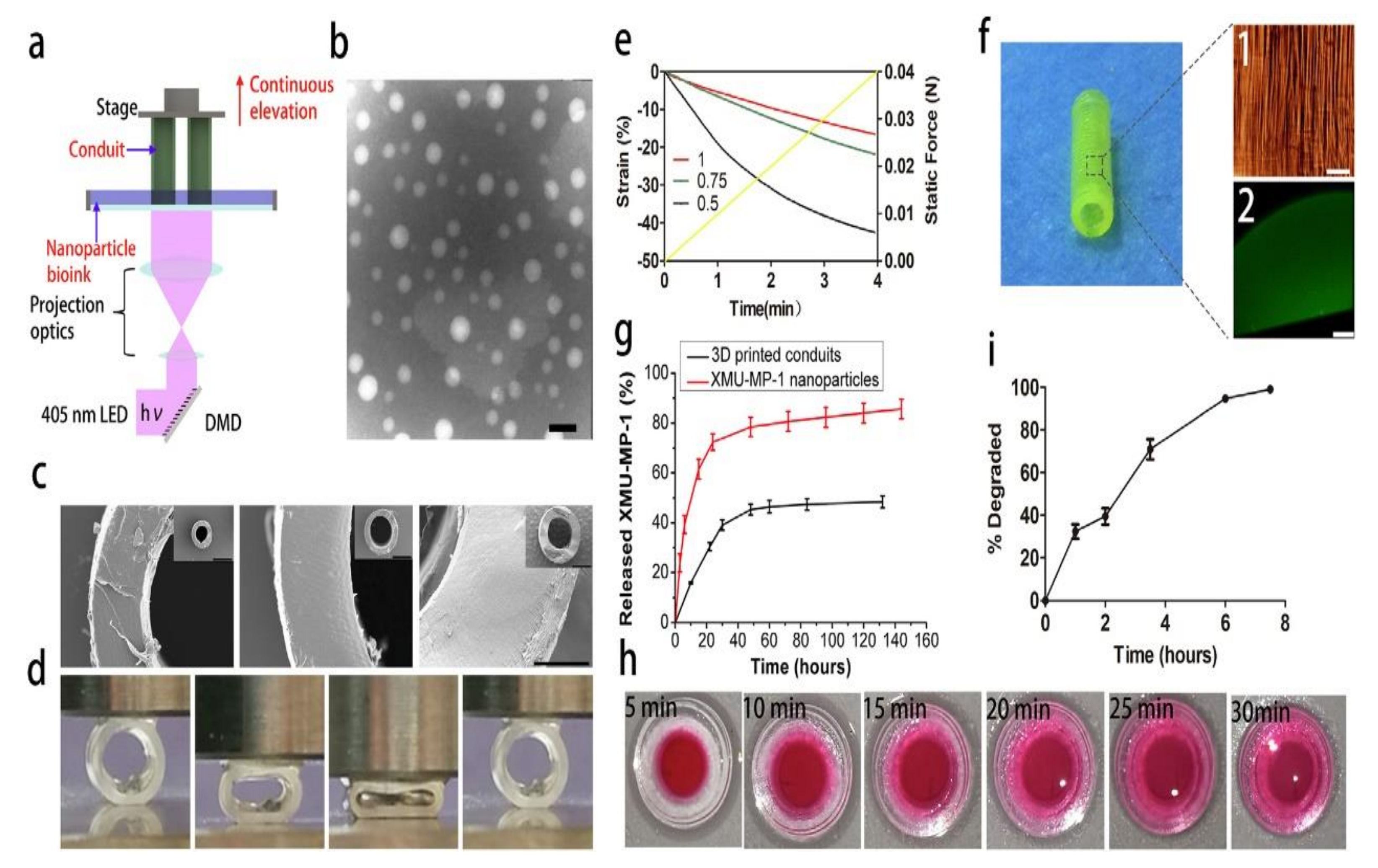
- Zhu, W.; Tringale, K.R.; Woller, S.A.; You, S.; Johnson, S.; Shen, H.; Chen, S. Rapid continuous 3D printing of customizable peripheral nerve guidance conduits. Mater. Today 2018, 21, 951–959. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, Y.; Gou, Z.; Tao, J.; Zhang, J.; Liu, Q.; Gou, M. 3D-engineering of Cellularized Conduits for Peripheral Nerve Regeneration. Sci. Rep. 2016, 6, 32184. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Zhang, J.; Du, T.; Xu, X.; Deng, X.; Chen, S.; Wei, Y. Rapid 3D printing of functional nanoparticle-enhanced conduits for effective nerve repair. Acta Biomater. 2019, 90, 49–59. [Google Scholar] [CrossRef]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.S.; Yuan, S.; Zhang, H.; Chen, S. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Li, J.; Leong, Y.J.; Rozen, I.; Qu, X.; Dong, R.; Chen, S. 3D-printed artificial microfish. Adv. Mater. 2015, 27, 4411–4417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolleboom, A.; De Ruiter, G.C.W.; Coert, J.H.; Tuk, B.; Holstege, J.C.; Van Neck, J.W. Novel experimental surgical strategy to prevent traumatic neuroma formation by combining a 3D-printed Y -tube with an autograft. J. Neurosurg. 2018, 130, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Harding, A.J.; Albadawi, E.; Boissonade, F.M.; Haycock, J.W.; Claeyssens, F. Additive manufactured biodegradable poly (glycerol sebacate methacrylate) nerve guidance conduits. Acta Biomater. 2018, 78, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Asikainen, S.; Teotia, A.K. Biomimetic Photocurable 3D Printed Nerve Guidance Channels with Aligned Cryomatrix Lumen for Peripheral Nerve Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 43327–43342. [Google Scholar] [CrossRef]
- Martine, Z.P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int. J. Pharm. 2017, 532, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Guo, H.; Gao, B.; Ruan, M.; Xu, L.; Wu, J.; Xue, W. Double network shape memory hydrogels activated by near-infrared with high mechanical toughness, nontoxicity, and 3D printability. Chem. Eng. J. 2019, 356, 934–949. [Google Scholar] [CrossRef]
- Darabi, M.A.; Khosrozadeh, A.; Mbeleck, R.; Liu, Y.; Chang, Q.; Jiang, J.; Xing, M. Skin-inspired multifunctional autonomic-intrinsic conductive self-healing hydrogels with pressure sensitivity, stretchability, and 3D printability. Adv. Mater. 2017, 29, 1700533. [Google Scholar] [CrossRef] [PubMed]














| Technique | Type of Materials | Effect of Adding Reinforcing Particles on Mechanical Properties | Effect of Fiber Reinforcement on Mechanical Properties | Effect of Nanoparticle Enhancement on Mechanical Properties |
|---|---|---|---|---|
| FDM |
|
|
|
|
| SLA |
|
|
| |
| DLP |
|
| ||
| Ink Direct Writing |
|
|
| |
| Inkjet Printing |
|
|
| Feature | 3D Hydrogel Printing | 4D Hydrogel Printing | SMP-Based 4D Hydrogel |
|---|---|---|---|
| Fabrication process | Builds layer by layer in an incremental process from the bottom to the top | Transforms 3D designs or constructs under certain external stimuli using smart materials | Transforms 3D designs or constructs under certain external stimuli using shape-memory polymers |
| Materials |
|
|
|
| Deformation characterization |
|
| |
| Toughness | No tunable toughness | Tunable toughness | Moderate toughness |
| Water content | Low | High | Low |
| Cost | High | Low | High |
| Shape | No change over time in response to trigger stimuli in the environment | Change occurs over time in response to trigger stimuli (physical, chemical, and biological stimuli) in the surrounding environment | Change occurs over time in response to trigger stimuli in the surrounding environment |
| Limitations |
|
|
|
| Advantages |
|
|
|
| Compressive Breaking Strain (%) | Compressive Strength (MPa) | |
|---|---|---|
| F127DA hydrogel in the dried state | 99 | 38.8 |
| F127DA/PLGA hydrogel in the dried state | 99 | 79.2 |
| F127DA/PLGA/GO hydrogel in the dried state | 99 | 76.5 |
| F127DA/PLGA hydrogel for shape-memory test | 99 | 64.2 |
| F127DA/PLGA/GO hydrogel for shape-memory test | 99 | 58.2 |
| F127DA hydrogel in swollen state | 70.2 | 0.22 |
| F127DA/PLGA hydrogel in swollen state | 68.3 | 3.56 |
| F127DA/PLGA/GO hydrogel in swollen state | 66.8 | 3.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Lv, Q.; Wu, W. Application and Prospects of Hydrogel Additive Manufacturing. Gels 2022, 8, 297. https://doi.org/10.3390/gels8050297
Zhao C, Lv Q, Wu W. Application and Prospects of Hydrogel Additive Manufacturing. Gels. 2022; 8(5):297. https://doi.org/10.3390/gels8050297
Chicago/Turabian StyleZhao, Changlong, Qiyin Lv, and Wenzheng Wu. 2022. "Application and Prospects of Hydrogel Additive Manufacturing" Gels 8, no. 5: 297. https://doi.org/10.3390/gels8050297
APA StyleZhao, C., Lv, Q., & Wu, W. (2022). Application and Prospects of Hydrogel Additive Manufacturing. Gels, 8(5), 297. https://doi.org/10.3390/gels8050297