Advances of Engineered Hydrogel Organoids within the Stem Cell Field: A Systematic Review
Abstract
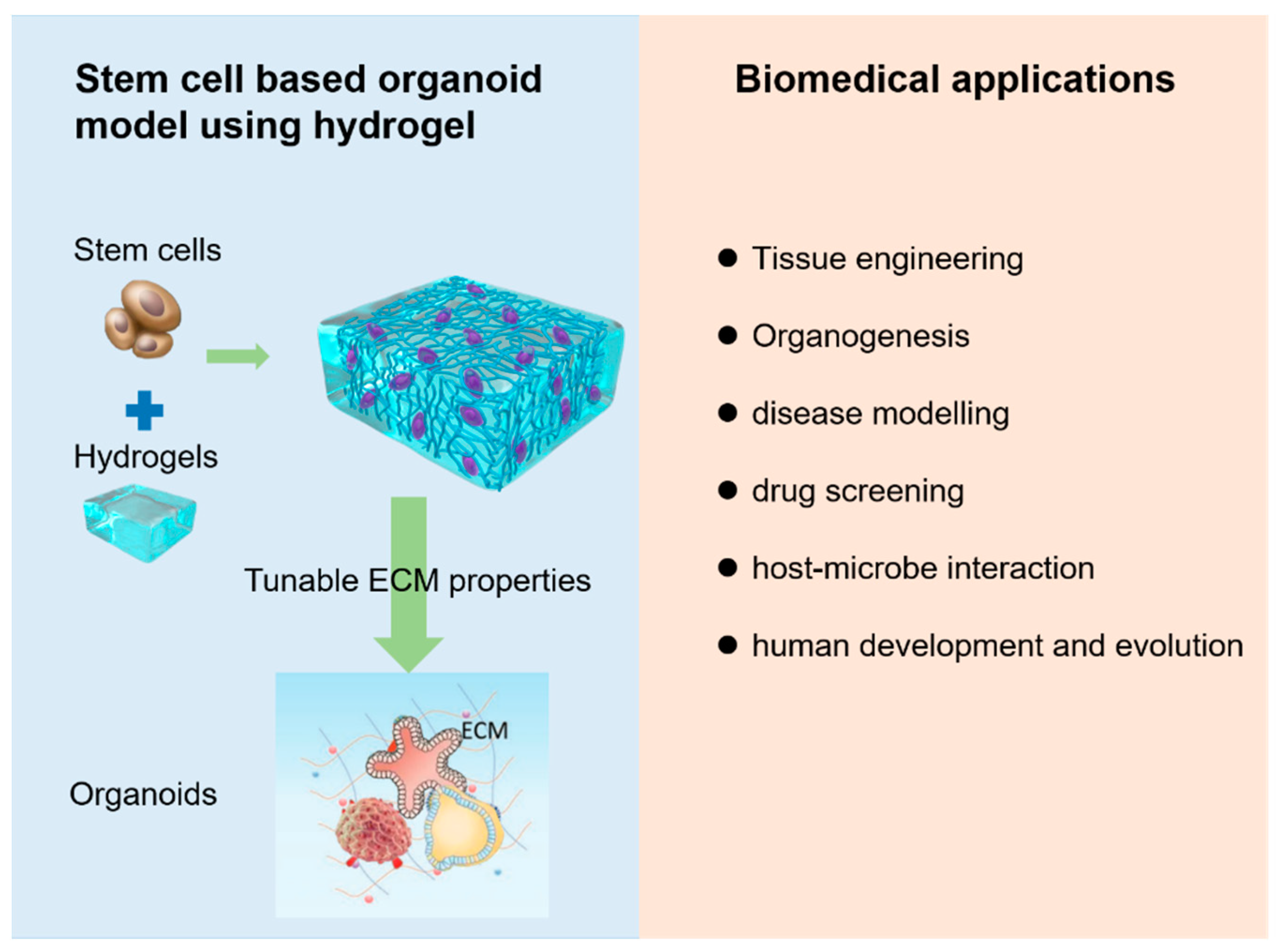
:1. Introduction
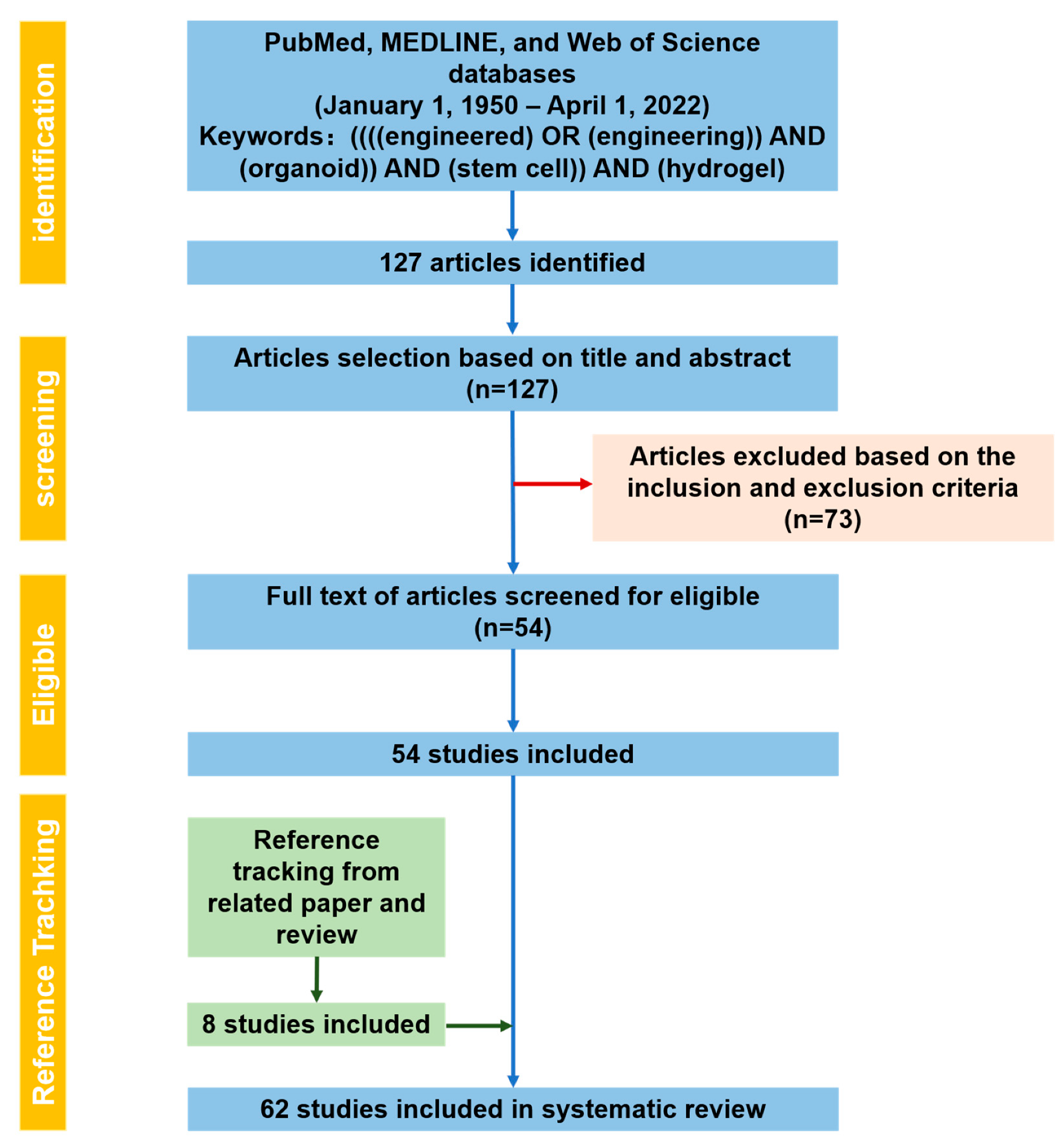
2. Methods
2.1. PRISMA Statement
2.2. Research Process
2.3. Risk of Bias
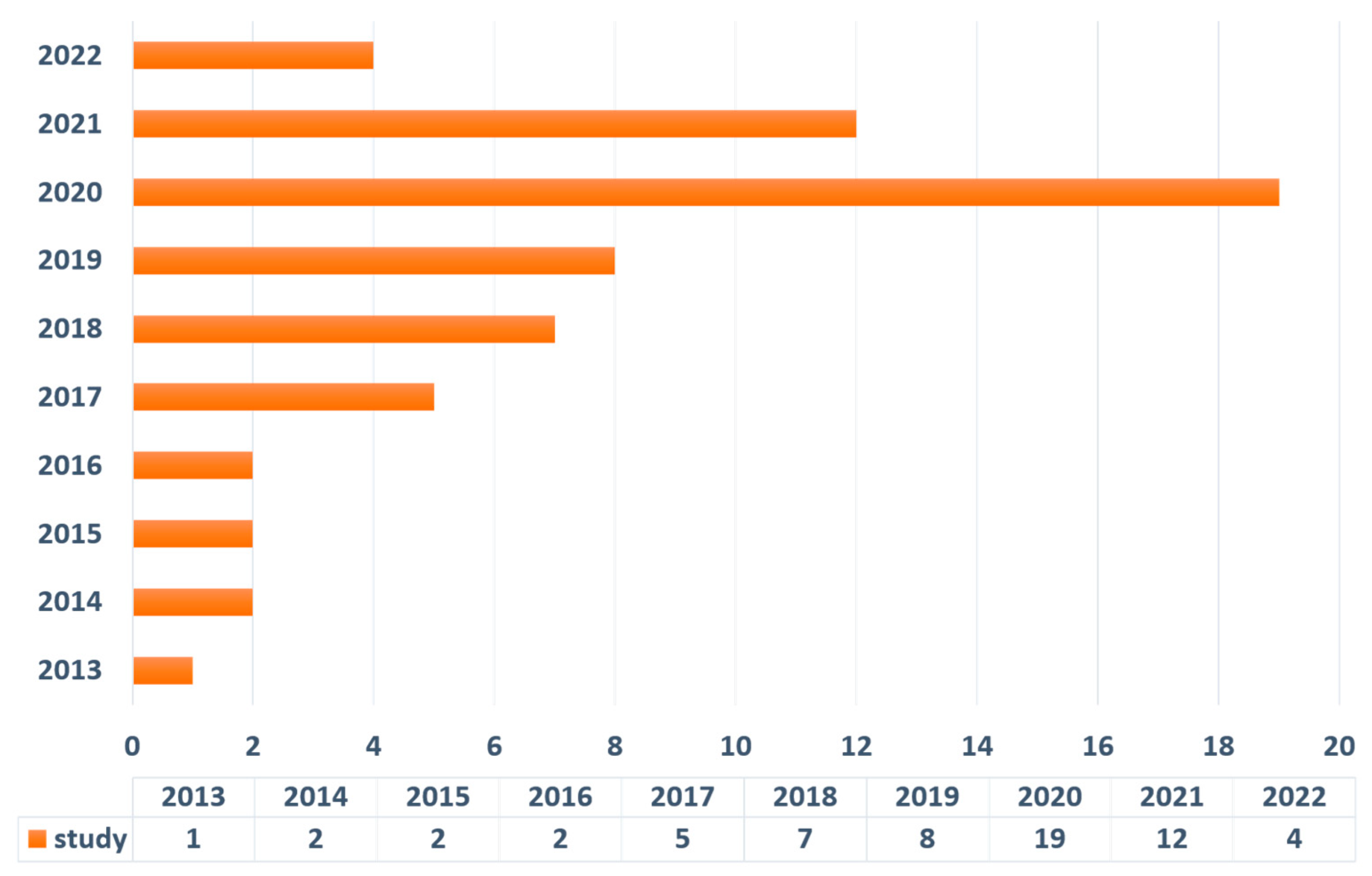
3. Results
Literature Search Output
4. Discussion
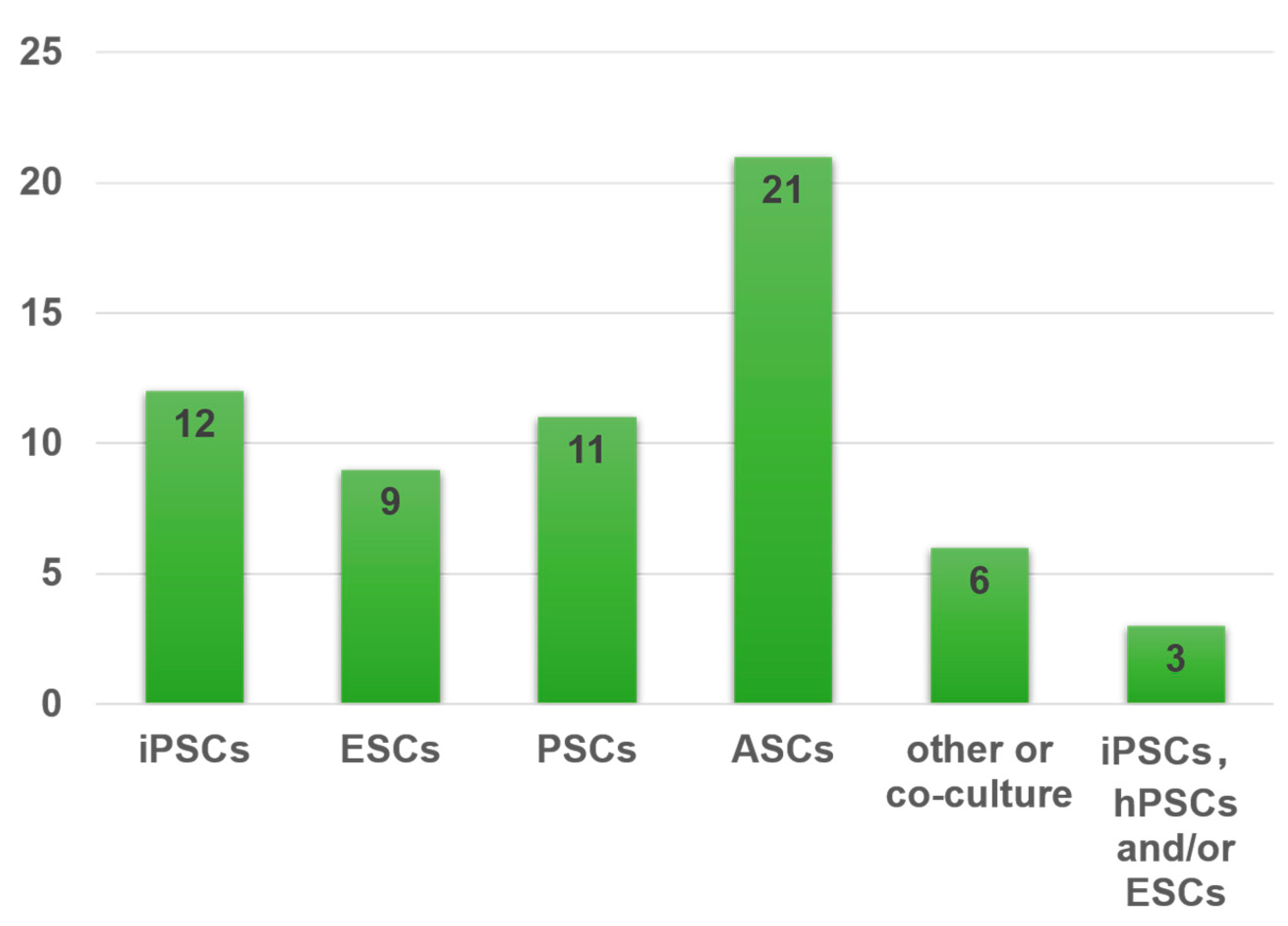
4.1. Representative Types of Stem Cells for Organoids
4.1.1. Pluripotent Stem Cells (PSCs)
4.1.2. Adult Stem Cells (ASCs) or Progenitor Cells
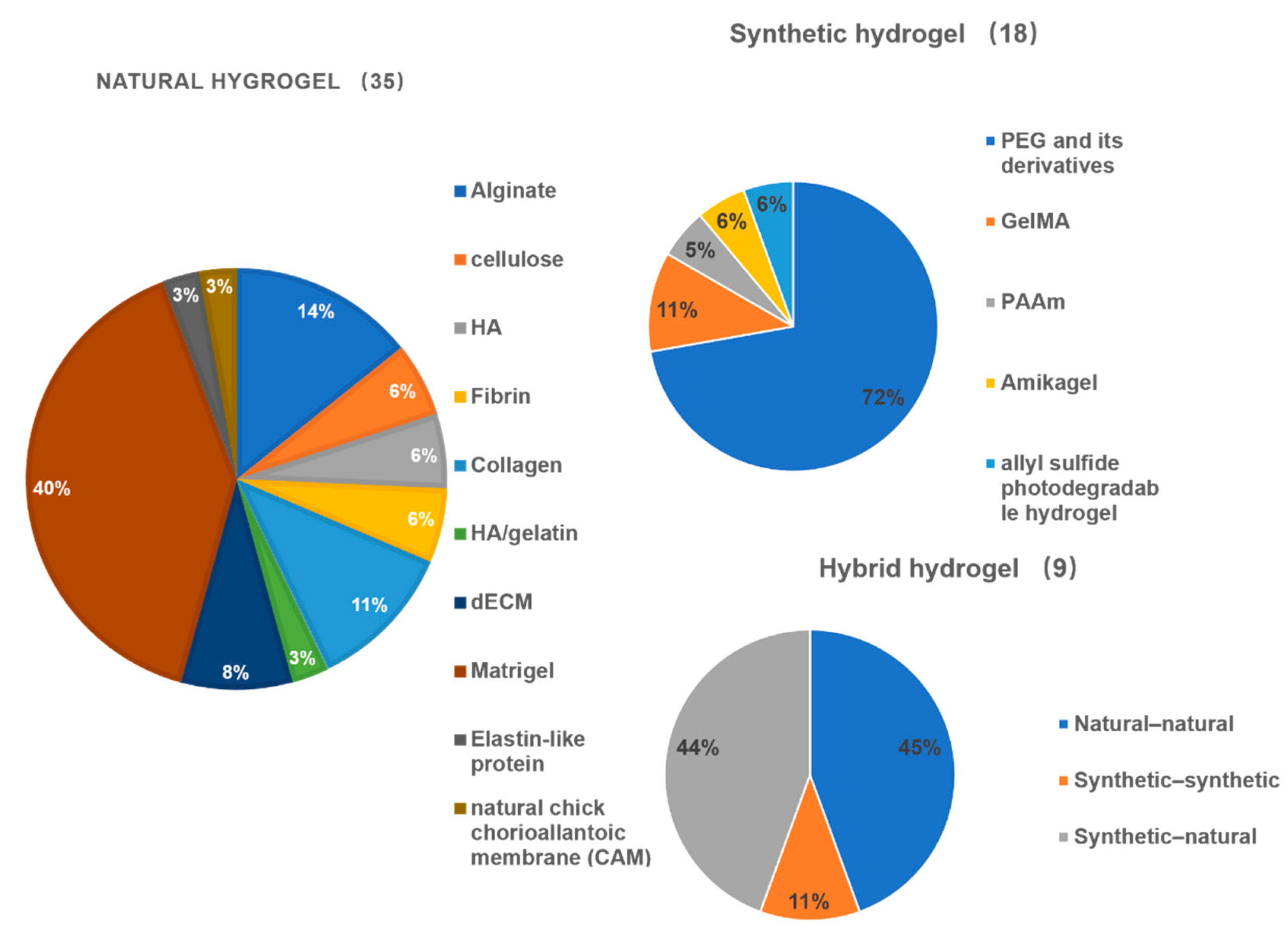
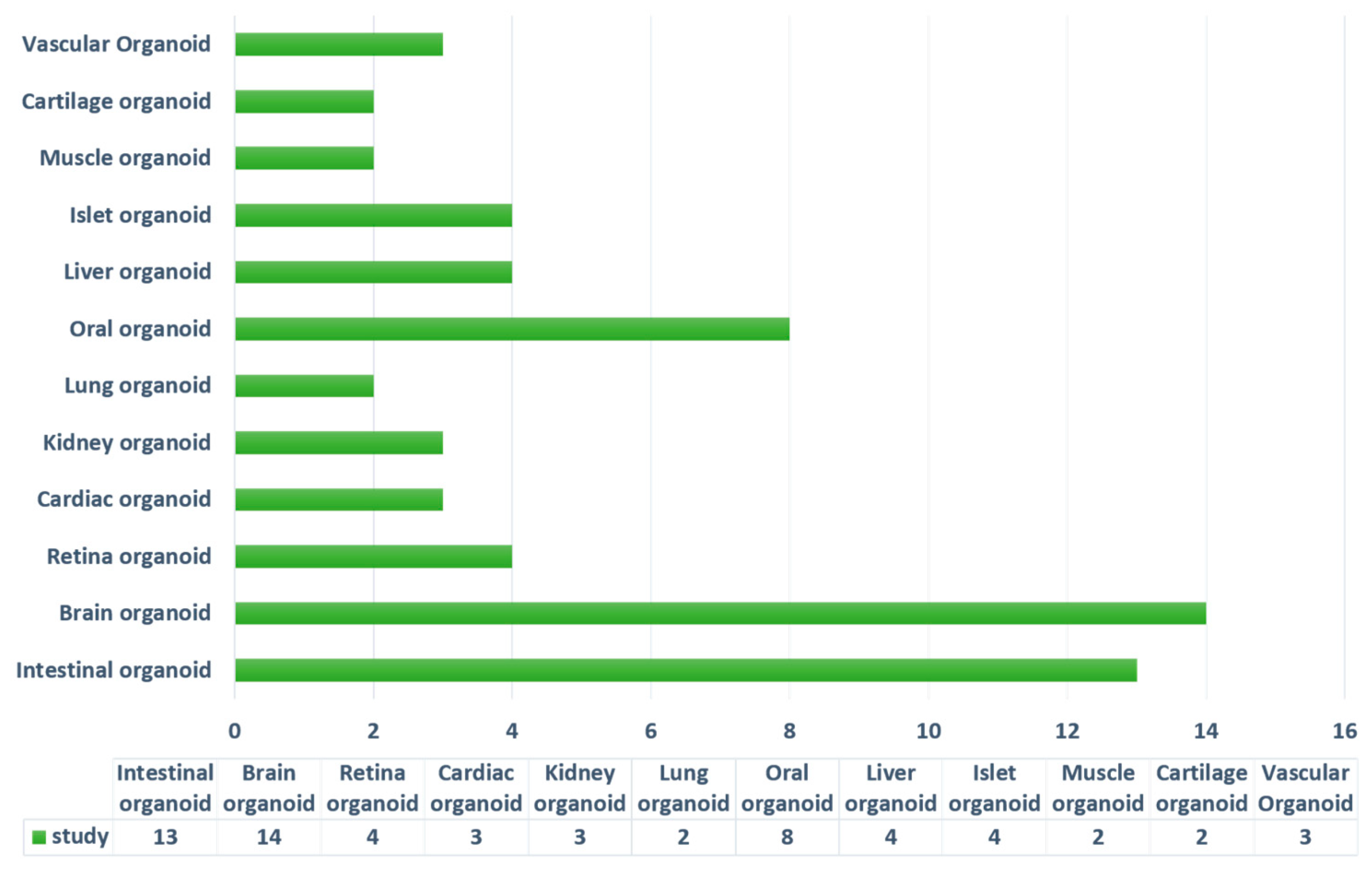
4.2. Current Stem-Cell-Derived Organoid Tissues Using Hydrogels
4.2.1. Intestinal Organoids
4.2.2. Brain and Neural Tube Organoids
4.2.3. Retina Organoids
4.2.4. Cardiac-Tissue Organoids
4.2.5. Kidney Organoids
4.2.6. Lung Organoids
4.2.7. Oral Organoids
Tooth Bud Germ
Salivary Glands
Lingual Epithelial Organoids
Taste Bud Organoids
4.2.8. Liver Organoids
4.2.9. Pancreas and Islet Organoids
4.2.10. Muscle and Cartilage Organoids
4.2.11. Vascular Network Construction in Organoids
5. Current Challenges and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mai, P.; Hampl, J.; Baca, M.; Brauer, D.; Singh, S.; Weise, F.; Borowiec, J.; Schmidt, A.; Küstner, J.M.; Klett, M.; et al. MatriGrid(®) Based Biological Morphologies: Tools for 3D Cell Culturing. Bioengineering 2022, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhang, J.; Qin, Y.; Tang, H.; Chen, Y.; Lin, W.; She, Y.; Zhang, K.; Yin, J.; Chen, C. A Simple and Efficient Strategy for Preparing a Cell-Spheroid-Based Bioink. Adv. Healthc. Mater. 2022; e2200648, ahead of print. [Google Scholar]
- Schneider, P.R.; Buhrmann, C.; Mobasheri, A.; Matis, U.; Shakibaei, M. Three-dimensional high-density co-culture with primary tenocytes induces tenogenic differentiation in mesenchymal stem cells. J. Orthop. Res. 2011, 29, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, M.; El Khoury, R.; Nagiah, N.; Thakur, V.; Chattopadhyay, M.; Joddar, B. 3D Biofabrication of a Cardiac Tissue Construct for Sustained Longevity and Function. ACS Appl. Mater. Interfaces 2022, 14, 21800–21813. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Blatchley, M.R.; Davidson, M.D.; Yavitt, F.M.; Cooke, M.E.; Anseth, K.S.; Burdick, J.A. Programming hydrogels to probe spatiotemporal cell biology. Cell Stem Cell 2022, 29, 678–691. [Google Scholar] [CrossRef]
- Shakibaei, M.; Shayan, P.; Busch, F.; Aldinger, C.; Buhrmann, C.; Lueders, C.; Mobasheri, A. Resveratrol mediated modulation of Sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: Potential role of Runx2 deacetylation. PLoS ONE 2012, 7, e35712. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Tang, H.; Xiahou, Z.; Zhang, J.; She, Y.; Zhang, K.; Hu, X.; Yin, J.; Chen, C. Solid multifunctional granular bioink for constructing chondroid basing on stem cell spheroids and chondrocytes. Biofabrication 2022, 14, 035003. [Google Scholar] [CrossRef]
- Willemse, J.; van der Laan, L.J.W.; de Jonge, J.; Verstegen, M.M.A. Design by Nature: Emerging Applications of Native Liver Extracellular Matrix for Cholangiocyte Organoid-Based Regenerative Medicine. Bioengineering 2022, 9, 9030110. [Google Scholar] [CrossRef]
- Heo, J.H.; Kang, D.; Seo, S.J.; Jin, Y. Engineering the Extracellular Matrix for Organoid Culture. Int. J. Stem Cells 2022, 15, 60–69. [Google Scholar] [CrossRef]
- Guan, J.; Wang, G.; Wang, J.; Zhang, Z.; Fu, Y.; Cheng, L.; Meng, G.; Lyu, Y.; Zhu, J.; Li, Y.; et al. Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature 2022, 605, 325–331. [Google Scholar] [CrossRef]
- Wang, Y.; Kankala, R.K.; Ou, C.; Chen, A.; Yang, Z. Advances in hydrogel-based vascularized tissues for tissue repair and drug screening. Bioact. Mater. 2022, 9, 198–220. [Google Scholar] [CrossRef]
- Han, Y.; Yang, L.; Lacko, L.A.; Chen, S. Human organoid models to study SARS-CoV-2 infection. Nat. Methods 2022, 19, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Curvello, R.; Kerr, G.; Micati, D.J.; Chan, W.H.; Raghuwanshi, V.S.; Rosenbluh, J.; Abud, H.E.; Garnier, G. Engineered Plant-Based Nanocellulose Hydrogel for Small Intestinal Organoid Growth. Adv. Sci. 2020, 8, 2002135. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gordillo, V.; Kassis, T.; Lampejo, A.; Choi, G.; Gamboa, M.E.; Gnecco, J.S.; Brown, A.; Breault, D.T.; Carrier, R.; Griffith, L.G. Fully synthetic matrices for in vitro culture of primary human intestinal enteroids and endometrial organoids. Biomaterials 2020, 254, 120125. [Google Scholar] [CrossRef] [PubMed]
- Pérez-González, C.; Ceada, G.; Greco, F.; Matejčić, M.; Gómez-González, M.; Castro, N.; Menendez, A.; Kale, S.; Krndija, D.; Clark, A.G.; et al. Mechanical compartmentalization of the intestinal organoid enables crypt folding and collective cell migration. Nat. Cell Biol. 2021, 23, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.P.; Liu, H.; Ciceri, G.; Jungverdorben, J.; Frishman, G.; Tchieu, J.; Cederquist, G.Y.; Rothenaigner, I.; Schorpp, K.; Klepper, L.; et al. Activation of HERV-K(HML-2) disrupts cortical patterning and neuronal differentiation by increasing NTRK3. Cell Stem Cell 2021, 28, 1566–1581.e8. [Google Scholar] [CrossRef]
- Zafeiriou, M.P.; Bao, G.; Hudson, J.; Halder, R.; Blenkle, A.; Schreiber, M.K.; Fischer, A.; Schild, D.; Zimmermann, W.H. Developmental GABA polarity switch and neuronal plasticity in Bioengineered Neuronal Organoids. Nat. Commun. 2020, 11, 3791. [Google Scholar] [CrossRef]
- Pollen, A.A.; Bhaduri, A.; Andrews, M.G.; Nowakowski, T.J.; Meyerson, O.S.; Mostajo-Radji, M.A.; di Lullo, E.; Alvarado, B.; Bedolli, M.; Dougherty, M.L.; et al. Establishing Cerebral Organoids as Models of Human-Specific Brain Evolution. Cell 2019, 176, 743–756.e17. [Google Scholar] [CrossRef] [Green Version]
- Cowan, C.S.; Renner, M.; de Gennaro, M.; Gross-Scherf, B.; Goldblum, D.; Hou, Y.; Munz, M.; Rodrigues, T.M.; Krol, J.; Szikra, T.; et al. Cell Types of the Human Retina and Its Organoids at Single-Cell Resolution. Cell 2020, 182, 1623–1640.e34. [Google Scholar] [CrossRef]
- Zheng, C.; Schneider, J.W.; Hsieh, J. Role of RB1 in human embryonic stem cell-derived retinal organoids. Dev. Biol. 2020, 462, 197–207. [Google Scholar] [CrossRef]
- Li, R.A.; Keung, W.; Cashman, T.J.; Backeris, P.C.; Johnson, B.V.; Bardot, E.S.; Wong, A.O.T.; Chan, P.K.W.; Chan, C.W.Y.; Costa, K.D. Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials 2018, 163, 116–127. [Google Scholar] [CrossRef]
- Lu, K.; Seidel, T.; Cao-Ehlker, X.; Dorn, T.; Batcha, A.M.N.; Schneider, C.M.; Semmler, M.; Volk, T.; Moretti, A.; Dendorfer, A.; et al. Progressive stretch enhances growth and maturation of 3D stem-cell-derived myocardium. Theranostics 2021, 11, 6138–6153. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.E.; Jodat, Y.A.; Samanipour, R.; Zorzi, G.; Zhu, K.; Hirano, M.; Chang, K.; Arnaout, A.; Hassan, S.; Matharu, N.; et al. Toward a neurospheroid niche model: Optimizing embedded 3D bioprinting for fabrication of neurospheroid brain-like co-culture constructs. Biofabrication 2020, 13, 015014. [Google Scholar] [CrossRef] [PubMed]
- Garreta, E.; Prado, P.; Tarantino, C.; Oria, R.; Fanlo, L.; Martí, E.; Zalvidea, D.; Trepat, X.; Roca-Cusachs, P.; Gavaldà-Navarro, A.; et al. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat. Mater. 2019, 18, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Nam, S.A.; Yi, J.; Kim, J.Y.; Lee, J.Y.; Park, S.Y.; Sen, T.; Choi, Y.M.; Lee, J.Y.; Kim, H.L.; et al. Kidney Decellularized Extracellular Matrix Enhanced the Vascularization and Maturation of Human Kidney Organoids. Adv. Sci. 2022, 9, e2103526. [Google Scholar] [CrossRef] [PubMed]
- Geuens, T.; Ruiter, F.A.A.; Schumacher, A.; Morgan, F.L.C.; Rademakers, T.; Wiersma, L.E.; van den Berg, C.W.; Rabelink, T.J.; Baker, M.B.; LaPointe, V.L.S. Thiol-ene cross-linked alginate hydrogel encapsulation modulates the extracellular matrix of kidney organoids by reducing abnormal type 1a1 collagen deposition. Biomaterials 2021, 275, 120976. [Google Scholar] [CrossRef]
- Dye, B.R.; Youngblood, R.L.; Oakes, R.S.; Kasputis, T.; Clough, D.W.; Spence, J.R.; Shea, L.D. Human lung organoids develop into adult airway-like structures directed by physico-chemical biomaterial properties. Biomaterials 2020, 234, 119757. [Google Scholar] [CrossRef]
- Dye, B.R.; Hill, D.R.; Ferguson, M.A.; Tsai, Y.H.; Nagy, M.S.; Dyal, R.; Wells, J.M.; Mayhew, C.N.; Nattiv, R.; Klein, O.D.; et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife 2015, 4, e05098. [Google Scholar] [CrossRef]
- Smith, E.E.; Zhang, W.; Schiele, N.R.; Khademhosseini, A.; Kuo, C.K.; Yelick, P.C. Developing a biomimetic tooth bud model. J. Tissue Eng. Regen. Med. 2017, 11, 3326–3336. [Google Scholar] [CrossRef]
- Hisha, H.; Tanaka, T.; Kanno, S.; Tokuyama, Y.; Komai, Y.; Ohe, S.; Yanai, H.; Omachi, T.; Ueno, H. Establishment of a novel lingual organoid culture system: Generation of organoids having mature keratinized epithelium from adult epithelial stem cells. Sci. Rep. 2013, 3, 3224. [Google Scholar] [CrossRef] [Green Version]
- Sui, Y.; Zhang, S.; Li, Y.; Zhang, X.; Hu, W.; Feng, Y.; Xiong, J.; Zhang, Y.; Wei, S. Generation of functional salivary gland tissue from human submandibular gland stem/progenitor cells. Stem Cell Res. Ther. 2020, 11, 127. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Lewandowski, B.C.; Watson, J.; Aihara, E.; Iwatsuki, K.; Bachmanov, A.A.; Margolskee, R.F.; Jiang, P. Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 16401–16406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorrentino, G.; Rezakhani, S.; Yildiz, E.; Nuciforo, S.; Heim, M.H.; Lutolf, M.P.; Schoonjans, K. Mechano-modulatory synthetic niches for liver organoid derivation. Nat. Commun. 2020, 11, 3416. [Google Scholar] [CrossRef] [PubMed]
- Gholobova, D.; Gerard, M.; Terrie, L.; Desender, L.; Shansky, J.; Vandenburgh, H.; Thorrez, L. Coculture Method to Obtain Endothelial Networks within Human Tissue-Engineered Skeletal Muscle. Methods Mol. Biol. 2019, 1889, 169–183. [Google Scholar] [PubMed]
- Xiahou, Z.; She, Y.; Zhang, J.; Qin, Y.; Li, G.; Zhang, L.; Fang, H.; Zhang, K.; Chen, C.; Yin, J. Designer Hydrogel with Intelligently Switchable Stem-Cell Contact for Incubating Cartilaginous Microtissues. ACS Appl. Mater. Interfaces 2020, 12, 40163–40175. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Min, S.; Choi, Y.S.; Jo, S.H.; Jung, J.H.; Han, K.; Kim, J.; An, S.; Ji, Y.W.; Kim, Y.G.; et al. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat. Commun. 2022, 13, 1692. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Hendriks, D.; Clevers, H.; Artegiani, B. CRISPR-Cas Tools and Their Application in Genetic Engineering of Human Stem Cells and Organoids. Cell Stem Cell 2020, 27, 705–731. [Google Scholar] [CrossRef]
- Yi, S.A.; Zhang, Y.; Rathnam, C.; Pongkulapa, T.; Lee, K.B. Bioengineering Approaches for the Advanced Organoid Research. Adv. Mater. 2021, 33, e2007949. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hunt, D.R.; Klett, K.C.; Mascharak, S.; Wang, H.; Gong, D.; Lou, J.; Li, X.; Cai, P.C.; Suhar, R.A.; Co, J.Y.; et al. Engineered Matrices Enable the Culture of Human Patient-Derived Intestinal Organoids. Adv. Sci. 2021, 8, 2004705. [Google Scholar] [CrossRef]
- Gjorevski, N.; Nikolaev, M.; Brown, T.E.; Mitrofanova, O.; Brandenberg, N.; DelRio, F.W.; Yavitt, F.M.; Liberali, P.; Anseth, K.S.; Lutolf, M.P. Tissue geometry drives deterministic organoid patterning. Science 2022, 375, eaaw9021. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Acuña, R.; Quirós, M.; Farkas, A.E.; Dedhia, P.H.; Huang, S.; Siuda, D.; García-Hernández, V.; Miller, A.J.; Spence, J.R.; Nusrat, A.; et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 2017, 19, 1326–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gjorevski, N.; Lutolf, M.P. Synthesis and characterization of well-defined hydrogel matrices and their application to intestinal stem cell and organoid culture. Nat. Protoc. 2017, 12, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Capeling, M.M.; Czerwinski, M.; Huang, S.; Tsai, Y.H.; Wu, A.; Nagy, M.S.; Juliar, B.; Sundaram, N.; Song, Y.; Han, W.M.; et al. Nonadhesive Alginate Hydrogels Support Growth of Pluripotent Stem Cell-Derived Intestinal Organoids. Stem Cell Rep. 2019, 12, 381–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrisnandy, A.; Blondel, D.; Rezakhani, S.; Broguiere, N.; Lutolf, M.P. Synthetic dynamic hydrogels promote degradation-independent in vitro organogenesis. Nat. Mater. 2022, 21, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordóñez-Morán, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef]
- Carberry, B.J.; Hergert, J.E.; Yavitt, F.M.; Hernandez, J.J.; Speckl, K.F.; Bowman, C.N.; McLeod, R.R.; Anseth, K.S. 3D printing of sacrificial thioester elastomers using digital light processing for templating 3D organoid structures in soft biomatrices. Biofabrication 2021, 13, 044104. [Google Scholar] [CrossRef]
- Wang, Y.; Gunasekara, D.B.; Reed, M.I.; DiSalvo, M.; Bultman, S.J.; Sims, C.E.; Magness, S.T.; Allbritton, N.L. A microengineered collagen scaffold for generating a polarized crypt-villus architecture of human small intestinal epithelium. Biomaterials 2017, 128, 44–55. [Google Scholar] [CrossRef]
- Yavitt, F.M.; Brown, T.E.; Hushka, E.A.; Brown, M.E.; Gjorevski, N.; Dempsey, P.J.; Lutolf, M.P.; Anseth, K.S. The Effect of Thiol Structure on Allyl Sulfide Photodegradable Hydrogels and their Application as a Degradable Scaffold for Organoid Passaging. Adv. Mater. 2020, 32, e1905366. [Google Scholar] [CrossRef]
- Capowski, E.E.; Samimi, K.; Mayerl, S.J.; Phillips, M.J.; Pinilla, I.; Howden, S.E.; Saha, J.; Jansen, A.D.; Edwards, K.L.; Jager, L.D.; et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development 2019, 146, dev171686. [Google Scholar] [CrossRef] [Green Version]
- Decembrini, S.; Hoehnel, S.; Brandenberg, N.; Arsenijevic, Y.; Lutolf, M.P. Hydrogel-based milliwell arrays for standardized and scalable retinal organoid cultures. Sci. Rep. 2020, 10, 10275. [Google Scholar] [CrossRef] [PubMed]
- Kanton, S.; Boyle, M.J.; He, Z.; Santel, M.; Weigert, A.; Sanchís-Calleja, F.; Guijarro, P.; Sidow, L.; Fleck, J.S.; Han, D.; et al. Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature 2019, 574, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.N.; Jin, Y.; An, Y.; Kim, J.; Choi, Y.S.; Lee, J.S.; Kim, J.; Choi, W.Y.; Koo, D.J.; Yu, W.; et al. Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat. Commun. 2021, 12, 4730. [Google Scholar] [CrossRef] [PubMed]
- Simsa, R.; Rothenbücher, T.; Gürbüz, H.; Ghosheh, N.; Emneus, J.; Jenndahl, L.; Kaplan, D.L.; Bergh, N.; Serrano, A.M.; Fogelstrand, P. Brain organoid formation on decellularized porcine brain ECM hydrogels. PLoS ONE 2021, 16, e0245685. [Google Scholar]
- Lindborg, B.A.; Brekke, J.H.; Vegoe, A.L.; Ulrich, C.B.; Haider, K.T.; Subramaniam, S.; Venhuizen, S.L.; Eide, C.R.; Orchard, P.J.; Chen, W.; et al. Rapid Induction of Cerebral Organoids From Human Induced Pluripotent Stem Cells Using a Chemically Defined Hydrogel and Defined Cell Culture Medium. Stem Cells Transl. Med. 2016, 5, 970–979. [Google Scholar] [CrossRef] [Green Version]
- Girgin, M.U.; Broguiere, N.; Mattolini, L.; Lutolf, M.P. Gastruloids generated without exogenous Wnt activation develop anterior neural tissues. Stem Cell Rep. 2021, 16, 1143–1155. [Google Scholar] [CrossRef]
- de Camps, C.C.; Aslani, S.; Stylianesis, N.; Nami, H.; Mohamed, N.V.; Durcan, T.M.; Moraes, C. Hydrogel Mechanics Influence the Growth and Development of Embedded Brain Organoids. ACS Appl. Bio. Mater. 2022, 5, 214–224. [Google Scholar] [CrossRef]
- Bejoy, J.; Wang, Z.; Bijonowski, B.; Yang, M.; Ma, T.; Sang, Q.X.; Li, Y. Differential Effects of Heparin and Hyaluronic Acid on Neural Patterning of Human Induced Pluripotent Stem Cells. ACS Biomater. Sci. Eng. 2018, 4, 4354–4366. [Google Scholar] [CrossRef]
- Fattah, A.R.A.; Daza, B.; Rustandi, G.; Berrocal-Rubio, M.; Gorissen, B.; Poovathingal, S.; Davie, K.; Barrasa-Fano, J.; Cóndor, M.; Cao, X.; et al. Actuation enhances patterning in human neural tube organoids. Nat. Commun. 2021, 12, 3192. [Google Scholar] [CrossRef]
- Ishihara, K.; Ranga, A.; Lutolf, M.P.; Tanaka, E.M.; Meinhardt, A. Reconstitution of a Patterned Neural Tube from Single Mouse Embryonic Stem Cells. Methods Mol. Biol. 2017, 1597, 43–55. [Google Scholar]
- McNulty, J.D.; Marti-Figueroa, C.; Seipel, F.; Plantz, J.Z.; Ellingham, T.; Duddleston, L.J.L.; Goris, S.; Cox, B.L.; Osswald, T.A.; Turng, L.S.; et al. Micro-injection molded, poly(vinyl alcohol)-calcium salt templates for precise customization of 3D hydrogel internal architecture. Acta Biomater. 2019, 95, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Shkumatov, A.; Baek, K.; Kong, H. Matrix rigidity-modulated cardiovascular organoid formation from embryoid bodies. PLoS ONE 2014, 9, e94764. [Google Scholar] [CrossRef]
- Tanaka, J.; Ogawa, M.; Hojo, H.; Kawashima, Y.; Mabuchi, Y.; Hata, K.; Nakamura, S.; Yasuhara, R.; Takamatsu, K.; Irié, T.; et al. Generation of orthotopically functional salivary gland from embryonic stem cells. Nat. Commun. 2018, 9, 4216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.S.; Hong, H.J.; Koh, W.G.; Lim, J.Y. Organotypic 3D Culture in Nanoscaffold Microwells Supports Salivary Gland Stem-Cell-Based Organization. ACS Biomater. Sci. Eng. 2018, 4, 4311–4320. [Google Scholar] [CrossRef] [PubMed]
- Aihara, E.; Mahe, M.M.; Schumacher, M.A.; Matthis, A.L.; Feng, R.; Ren, W.; Noah, T.K.; Matsu-ura, T.; Moore, S.R.; Hong, C.I.; et al. Characterization of stem/progenitor cell cycle using murine circumvallate papilla taste bud organoid. Sci. Rep. 2015, 5, 17185. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Achoute, L.; Margolskee, R.F.; Jiang, P.; Wang, H. Lipopolysaccharide-Induced Inflammatory Cytokine Expression in Taste Organoids. Chem. Senses 2020, 45, 187–194. [Google Scholar] [CrossRef]
- Zhang, W.; Lanzoni, G.; Hani, H.; Overi, D.; Cardinale, V.; Simpson, S.; Pitman, W.; Allen, A.; Yi, X.; Wang, X.; et al. Patch grafting, strategies for transplantation of organoids into solid organs such as liver. Biomaterials 2021, 277, 121067. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Zhang, M.; Wang, H.; Chen, W.; Qin, J. One-step synthesis of composite hydrogel capsules to support liver organoid generation from hiPSCs. Biomater. Sci. 2020, 8, 5476–5488. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, K.; Yoon, S.; Kim, J.S.; Park, S.A.; Kim, W.D.; Lee, S.B.; Ryu, K.Y.; Jeong, J.; Choi, D. Prolongation of liver-specific function for primary hepatocytes maintenance in 3D printed architectures. Organogenesis 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Augsornworawat, P.; Velazco-Cruz, L.; Song, J.; Millman, J.R. A hydrogel platform for in vitro three dimensional assembly of human stem cell-derived islet cells and endothelial cells. Acta Biomater. 2019, 97, 272–280. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Wang, H.; Zhao, M.; Tao, T.; Zhang, X.; Qin, J. A Droplet Microfluidic System to Fabricate Hybrid Capsules Enabling Stem Cell Organoid Engineering. Adv. Sci. 2020, 7, 1903739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candiello, J.; Grandhi, T.S.P.; Goh, S.K.; Vaidya, V.; Lemmon-Kishi, M.; Eliato, K.R.; Ros, R.; Kumta, P.N.; Rege, K.; Banerjee, I. 3D heterogeneous islet organoid generation from human embryonic stem cells using a novel engineered hydrogel platform. Biomaterials 2018, 177, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, H.; Zhang, X.; Wang, Y.; Zhao, M.; Chen, W.; Qin, J. One-Step Generation of Aqueous-Droplet-Filled Hydrogel Fibers as Organoid Carriers Using an All-in-Water Microfluidic System. ACS Appl. Mater. Interfaces 2021, 13, 3199–3208. [Google Scholar] [CrossRef] [PubMed]
- Maffioletti, S.M.; Sarcar, S.; Henderson, A.B.H.; Mannhardt, I.; Pinton, L.; Moyle, L.A.; Steele-Stallard, H.; Cappellari, O.; Wells, K.E.; Ferrari, G.; et al. Three-Dimensional Human iPSC-Derived Artificial Skeletal Muscles Model Muscular Dystrophies and Enable Multilineage Tissue Engineering. Cell Rep. 2018, 23, 899–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, D.; Liu, W.; Li, J.J.; Liu, L.; Guo, A.; Wang, B.; Yu, H.; Zhao, Y.; Chen, Y.; You, Z.; et al. Engineering 3D functional tissue constructs using self-assembling cell-laden microniches. Acta Biomater. 2020, 114, 170–182. [Google Scholar] [CrossRef]
- Markou, M.; Kouroupis, D.; Badounas, F.; Katsouras, A.; Kyrkou, A.; Fotsis, T.; Murphy, C.; Bagli, E. Tissue Engineering Using Vascular Organoids From Human Pluripotent Stem Cell Derived Mural Cell Phenotypes. Front Bioeng. Biotechnol. 2020, 8, 278. [Google Scholar] [CrossRef]
- Rossen, N.S.; Anandakumaran, P.N.; Nieden, R.Z.; Lo, K.; Luo, W.; Park, C.; Huyan, C.; Fu, Q.; Song, Z.; Singh-Moon, R.P.; et al. Injectable Therapeutic Organoids Using Sacrificial Hydrogels. iScience 2020, 23, 101052. [Google Scholar] [CrossRef]
- Petta, D.; Basoli, V.; Pellicciotta, D.; Tognato, R.; Barcik, J.; Arrigoni, C.; Bella, E.D.; Armiento, A.R.; Candrian, C.; Richards, R.G.; et al. Sound-induced morphogenesis of multicellular systems for rapid orchestration of vascular networks. Biofabrication 2020, 13, 015004. [Google Scholar] [CrossRef]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef]
- Gao, X.; Wu, Y.; Liao, L.; Tian, W. Oral Organoids: Progress and Challenges. J. Dent. Res. 2021, 100, 454–463. [Google Scholar] [CrossRef]
- Liu, L.P.; Zheng, Y.W. Predicting differentiation potential of human pluripotent stem cells: Possibilities and challenges. World J. Stem Cells 2019, 11, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; van Es, J.H.; van den Brink, S.; van Houdt, W.J.; Pronk, A.; van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Bartfeld, S.; Clevers, H. Stem cell-derived organoids and their application for medical research and patient treatment. J. Mol. Med. 2017, 95, 729–738. [Google Scholar] [CrossRef]
- Rahmani, S.; Breyner, N.M.; Su, H.M.; Verdu, E.F.; Didar, T.F. Intestinal organoids: A new paradigm for engineering intestinal epithelium in vitro. Biomaterials 2019, 194, 195–214. [Google Scholar] [CrossRef]
- Qian, X.; Song, H.; Ming, G.L. Brain organoids: Advances, applications and challenges. Development 2019, 146, dev166074. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, Y.; Cui, K.; Guo, Y.; Zhang, X.; Qin, J. Advances in Hydrogels in Organoids and Organs-on-a-Chip. Adv. Mater. 2019, 31, e1902042. [Google Scholar] [CrossRef]
- Foltz, L.P.; Clegg, D.O. Patient-derived induced pluripotent stem cells for modelling genetic retinal dystrophies. Prog. Retin. Eye Res. 2019, 68, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Kruczek, K.; Swaroop, A. Pluripotent stem cell-derived retinal organoids for disease modeling and development of therapies. Stem Cells 2020, 38, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Rafatian, N.; Feric, N.T.; Cox, B.J.; Aschar-Sobbi, R.; Wang, E.Y.; Aggarwal, P.; Zhang, B.; Conant, G.; Ronaldson-Bouchard, K.; et al. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 2019, 176, 913–927.e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018, 556, 239–243. [Google Scholar] [CrossRef]
- Nishinakamura, R. Human kidney organoids: Progress and remaining challenges. Nat. Rev. Nephrol. 2019, 15, 613–624. [Google Scholar] [CrossRef]
- van der Vaart, J.; Lamers, M.M.; Haagmans, B.L.; Clevers, H. Advancing lung organoids for COVID-19 research. Dis. Model Mech. 2021, 14, dmm049060. [Google Scholar] [CrossRef]
- Clevers, H. COVID-19: Organoids go viral. Nat. Rev. Mol. Cell Biol. 2020, 21, 355–356. [Google Scholar] [CrossRef]
- Tang, Z.; Kong, N.; Zhang, X.; Liu, Y.; Hu, P.; Mou, S.; Liljeström, P.; Shi, J.; Tan, W.; Kim, J.S.; et al. A materials-science perspective on tackling COVID-19. Nat. Rev. Mater. 2020, 5, 847–860. [Google Scholar] [CrossRef]
- Ikeda, E.; Morita, R.; Nakao, K.; Ishida, K.; Nakamura, T.; Takano-Yamamoto, T.; Ogawa, M.; Mizuno, M.; Kasugai, S.; Tsuji, T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 13475–13480. [Google Scholar] [CrossRef] [Green Version]
- Nakao, K.; Morita, R.; Saji, Y.; Ishida, K.; Tomita, Y.; Ogawa, M.; Saitoh, M.; Tomooka, Y.; Tsuji, T. The development of a bioengineered organ germ method. Nat. Methods 2007, 4, 227–230. [Google Scholar] [CrossRef]
- Luo, X.; Okubo, T.; Randell, S.; Hogan, B.L. Culture of endodermal stem/progenitor cells of the mouse tongue. In Vitro Cell Dev. Biol. Anim. 2009, 45, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhou, Z.; Shi, L.; Yang, X.; Liu, W. Cancer stem cell markers ALDH1 and Bmi1 expression in oral erythroplakia revisited: Implication for driving the process of field cancerization. J. Oral Pathol. Med. 2020, 49, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Prior, N.; Inacio, P.; Huch, M. Liver organoids: From basic research to therapeutic applications. Gut 2019, 68, 2228–2237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balak, J.R.A.; Juksar, J.; Carlotti, F.; Nigro, A.L.; de Koning, E.J.P. Organoids from the Human Fetal and Adult Pancreas. Curr. Diab. Rep. 2019, 19, 160. [Google Scholar] [CrossRef] [Green Version]
- Jalal, S.; Dastidar, S.; Tedesco, F.S. Advanced models of human skeletal muscle differentiation, development and disease: Three-dimensional cultures, organoids and beyond. Curr. Opin. Cell Biol. 2021, 73, 92–104. [Google Scholar] [CrossRef]
- Schechner, J.S.; Nath, A.K.; Zheng, L.; Kluger, M.S.; Hughes, C.C.; Sierra-Honigmann, M.R.; Lorber, M.I.; Tellides, G.; Kashgarian, M.; Bothwell, A.L.; et al. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc. Natl. Acad. Sci. USA 2000, 97, 9191–9196. [Google Scholar] [CrossRef] [Green Version]
- Au, P.; Tam, J.; Duda, D.G.; Lin, P.C.; Munn, L.L.; Fukumura, D.; Jain, R.K. Paradoxical effects of PDGF-BB overexpression in endothelial cells on engineered blood vessels in vivo. Am. J. Pathol. 2009, 175, 294–302. [Google Scholar] [CrossRef] [Green Version]
- Huch, M.; Gehart, H.; van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.; Ellis, E.; van Wenum, M.; Fuchs, S.A.; de Ligt, J.; et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 2015, 160, 299–312. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
| Tissue | Stem Cell Type | Hydrogel | Hydrogel Type | Mechanism | Ref. |
|---|---|---|---|---|---|
| Intestine | mISCs | HELP | Natural hydrogel | High matrix stiffness significantly enhanced ISC expansion through YAP1-dependent mechanism | [41] |
| Human tissue-derived stem/progenitor epithelial cells | 20 kDa 8-arm PEG macromer | synthetic hydrogel | NA | [14] | |
| Mouse stem cells (labelled with Lgr5 and Olfm4) | Soft (5 kPa) PAAm | Synthetic hydrogel | The stem cell compartment pushes the ECM and folds through apical constriction, whereas the transit-amplifying zone pulls the ECM and elongates through basal constriction | [15] | |
| mISCs | RGD–laminin-1–PEG | Synthetic hydrogel | YAP mechanosensing/transduction and Notch signaling | [42] | |
| hPSCs | Four-armed, maleimide-terminated PEG macromer | Synthetic hydrogel | NA | [43] | |
| ISCs | RGD-lamin-111-PEG | synthetic hydrogel | NA | [44] | |
| Mouse small intestine crypts which exhibit revival stem cells markers | Nanocellulose hydrogel | Natural hydrogel | Combined with laminin-1 and supplemented with IGF-1 | [13] | |
| hPSCs | Alginate | Natural hydrogel | RNA-seq | [45] | |
| ISCs | Hybrid PEG hydrogels | Synthetic hydrogel | Increased symmetry breaking and Paneth cell formation dependent on YAP1 | [46] | |
| mISCs and hISCs | RGD-functionalized PEG gels (with laminin-111, collagen IV, HA acid, and perlecan) | Synthetic hydrogel | High matrix stiffness significantly enhanced ISCs expansion through a YAP-dependent mechanism | [47] | |
| ISCs | 3D printing: PEG elastomers; matrices: patterned Matrigel | Hybrid hydrogel (synthetic-natural) | NA | [48] | |
| ASCs | A cross-linked collagen hydrogel | Natural hydrogel | Activation of the Wnt signaling pathway to support stem cells while Noggin inhibits BMP to block differentiation | [49] | |
| ISCs | Allyl sulfide photodegradable hydrogel | Synthetic hydrogel | NA | [50] | |
| Retina | iPSCs | Matrigel | Natural hydrogel | NA | [19] |
| hPSCs, including hESC and hiPSC | Matrigel | Natural hydrogel | Exposure to BMP4 | [51] | |
| mESCs | Vinyl sulfone-functionalized 4-arm and 8-arm PEG macromers | Synthetic hydrogel | NA | [52] | |
| hESCs | Matrigel | Natural hydrogel | Depletion of RB using CRISPR/Cas9 system | [20] | |
| Brain or neural tube | hESCs and iPSC | Matrigel | Natural hydrogel | Human-specific expression was mapped in adult prefrontal cortex using single-nucleus RNA sequencing analysis and identification of developmental differences that persist into adulthood | [53] |
| hiPSCs | Matrigel | Natural hydrogel | PI3K-AKT-mTOR signaling | [18] | |
| Human-induced pluripotent stem cells (iPSCs) | Decellularized human BEM-incorporated hydrogel | Natural hydrogel | RNA-SEQ | [54] | |
| hESCs | B-ECM hydrogel | Natural hydrogel | protein analysis | [55] | |
| hPSCs | Cell-Mate 3D hydrogels (HA-Na and chitosan) | Hybrid hydrogel (synthetic-natural) | NA | [56] | |
| NSCs | GelMA | Synthetic hydrogel | mimic the mechanical modulus of soft tissue while supporting the formation of self-organizing neurospheroids within elaborate 3D networks | [23] | |
| iPSCs | Collagen hydrogel | Natural hydrogel | RNA-SEQ | [17] | |
| mESCs | PEG microwells | Synthetic hydrogel | WNT signaling | [57] | |
| iPSCs | Matrigel was modified with an IPN of alginate | Hybrid hydrogel (natural-natural) | Stiffer matrices skewed cell populations towards mature neuronal phenotype | [58] | |
| hiPSCs | Heparin and HA Acid | Natural hydrogel | Wnt and Hippo/YAP signaling | [59] | |
| hPSCs | Matrigel | Natural hydrogel | CRISPR/Cas9; HML-2 activation leads to defective forebrain organoid patterning | [16] | |
| hPSCs | PEG | Synthetic hydrogel | Organoid response to stretch is mediated extracellularly by matrix stiffness and intracellularly by cytoskeleton contractility and planar cell polarity | [60] | |
| mESCs | PEG | Synthetic hydrogel | NA | [61] | |
| hESCs | Alginate | Natural hydrogel | NA | [62] | |
| Heart or cardiovascular organoid | hiPSCs | Collagen | Natural hydrogel | NA | [22] |
| hPSCs | Collagen-based ECM hydrogel | Natural hydrogel | Upregulation of key Ca2+-handling, ion channel, and cardiac-specific proteins | [21] | |
| ESCs | Collagen-conjugated PAAm hydrogels (natural-synthetic) | Hybrid hydrogel (synthetic-natural) | Modulating the stiffness of a cell adherent hydrogel | [63] | |
| Kidney | hiPSCs | Thiol–ene cross-linked alginate hydrogel | Synthetic hydrogel | A reduction of abnormal type 1a1 collagen expression | [26] |
| hPSCs | A kidney dECM hydrogel | Natural hydrogel | CRISPR/Cas9 | [25] | |
| hPSCs | Natural chick CAM | Natural hydrogel | Physiologically relevant soft microenvironment could favor the differentiation of kidney organoids | [24] | |
| Lung | hPSCs | PEG; PCL or PLG | Synthetic hydrogel | Microporous scaffold can affect lung airway formation, airway size, and explant size | [27] |
| hPSCs, hESCs and hiPSCs | Matrigel | Natural hydrogel | RNA-SEQ | [28] | |
| Tooth germ organoids | pDM progenitor cells | GelMA | Synthetic hydrogel | NA | [29] |
| Salivary gland | ESCs | Matrigel | Natural hydrogel | Sox9 and Foxc1 | [64] |
| Human single clonal SGSCs | PEG with an electrospun PCLnanofibrous scaffold | Synthetic hydrogel | NA | [65] | |
| hSMGepiS/PCs | Matrigel | Natural hydrogel | FGF10 | [31] | |
| Lingual epithelium organoids | Adult epithelial stem cells | Matrigel | Natural hydrogel | EGF, noggin, and R-spondin 1 | [30] |
| Taste bud organoids | Lgr5+ or Lgr6+ taste bud stem cells | Matrigel | Natural hydrogel | R-spondin 1, Noggin, Jagged 1, Y27632, N-acetylcysteine, EGF, N2, and B27 | [66] |
| Adult taste stem/progenitor cells | Matrigel | Natural hydrogel | Wnt, EGF, R-spondin 1, and Noggin | [32] | |
| Mouse circumvallate stem cells | Matrigel | Natural hydrogel | Toll-like receptors (TLRs)-mediated inflammatory cytokine expression | [67] | |
| Liver | Liver progenitor cells | PEG-RGD | Synthetic hydrogel | Organoid growth is stiffness-sensitive, independent of actomyosin contractility, and requiring instead activation of the SFKs and YAP | [33] |
| Porcine liver-derived MSCs | HA hydrogels | Natural hydrogel | Expression of MMPs | [68] | |
| hiPSCs | CHCs composed of a fibrin hydrogel core and an alginate-chitosan composite shell | Hybrid hydrogel (natural-natural) | NA | [69] | |
| MSCs | alginate hydrogels | Natural hydrogel | NA | [70] | |
| Pancreas and islet | hESCs | Matrigel | Natural hydrogel | Physical culture conditions greatly influence the interactions among these cell types | [71] |
| hiPSCs | Na-alginate (NaA) and chitosan | Hybrid hydrogel (natural-natural) | NA | [72] | |
| hPSCs | amikagel | Synthetic hydrogel | Amikagel-induced hESC-PP spheroid formation enhanced pancreatic islet-specific Pdx-1 and NKX6.1 gene and protein expression while also increasing the percentage of committed population | [73] | |
| hiPSCs | ADHFs | Natural hydrogel | The established system enabled the formation of functional human islet organoids in situ by encapsulating pancreatic endocrine progenitor cells within microfibers | [74] | |
| Muscle | iPSCs | Fibrin hydrogels | Natural hydrogel | NA | [75] |
| human muscle progenitor cells are cocultured with HUVECs | Fibrin hydrogel | Natural hydrogel | NA | [34] | |
| Cartilage | AdSCs | PLA, ADH | Hybrid hydrogel (synthetic-synthetic) | TGF-β1 and IGF-1 | [35] |
| MSCs | Gelatin-based microscopic hydrogel (microcryogels) | Natural hydrogel | Self-assembly was induced by the connection of microniches through ECM accumulation secreted by MSCs | [76] | |
| Vascular organoids | hPSCs | Methylcellulose-based hydrogel system | Natural hydrogel | NA | [77] |
| co-culture of ECs and MSCs (of either mouse or human origin) | Alginate microwells | Natural hydrogel | NA | [78] | |
| HUVEC and hMSCs | GelMA and fibrin gel, with TCP particles | Hybrid hydrogel (synthetic-natural) | NA | [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yue, M.; Liu, Y.; Zhang, P.; Qing, J.; Liu, H.; Zhou, Y. Advances of Engineered Hydrogel Organoids within the Stem Cell Field: A Systematic Review. Gels 2022, 8, 379. https://doi.org/10.3390/gels8060379
Li Z, Yue M, Liu Y, Zhang P, Qing J, Liu H, Zhou Y. Advances of Engineered Hydrogel Organoids within the Stem Cell Field: A Systematic Review. Gels. 2022; 8(6):379. https://doi.org/10.3390/gels8060379
Chicago/Turabian StyleLi, Zheng, Muxin Yue, Yunsong Liu, Ping Zhang, Jia Qing, Hao Liu, and Yongsheng Zhou. 2022. "Advances of Engineered Hydrogel Organoids within the Stem Cell Field: A Systematic Review" Gels 8, no. 6: 379. https://doi.org/10.3390/gels8060379
APA StyleLi, Z., Yue, M., Liu, Y., Zhang, P., Qing, J., Liu, H., & Zhou, Y. (2022). Advances of Engineered Hydrogel Organoids within the Stem Cell Field: A Systematic Review. Gels, 8(6), 379. https://doi.org/10.3390/gels8060379







