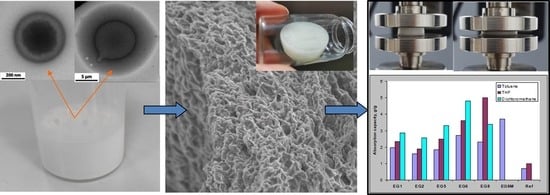
Emulsion Gels as Precursors for Porous Silicones and All-Polymer Composites—A Proof of Concept Based on Siloxane Stabilizers
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
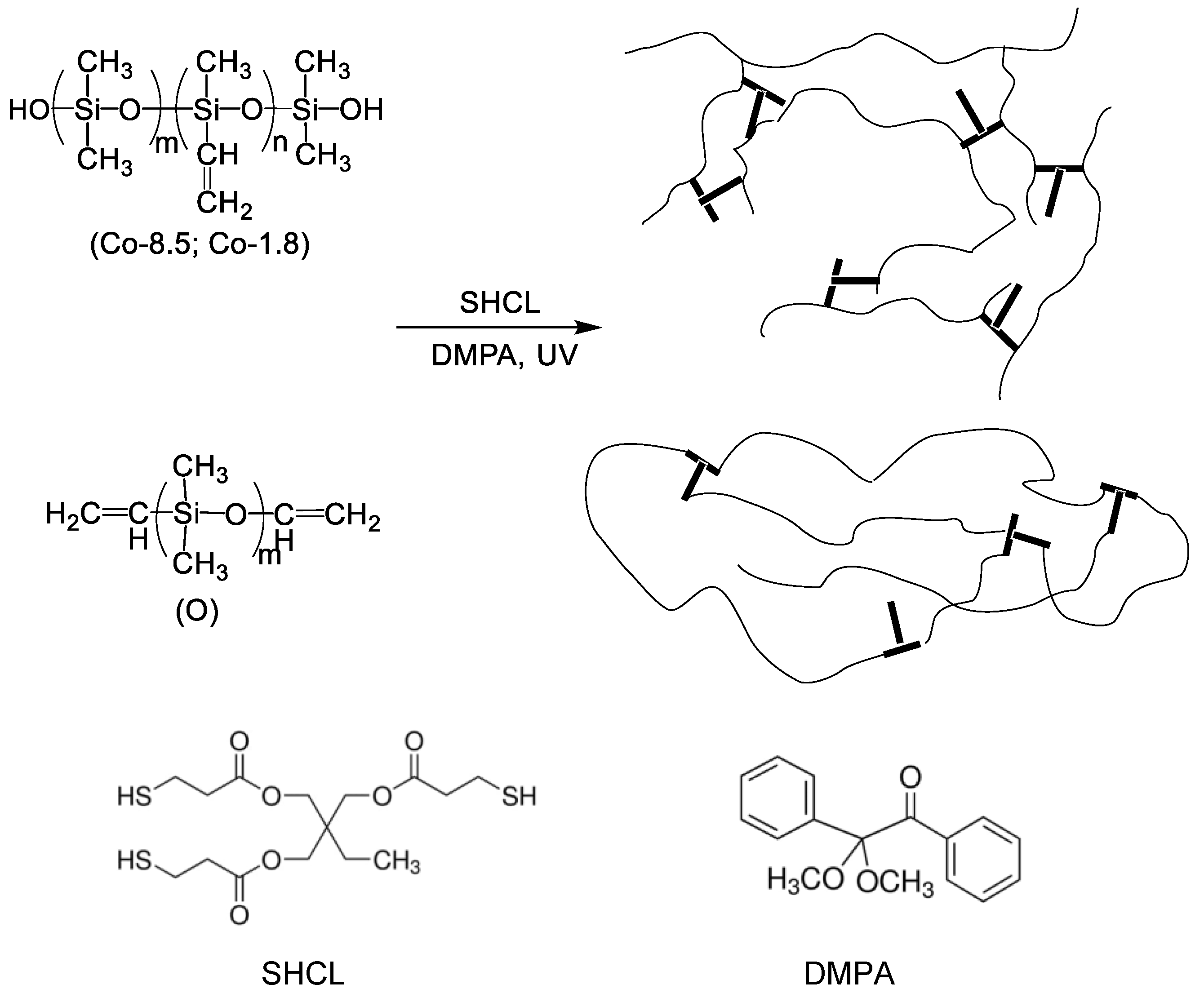
2.3. Preparation of Emulsion Gels and Xerogels
3. Results and Discussion
3.1. Surface Properties of the Stabilizers
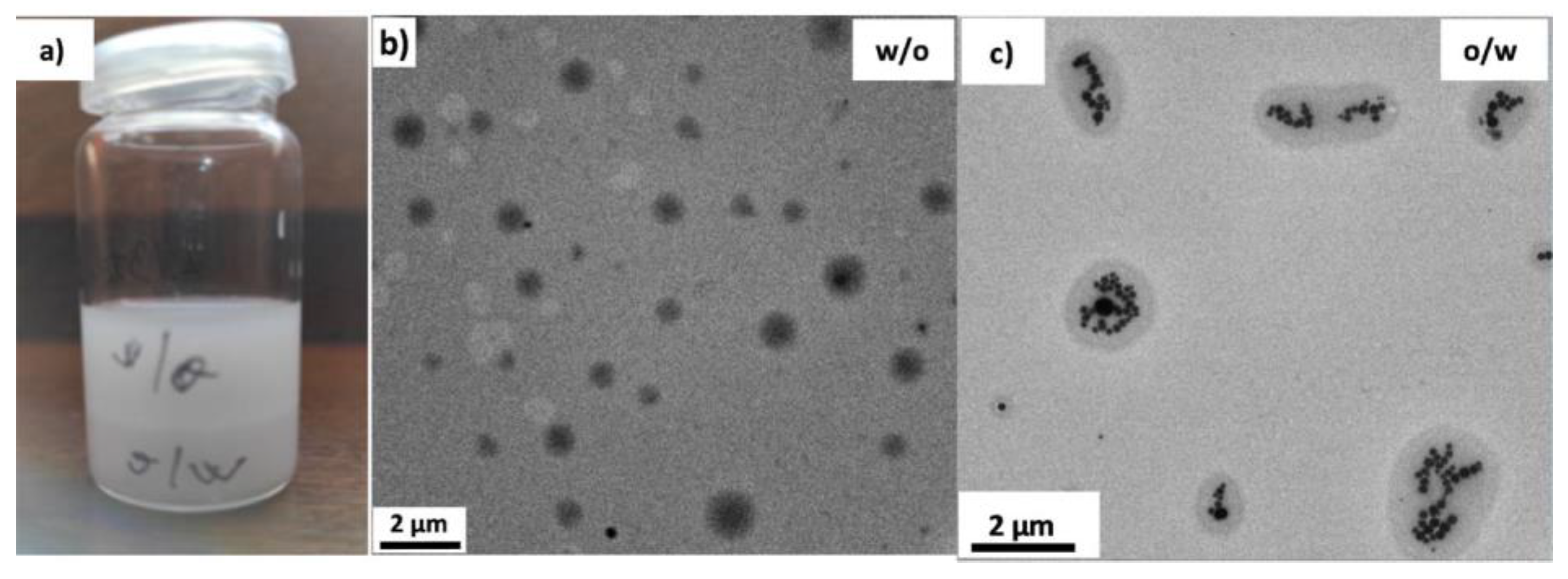
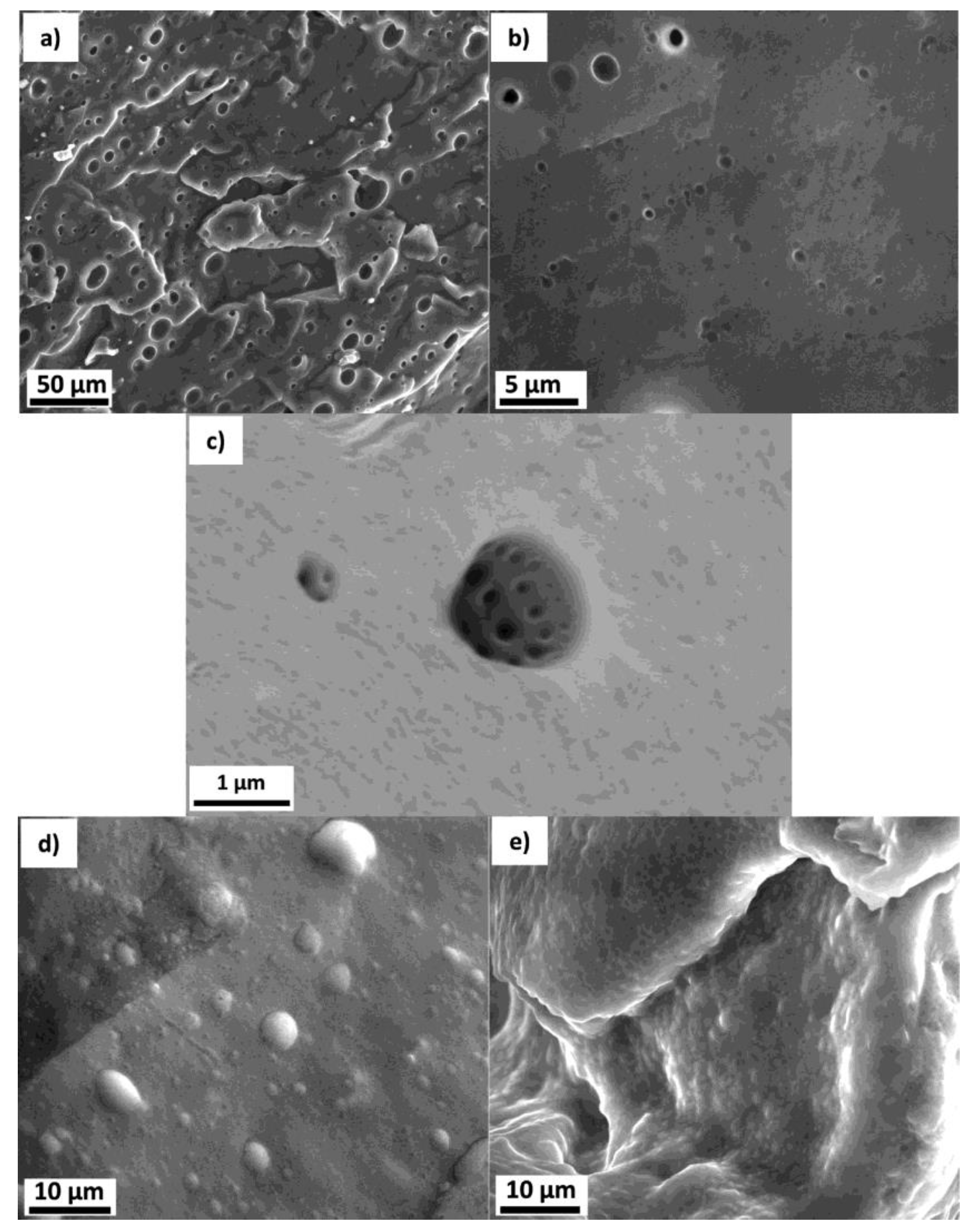
3.2. Morphology of the Emulsions and Templated Xerogels
3.3. Brief Characterization of the Obtained Xerogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mudassir, M.A.; Aslam, H.Z.; Ansari, T.M.; Zhang, H.; Hussain, I. Fundamentals and design-led synthesis of emulsion-templated porous materials for environmental applications. Adv. Sci. 2021, 8, 2102540. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Cui, J.; Zhang, W.; Feng, K.; Liang, Y.; Wang, S.; Wang, P.; Zhou, K.; Li, G. Observation of osmotically driven, highly controllable and reconfigurable oil/water phase separation. Chem. Sci. 2019, 10, 7887–7897. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mao, L.; Hou, Z.; Miao, S.; Gao, Y. Development of emulsion gels for the delivery of functional food ingredients: From structure to functionality. Food Eng. Rev. 2019, 11, 245–258. [Google Scholar] [CrossRef]
- Chen, X.; Liu, K.; He, P.; Zhang, H.; Fang, Y. Preparation of novel W/O gel-emulsions and their application in the preparation of low-density materials. Langmuir 2012, 28, 9275–9281. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Peng, J.; Fang, Y. Molecular gels as intermediates in the synthesis of porous materials and fluorescent films: Concepts and applications. Langmuir 2017, 33, 10419–10428. [Google Scholar] [CrossRef]
- Soldatov, M.; Liu, H. Hybrid porous polymers based on cage-like organosiloxanes: Synthesis, properties and applications. Prog. Polym. Sci. 2021, 119, 101419. [Google Scholar] [CrossRef]
- Si, J.; Cui, Z.; Xie, P.; Song, L.; Wang, Q.; Liu, Q.; Liu, C. Characterization of 3D elastic porous polydimethylsiloxane (PDMS) cell scaffolds fabricated by VARTM and particle leaching. J. Appl. Polym. Sci. 2016, 133, 42909. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, D.; Wang, J.; Shao, L.-H. Ultrahigh flexoelectric effect of 3D interconnected porous polymers: Modelling and verification. J. Mech. Phys. Solids 2021, 151, 104396. [Google Scholar] [CrossRef]
- Rinaldi, A.; Tamburrano, A.; Fortunato, M.; Sarto, M.S. A flexible and highly sensitive pressure sensor based on a PDMS foam coated with graphene nanoplatelets. Sensors 2016, 16, 2148. [Google Scholar] [CrossRef]
- Zimny, K.; Merlin, A.; Ba, A.; Aristégui, C.; Brunet, T.; Mondain-Monval, O. Soft porous silicon rubbers as key elements for the realization of acoustic metamaterials. Langmuir 2015, 31, 3215–3221. [Google Scholar] [CrossRef]
- Ba, A.; Kovalenko, A.; Aristégui, C.; Mondain-Monval, O.; Brunet, T. Soft porous silicone rubbers with ultra-low sound speeds in acoustic metamaterials. Sci. Rep. 2017, 7, 40106. [Google Scholar] [CrossRef] [PubMed]
- Ganachaud, F.; Fleury, E.; Larribe, G.; Mariot, D.; Marchal, F. Method for Producing Porous Silicone Materials. U.S. Patent Application No. 17/250,067 US20210206938 A1, 8 July 2021. [Google Scholar]
- Zhang, D.; Xia, Y.; Chen, X.; Shi, S.; Lei, L. PDMS infused poly (high internal phase emulsion) template for the construction of slippery liquid-infused porous surface with self-cleaning and self-repairing properties. Langmuir 2019, 35, 8276–8284. [Google Scholar] [CrossRef] [PubMed]
- Salager, J.-L.; Forgiarini, A.M.; Márquez, L.; Manchego, L.; Bullón, J. How to attain an ultralow interfacial tension and a three-phase behavior with a surfactant formulation for enhanced oil recovery: A review. Part 2. Performance improvement trends from Winsor’s premise to currently proposed inter- and intra-molecular mixtures. J. Surfact. Deterg. 2013, 16, 631–663. [Google Scholar] [CrossRef]
- Tolman, R.C. Colloids and negative surface tension. Science 1916, 44, 565–567. [Google Scholar] [CrossRef][Green Version]
- Kimball, A.L. Negative surface tension. Science 1917, 45, 85–87. [Google Scholar] [CrossRef]
- van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Monopolar surfaces. Adv. Colloid Interface Sci. 1987, 28, 35–64. [Google Scholar] [CrossRef]
- Kaptay, G. On the negative surface tension of solutions and on spontaneous emulsification. Langmuir 2017, 33, 10550–10560. [Google Scholar] [CrossRef]
- van Oss, C.J.; Ju, L.; Chaudhury, M.K.; Good, R.J. Estimation of the polar parameters of the surface tension of liquids by contact angle measurements on gels. J. Colloid. Interface Sci. 1989, 128, 313–319. [Google Scholar] [CrossRef]
- Peddireddy, K.; Čopar, S.; Le, K.V.; Muševič, I.; Bahr, C.; Jampani, V.S.R. Self-shaping liquid crystal droplets by balancing bulk elasticity and interfacial tension. Proc. Natl. Acad. Sci. USA 2021, 118, e2011174118. [Google Scholar] [CrossRef]
- Prince, L.M. A theory of aqueous emulsions I. Negative interfacial tension at the oil/water interface. J. Colloid. Interface Sci. 1967, 23, 165–173. [Google Scholar] [CrossRef]
- Racles, C.; Dascalu, M.; Bele, A.; Cazacu, M. Reactive and functional silicones for special applications. In Reactive and Functional Polymers; Guitierrez, T., Ed.; Springer: Cham Switzerland, 2020; Volume 1, pp. 235–291. [Google Scholar] [CrossRef]
- Czajka, A.; Hazell, G.; Eastoe, J. Surfactants at the design limit. Langmuir 2015, 31, 8205–8217. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.M. (Ed.) Silicone Surfactants; Marcel Dekker: New York, NY, USA, 1999. [Google Scholar]
- Bao, Y.; Guo, J.; Ma, J.; Liu, P.; Kang, Q.; Zhang, J. Cationic silicon-based gemini surfactants: Effect of hydrophobic chains on surface activity, physic-chemical properties and aggregation behaviors. J. Ind. Eng. Chem. 2017, 53, 51–61. [Google Scholar] [CrossRef]
- Racles, C.; Silion, M.; Sacarescu, L. Multi-tasking pyridyl-functionalized siloxanes. Colloids Surf. A Physicochem. Eng. Asp. 2018, 547, 102–110. [Google Scholar] [CrossRef]
- Cazacu, M.; Marcu, M.; Holerca, M.; Petrovan, S.; Lazarescu, S. Heterogeneous catalyzed copolymerization of octamethylcyclotetrasiloxane with 1,3,5,7-tetravinyl-1,3,5,7-tetramethylcyclo-tetrasiloxane. J. Macromol. Sci.-Pure and Appl. Chem. A 1996, 33, 65–76. [Google Scholar] [CrossRef]
- Northrop, B.H.; Coffey, R.N. Thiol-ene click chemistry: Computational and kinetic analysis of the influence of alkene functionality. J. Am. Chem. Soc. 2012, 134, 13804–13817. [Google Scholar] [CrossRef] [PubMed]
- Derboven, P.; D’hooge, D.R.; Stamenovic, M.M.; Espeel, P.; Marin, G.B.; Du Prez, F.E.; Reyniers, M.-F. Kinetic modeling of radical thiol-ene chemistry for macromolecular design: Importance of side reactions and diffusional limitations. Macromolecules 2013, 46, 1732–1742. [Google Scholar] [CrossRef]
- Opris, D.; Dunki, S.; Racles, C.; Bele, A.; Cazacu, M. High Permittivity Polymers Based on Functionalized Silicones. Patent No. WO2015135086A1, 13 March 2015. [Google Scholar]
- Racles, C.; Cozan, V.; Bele, A.; Dascalu, M. Polar silicones: Structure-dielectric properties relationship. Des. Monomers Polym. 2016, 19, 496–507. [Google Scholar] [CrossRef]
- Cozma, V.; Rosca, I.; Radulescu, L.; Martu, C.; Nastasa, V.; Varganici, C.D.; Ursu, E.L.; Doroftei, F.; Pinteala, M.; Racles, C. Antibacterial polysiloxane polymers and coatings for cochlear implants. Molecules 2021, 26, 4892. [Google Scholar] [CrossRef]
- Di Luca, A.; Ostrowska, B.; Lorenzo-Moldero, I.; Lepedda, A.; Swieszkowski, W.; Van Blitterswijk, C.; Moroni, L. Gradients in pore size enhance the osteogenic differentiation of human mesenchymal stromal cells in three-dimensional scaffolds. Sci. Rep. 2016, 6, 22898. [Google Scholar] [CrossRef]
- Bronner, H.; Holzer, A.-K.; Finke, A.; Kunkel, M.; Marx, A.; Leist, M.; Polarz, S. The influence of structural gradients in large pore organosilica materials on the capabilities for hosting cellular communities. RSC Adv. 2020, 10, 17327–17335. [Google Scholar] [CrossRef] [PubMed]
- Abshirini, M.; Saha, M.C.; Cummings, L.; Robison, T. Synthesis and characterization of porous polydimethylsiloxane structures with adjustable porosity and pore morphology using emulsion templating technique. Polym Eng. Sci. 2021, 61, 1943–1955. [Google Scholar] [CrossRef]
- Zhao, X.; Li, L.; Li, B.; Zhang, J.; Wang, A. Durable superhydrophobic / superoleophilic PDMS sponges and their applications in selective oil absorption and in plugging oil leakages. Mater. Chem. A 2014, 2, 18281–18287. [Google Scholar] [CrossRef]
- Yan, H.; Wang, K.; Zhao, Y. Fabrication of silicone rubber foam with tailored porous structures by supercritical CO2. Macromol. Mater. Eng. 2016, 302, 1600377. [Google Scholar] [CrossRef]


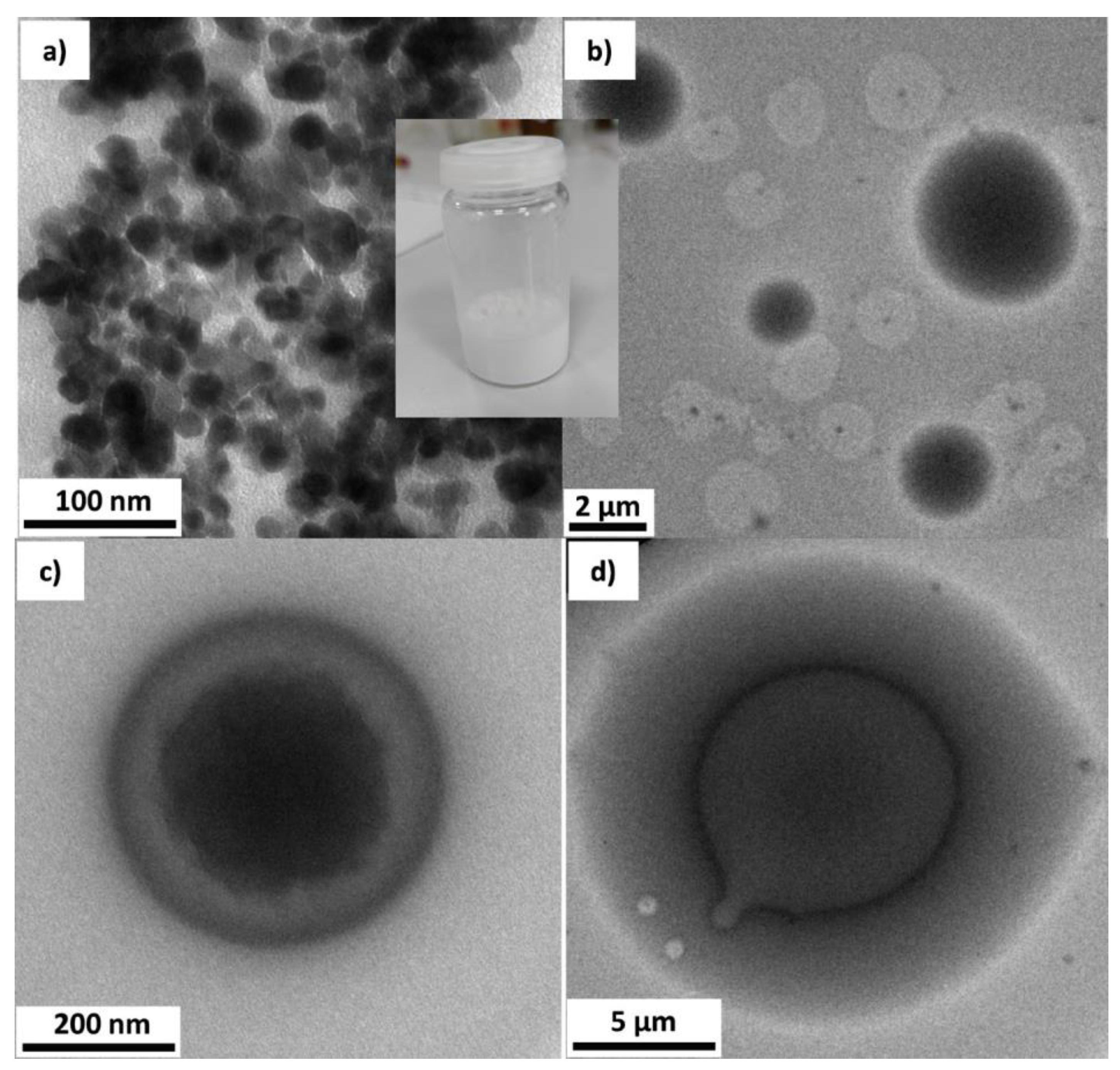




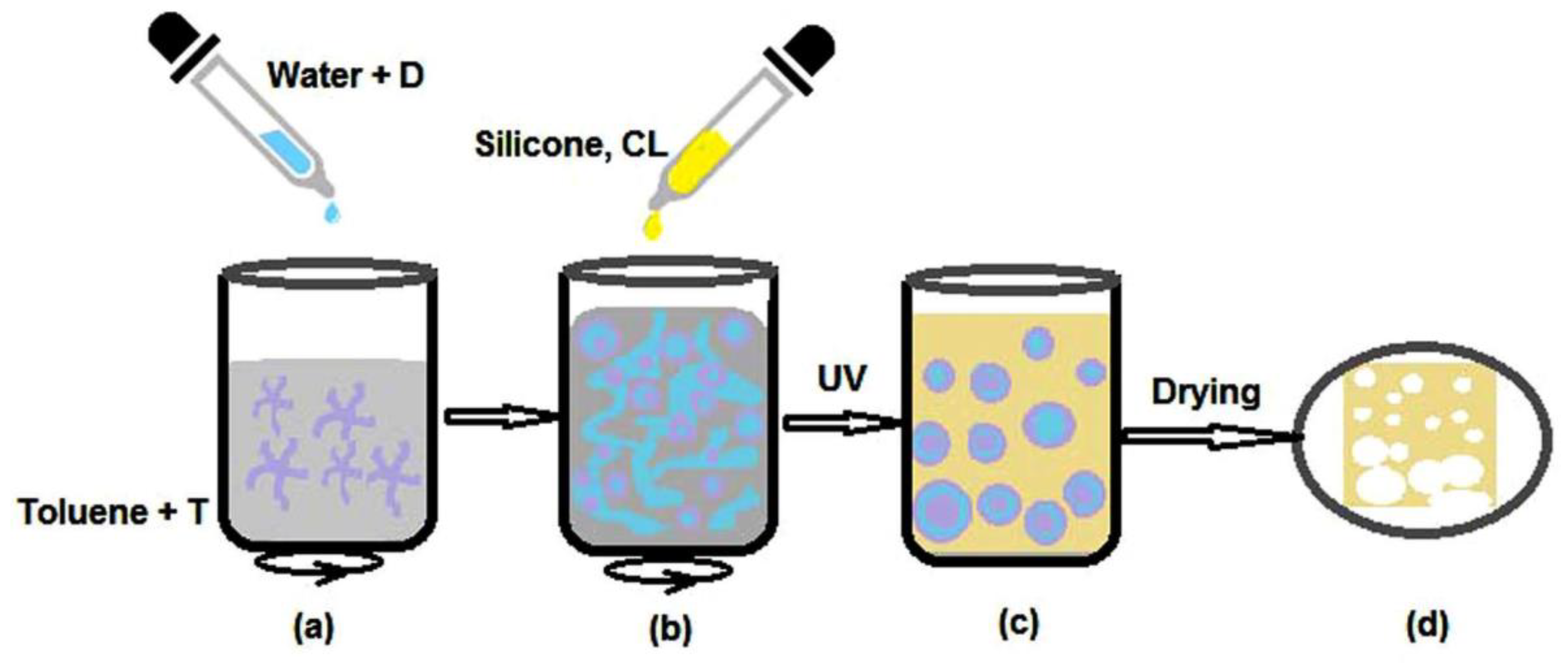

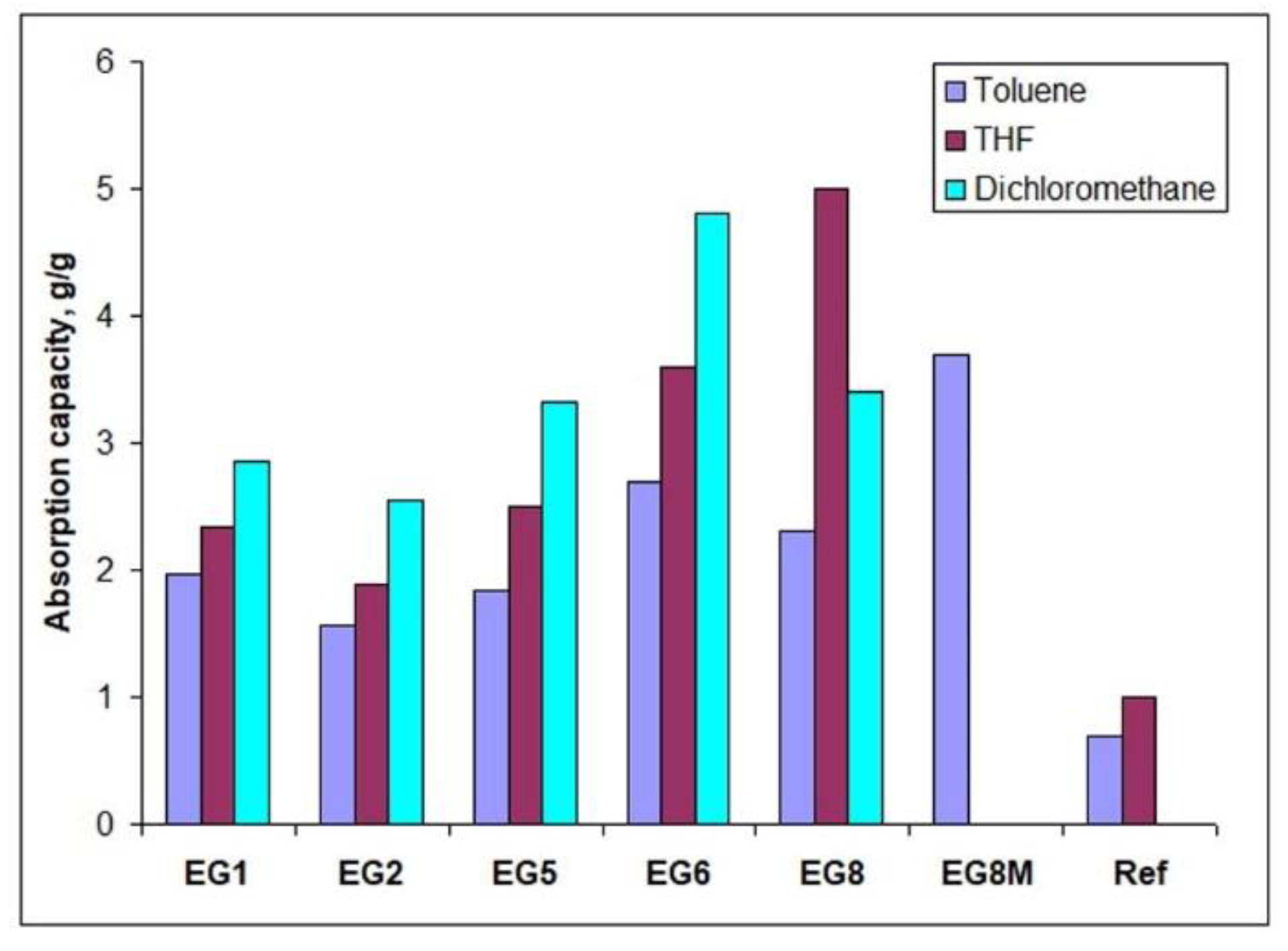
| Code | Surfactants, Conc. | Silicone Type, Conc. (w/v) a | Water Content (f) b | Additive, Conc. c/ Other Conditions |
|---|---|---|---|---|
| PE1 | T, 1 g/L | Co-8.5, 20% | 0.1 | |
| PE2 | T, 1% | Co-8.5, 20% | 0.16 | |
| PE3 | T, 1% | Co-8.5, 20% | 0.1 | PEG, 10% |
| EG1 | T, 1% and D, 1% | Co-8.5, 20% | 0.16 | |
| EG2 | T, 1% and D, 1% | Co-8.5, 20% | 0.25 | |
| EG3 | T, 1% and D, 1% | Co-8.5, 20% | 0.25 | PEG 10% |
| EG4 | T, 1% and D, 1% | Co-8.5, 20% | 0.33 | PEG 20% |
| EG5 | T, 1% and D, 1% | Co-8.5, 20% | 0.33 | |
| EG6 | T, 1% and D, 1% | Co-8.5, 20% | 0.5 | Very porous film separated |
| EG6M | Monolith | |||
| EG7 | T, 5% and D,1% | O, 110% | 0.82 | Phase separation: thin film and latex |
| EG8 | T, 1% and D, 1% | Co-1.8, 20% | 0.5 | Film separated from liquid |
| EG8M | Monolith | |||
| EG9M | T, 1% in D4 and D, 1% | Co-1.8, 20% | 0.5 (vs. D4) | Monolith |
| EG10M | T, 1% and D, 1% | Co-1.8, 10% | 0.5 | Monolith |
| Sample | Surface Tension, γ (mN/m) | Interfacial Tension, σ (mN/m) | Surface Tension of Emulsion, γ (mN/m) |
|---|---|---|---|
| D (water) | 35.65 (±0.27) | −4.21 (±0.3) | 26.37 (±3.5) o/w |
| T (toluene) | 25.28 (±0.025) | −2.95 (±0.15) | 25.33 (±0.21) w/o 25.91 (±0.9) o/w |
| Sample | CA Upper Side (°) | CA Bottom Side (°) |
|---|---|---|
| EG4 | 117.2 ± 6.5 | 93.8 ± 3.1 |
| EG5 | 111.7 ± 4.6 | 66.8 ± 5.8 |
| EG6 | 75 ± 18 | 73.7 ± 14 |
| EG8 | 124.3 ± 6.1 | 97.1 ± 6.3 |
| Code | Composition | Y, MPa | Obs. |
|---|---|---|---|
| EG1 | f = 0.16 | 0.64 | film |
| EG2 | f = 0.25 | 0.70 | film |
| EG3 | f = 0.25; PEG 10% | 0.65 | film |
| EG4 | f = 0.33; PEG 20% | 0.49 | film |
| EG5 | f = 0.33 | 0.44 | film |
| EG6 | f = 0.5 | 0.45 | film |
| EG8 | f = 0.5 (Co-1.8) | 0.33 | film |
| EG6M | f = 0.5 (Co-8.5) | 0.032 | Dried monolith |
| EG9M | f = 0.5 (Co-1.8; D4) | 0.010 | Monolith swollen in D4 |
| EG10M | f = 0.5 (Co-1.8, 10%) | 0.008 | Swollen monolith |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racles, C.; Bele, A.; Vasiliu, A.-L.; Sacarescu, L. Emulsion Gels as Precursors for Porous Silicones and All-Polymer Composites—A Proof of Concept Based on Siloxane Stabilizers. Gels 2022, 8, 377. https://doi.org/10.3390/gels8060377
Racles C, Bele A, Vasiliu A-L, Sacarescu L. Emulsion Gels as Precursors for Porous Silicones and All-Polymer Composites—A Proof of Concept Based on Siloxane Stabilizers. Gels. 2022; 8(6):377. https://doi.org/10.3390/gels8060377
Chicago/Turabian StyleRacles, Carmen, Adrian Bele, Ana-Lavinia Vasiliu, and Liviu Sacarescu. 2022. "Emulsion Gels as Precursors for Porous Silicones and All-Polymer Composites—A Proof of Concept Based on Siloxane Stabilizers" Gels 8, no. 6: 377. https://doi.org/10.3390/gels8060377
APA StyleRacles, C., Bele, A., Vasiliu, A.-L., & Sacarescu, L. (2022). Emulsion Gels as Precursors for Porous Silicones and All-Polymer Composites—A Proof of Concept Based on Siloxane Stabilizers. Gels, 8(6), 377. https://doi.org/10.3390/gels8060377