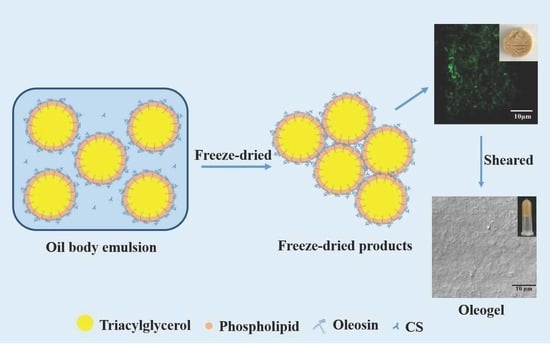
Gel Properties and Formation Mechanism of Camellia Oil Body-Based Oleogel Improved by Camellia Saponin
Abstract
:1. Introduction
2. Results and Discussion
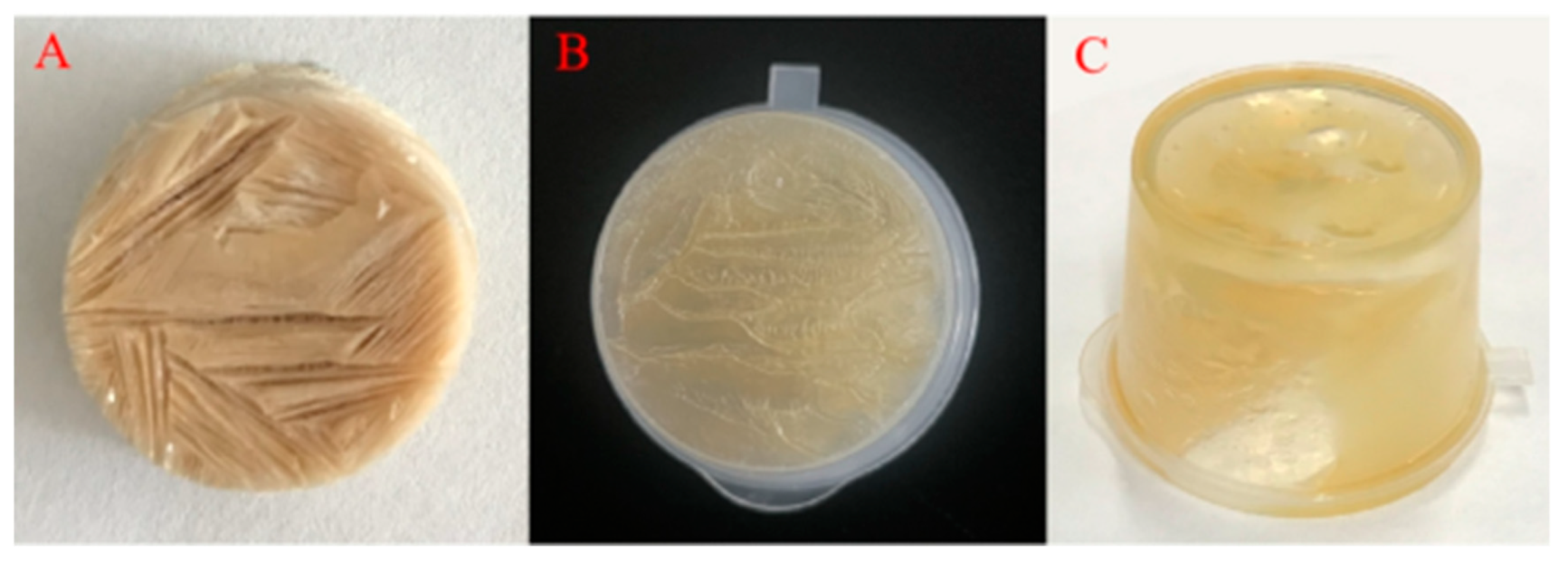
2.1. Formation of COBO
2.2. Microstructure and Texture of Freeze-Dried Products
2.3. Characterization of COBO
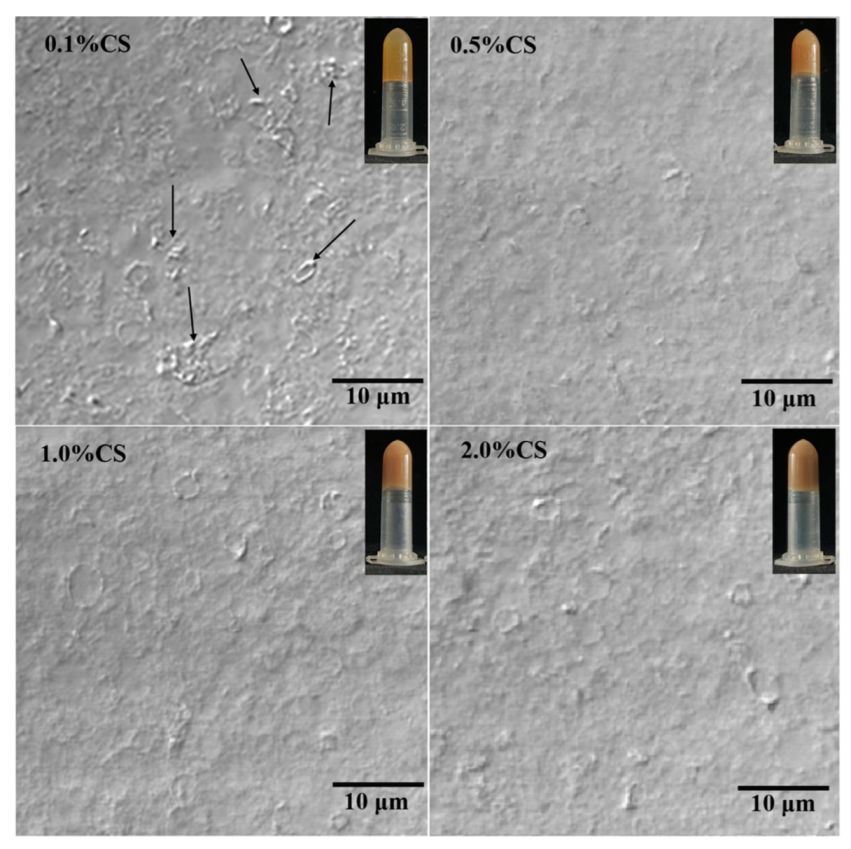
2.3.1. Appearance and Microstructure of COBO
2.3.2. Rheological Properties of COBO
2.3.3. Thermal Stability of COBO
2.3.4. LF-NMR of COBO
2.4. Spectral Characteristics of COBO
2.5. The Mechanism of COBO Formation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Isolation and Purification of Camellia Oil Body (COB)
4.3. Preparation of COBO
4.4. Microstructure Observation
4.5. Texture Test
4.6. Rheological Analysis of COBO
4.7. Thermogravimetric (TG) Analysis
4.8. LF-NMR
4.9. Spectroscopic Analysis
4.9.1. Front-Face Fluorescence Spectroscopy (FFFS)
4.9.2. FTIR Spectroscopy
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, A.R.; Dewettinck, K. Comparative evaluation of structured oil systems-Shellac oleogel, HPMC oleogel, and HIPE gel. Eur. J. Lipid Sci. Technol. 2015, 117, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Qiu, Z.; Chen, Z.; Liu, L.; Wang, J.; Jiang, H.; Zhang, H.; Liu, G.-Q. Antioxidant properties of blueberry extract in different oleogel systems. LWT 2021, 137, 110364. [Google Scholar] [CrossRef]
- Zhang, R.; Cui, M.; Ye, J.; Yuan, D.; Mao, L. Physicochemical stability of oleogel-in-water emulsions loaded with β-carotene against environmental stresses. LWT 2022, 155, 112965. [Google Scholar] [CrossRef]
- Sun, P.; Xia, B.; Ni, Z.-J.; Wang, Y.; Elam, E.; Thakur, K.; Ma, Y.-L.; Wei, Z.-J. Characterization of functional chocolate formulated using oleogels derived from β-sitosterol with γ-oryzanol/lecithin/stearic acid. Food Chem. 2021, 360, 130017. [Google Scholar] [CrossRef] [PubMed]
- Moghtadaei, M.; Soltanizadeh, N.; Goli, S.A.H.; Sharifimehr, S. Physicochemical properties of beef burger after partial incorporation of ethylcellulose oleogel instead of animal fat. J. Food Sci. Technol. 2021, 58, 4775–4784. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.R.; Ghosh, S. Canola protein thermal denaturation improved emulsion-templated oleogelation and its cake-baking application. RSC Adv. 2021, 11, 25141–25157. [Google Scholar] [CrossRef]
- Li, L.; Liu, G. Corn oil-based oleogels with different gelation mechanisms as novel cocoa butter alternatives in dark chocolate. J. Food Eng. 2019, 263, 114–122. [Google Scholar] [CrossRef]
- Martins, A.J.; Vicente, A.A.; Cunha, R.L.; Cerqueira, M.A. Edible oleogels: An opportunity for fat replacement in foods. Food Funct. 2018, 9, 758–773. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wijarnprecha, K.; Sonwai, S.; Rousseau, D. Oleogelation of emulsified oil delays in vitro intestinal lipid digestion. Food Res. Int. 2019, 119, 805–812. [Google Scholar] [CrossRef]
- Vellido-Perez, J.A.; Ochando-Pulido, J.M.; la Fuente, E.B.; Martinez-Ferez, A. Effect of operating parameters on the physical and chemical stability of an oil gelled-in-water emulsified curcumin delivery system. J. Sci. Food Agric. 2021, 101, 6395–6406. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Auzanneau, F.-I.; Rogers, M. Advances in edible oleogel technologies–A decade in review. Food Res. Int. 2017, 97, 307–317. [Google Scholar] [CrossRef]
- Rosen-Kligvasser, J.; Davidovich-Pinhas, M. The role of hydrogen bonds in TAG derivative-based oleogel structure and properties. Food Chem. 2021, 334, 127585. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Ramírez, D.; Reyes, I.; Lobato-Calleros, C.; Vernon-Carter, E.; Alvarez-Ramirez, J. Chia seed oil-candelilla wax oleogels structural features and viscoelasticity are enhanced by annealing. LWT 2022, 153, 112433. [Google Scholar] [CrossRef]
- Han, W.; Chai, X.; Liu, Y.; Xu, Y.; Tan, C.-P. Crystal network structure and stability of beeswax-based oleogels with different polyunsaturated fatty acid oils. Food Chem. 2021, 381, 131745. [Google Scholar] [CrossRef]
- Gaudino, N.; Ghazani, S.M.; Clark, S.; Marangoni, A.G.; Acevedo, N.C. Development of lecithin and stearic acid based oleogels and oleogel emulsions for edible semisolid applications. Food Res. Int. 2019, 116, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, W.; Wang, X.; Cheng, S.; Zhou, J.; Wu, Z.; Li, Y. Fabrication and physicochemical and antibacterial properties of ethyl cellulose-structured cinnamon oil oleogel: Relation between ethyl cellulose viscosity and oleogel performance. J. Sci. Food Agric. 2019, 99, 4063–4071. [Google Scholar] [CrossRef] [PubMed]
- Bin Sintang, M.D.; Danthine, S.; Patel, A.R.; Rimaux, T.; Van De Walle, D.; Dewettinck, K. Mixed surfactant systems of sucrose esters and lecithin as a synergistic approach for oil structuring. J. Colloid Interface Sci. 2017, 504, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Lo, Y.M.; Yan, M.; Li, P.; Cao, Y. Characterization of thermo-oxidative behavior of ethylcellulose oleogels. Food Chem. 2020, 305, 125470. [Google Scholar] [CrossRef]
- Tavernier, I.; Patel, A.R.; Van der Meeren, P.; Dewettinck, K. Emulsion-templated liquid oil structuring with soy protein and soy protein: κ-carrageenan complexes. Food Hydrocoll. 2017, 65, 107–120. [Google Scholar] [CrossRef]
- Zheng, H.; Mao, L.; Cui, M.; Liu, J.; Gao, Y. Development of food-grade bigels based on κ-carrageenan hydrogel and monoglyceride oleogels as carriers for β-carotene: Roles of oleogel fraction. Food Hydrocoll. 2020, 105, 105855. [Google Scholar] [CrossRef]
- Luo, S.-Z.; Hu, X.-F.; Jia, Y.-J.; Pan, L.-H.; Zheng, Z.; Zhao, Y.-Y.; Mu, D.-D.; Zhong, X.-Y.; Jiang, S.-T. Camellia oil-based oleogels structuring with tea polyphenol-palmitate particles and citrus pectin by emulsion-templated method: Preparation, characterization and potential application. Food Hydrocoll. 2019, 95, 76–87. [Google Scholar] [CrossRef]
- Plazzotta, S.; Calligaris, S.; Manzocco, L. Structural characterization of oleogels from whey protein aerogel particles. Food Res. Int. 2020, 132, 109099. [Google Scholar] [CrossRef] [PubMed]
- Phoon, P.Y.; Henry, C.J. Fibre-based oleogels: Effect of the structure of insoluble fibre on its physical properties. Food Funct. 2020, 11, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Qi, K.; Guo, Y.; Wang, Y.; Liu, Y. Effects of thickening agents on the formation and properties of edible oleogels based on hydroxypropyl methyl cellulose. Food Chem. 2018, 246, 137–149. [Google Scholar] [CrossRef]
- Nikiforidis, C.; Kiosseoglou, V.; Scholten, E. Oil bodies: An insight on their microstructure—Maize germ vs sunflower seed. Food Res. Int. 2013, 52, 136–141. [Google Scholar] [CrossRef]
- Ishii, T.; Matsumiya, K.; Matsumura, Y. Combinational effects of acid and salt addition on colloidal, interfacial, and emulsifying properties of purified soybean oil bodies. Food Hydrocoll. 2021, 111, 106213. [Google Scholar] [CrossRef]
- Zhu, Z.; Wen, Y.; Yi, J.; Cao, Y.; Liu, F.; McClements, D.J. Comparison of natural and synthetic surfactants at forming and stabilizing nanoemulsions: Tea saponin, Quillaja saponin, and Tween 80. J. Colloid Interface Sci. 2019, 536, 80–87. [Google Scholar] [CrossRef]
- Zhang, S.; Tian, L.; Yi, J.; Zhu, Z.; Decker, E.A.; McClements, D.J. Mixed plant-based emulsifiers inhibit the oxidation of proteins and lipids in walnut oil-in-water emulsions: Almond protein isolate-camellia saponin. Food Hydrocoll. 2020, 109, 106136. [Google Scholar] [CrossRef]
- Ma, M.; Yuan, Y.; Yang, S.; Wang, Y.; Lv, Z. Fabrication and characterization of zein/tea saponin composite nanoparticles as delivery vehicles of lutein. LWT 2020, 125, 109270. [Google Scholar] [CrossRef]
- Yang, Z.; Li, W. Extraction and surface activity of tea saponin from Pu’er tea seeds. IOP Conf. Series Earth Environ. Sci. 2020, 585, 012149. [Google Scholar] [CrossRef]
- Tsibranska, S.; Tcholakova, S.; Golemanov, K.; Denkov, N.; Pelan, E.; Stoyanov, S.D. Role of interfacial elasticity for the rheological properties of saponin-stabilized emulsions. J. Colloid Interface Sci. 2020, 564, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Zhong, F.; Zhu, S.; Huang, D.; Li, Y. Formation, structural characteristics and physicochemical properties of beeswax oleogels prepared with tea polyphenol loaded gelators. Food Funct. 2021, 12, 1662–1671. [Google Scholar] [CrossRef]
- Miklos, R.; Cheong, L.-Z.; Xu, X.; Lametsch, R.; Larsen, F.H. Water and Fat Mobility in Myofibrillar Protein gels Explored by Low-Field NMR. Food Biophys. 2015, 10, 316–323. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Liu, C.; Liu, C. Application of LF-NMR to the characterization of camellia oil-loaded pickering emulsion fabricated by soy protein isolate. Food Hydrocoll. 2021, 112, 106329. [Google Scholar] [CrossRef]
- Kaspchak, E.; Kayukawa, C.T.M.; Silveira, J.L.M.; Igarashi-Mafra, L.; Mafra, M.R. Interaction of Quillaja bark saponin and bovine serum albumin: Effect on secondary and tertiary structure, gelation and in vitro digestibility of the protein. LWT 2020, 121, 108970. [Google Scholar] [CrossRef]
- Li, J.; Yu, H.; Yang, Y.; Drummond, C.J.; Conn, C.E. Effect of Crystallization State on the Gel Properties of Oleogels Based on β-sitosterol. Food Biophys. 2020, 16, 48–57. [Google Scholar] [CrossRef]
- Zhai, J.; Wooster, T.J.; Hoffmann, S.V.; Lee, T.-H.; Augustin, M.A.; Aguilar, M.-I. Structural Rearrangement of β-Lactoglobulin at Different Oil–Water Interfaces and Its Effect on Emulsion Stability. Langmuir 2011, 27, 9227–9236. [Google Scholar] [CrossRef]
- Matsakidou, A.; Tsimidou, M.Z.; Kiosseoglou, V. Storage behavior of caseinate-based films incorporating maize germ oil bodies. Food Res. Int. 2019, 116, 1031–1040. [Google Scholar] [CrossRef]
- Lavigne, P.; Tancrede, P.; Lamarche, F.; Max, J.J. The packing of hydrophobic. alpha.-helices: A study at the air/water interface. Langmuir 1992, 8, 1988–1993. [Google Scholar] [CrossRef]
- Tzen, J.T.; Huang, A.H. Surface structure and properties of plant seed oil bodies. J. Cell Biol. 1992, 117, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Rampon, V.; Riaublanc, A.; Anton, M.; Genot, A.C.; McClements, D.J. Evidence that Homogenization of BSA-Stabilized Hexadecane-in-Water Emulsions Induces Structure Modification of the Nonadsorbed Protein. J. Agric. Food Chem. 2003, 51, 5900–5905. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Xu, Z.; Qi, B.; Jiang, L.; Sui, X. Physicochemical and oxidative stability of a soybean oleosome-based emulsion and itsin vitrodigestive fate as affected by (−)-epigallocatechin-3-gallate. Food Funct. 2018, 9, 6146–6154. [Google Scholar] [CrossRef]
- Meng, Z.; Qi, K.; Guo, Y.; Wang, Y.; Liu, Y. Macro-micro structure characterization and molecular properties of emulsion-templated polysaccharide oleogels. Food Hydrocoll. 2018, 77, 17–29. [Google Scholar] [CrossRef]
- Xiao, Y.; Kang, S.; Liu, Y.; Guo, X.; Li, M.; Xu, H. Effect and mechanism of calcium ions on the gelation properties of cellulose nanocrystals-whey protein isolate composite gels. Food Hydrocoll. 2021, 111, 106401. [Google Scholar] [CrossRef]




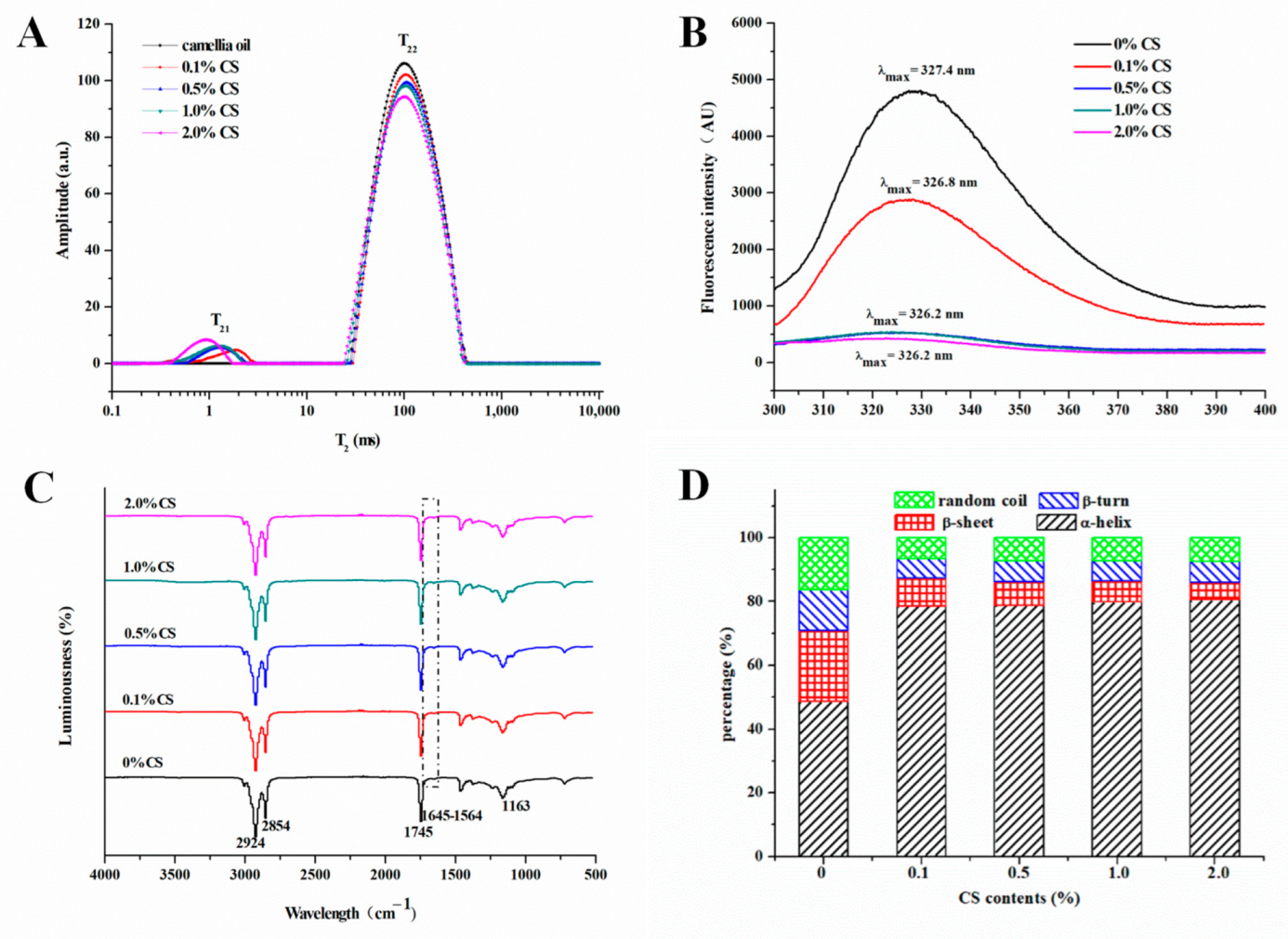

| CS Content (%) | T21 (s) | T22 (s) | RC21 (%) | RC22 (%) | Oil Percentages (%) |
|---|---|---|---|---|---|
| 0 0.1 | - 0.595 ± 0.035 a | 29.392 ± 0.783 a 30.366 ± 0.646 a | - 0.0248 ± 0.0004 c | 0.9985 ± 0.0007 a 0.9752 ± 0.0004 b | - 95.19 ± 0.05 a |
| 0.5 | 0.565 ± 0.007 a | 29.134 ± 0.537 a | 0.0293 ± 0.0008 b | 0.9707 ± 0.0008 c | 93.45 ± 0.09 b |
| 1.0 | 0.430 ± 0.011 b | 26.373 ± 0.652 b | 0.0298 ± 0.0010 b | 0.9702 ± 0.0009 c | 91.42 ± 0.08 c |
| 2.0 | 0.400 ± 0.014 b | 24.827 ± 0.905 b | 0.0392 ± 0.0004 a | 0.9608 ± 0.0004 d | 88.23 ± 0.05 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Hu, L.; Xiao, Y.; Liu, Y.; Li, S.; Zheng, M.; Yu, Z.; Liu, K.; Zhou, Y. Gel Properties and Formation Mechanism of Camellia Oil Body-Based Oleogel Improved by Camellia Saponin. Gels 2022, 8, 499. https://doi.org/10.3390/gels8080499
Liu J, Hu L, Xiao Y, Liu Y, Li S, Zheng M, Yu Z, Liu K, Zhou Y. Gel Properties and Formation Mechanism of Camellia Oil Body-Based Oleogel Improved by Camellia Saponin. Gels. 2022; 8(8):499. https://doi.org/10.3390/gels8080499
Chicago/Turabian StyleLiu, Jing, Lili Hu, Yaqing Xiao, Yingnan Liu, Songnan Li, Mingming Zheng, Zhenyu Yu, Kang Liu, and Yibin Zhou. 2022. "Gel Properties and Formation Mechanism of Camellia Oil Body-Based Oleogel Improved by Camellia Saponin" Gels 8, no. 8: 499. https://doi.org/10.3390/gels8080499