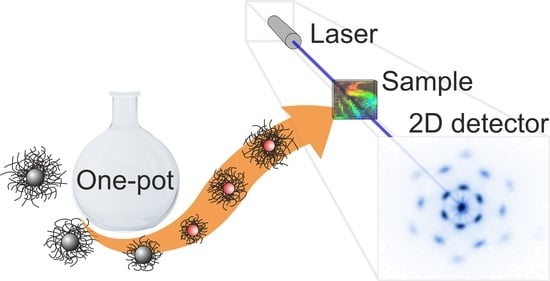
Micron-Sized Silica-PNIPAM Core-Shell Microgels with Tunable Shell-To-Core Ratio
Abstract
:1. Introduction
2. Results and Discussion
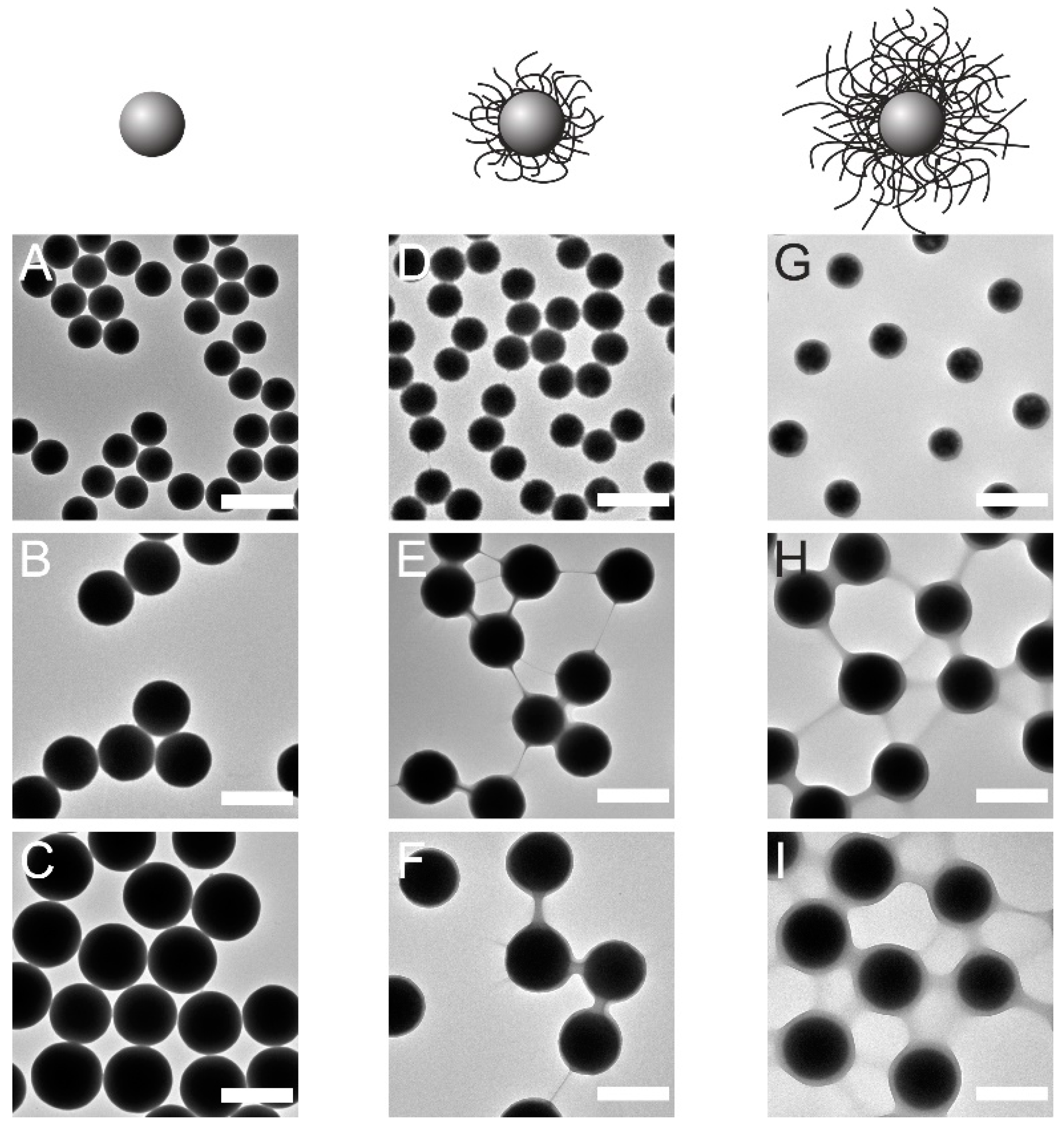
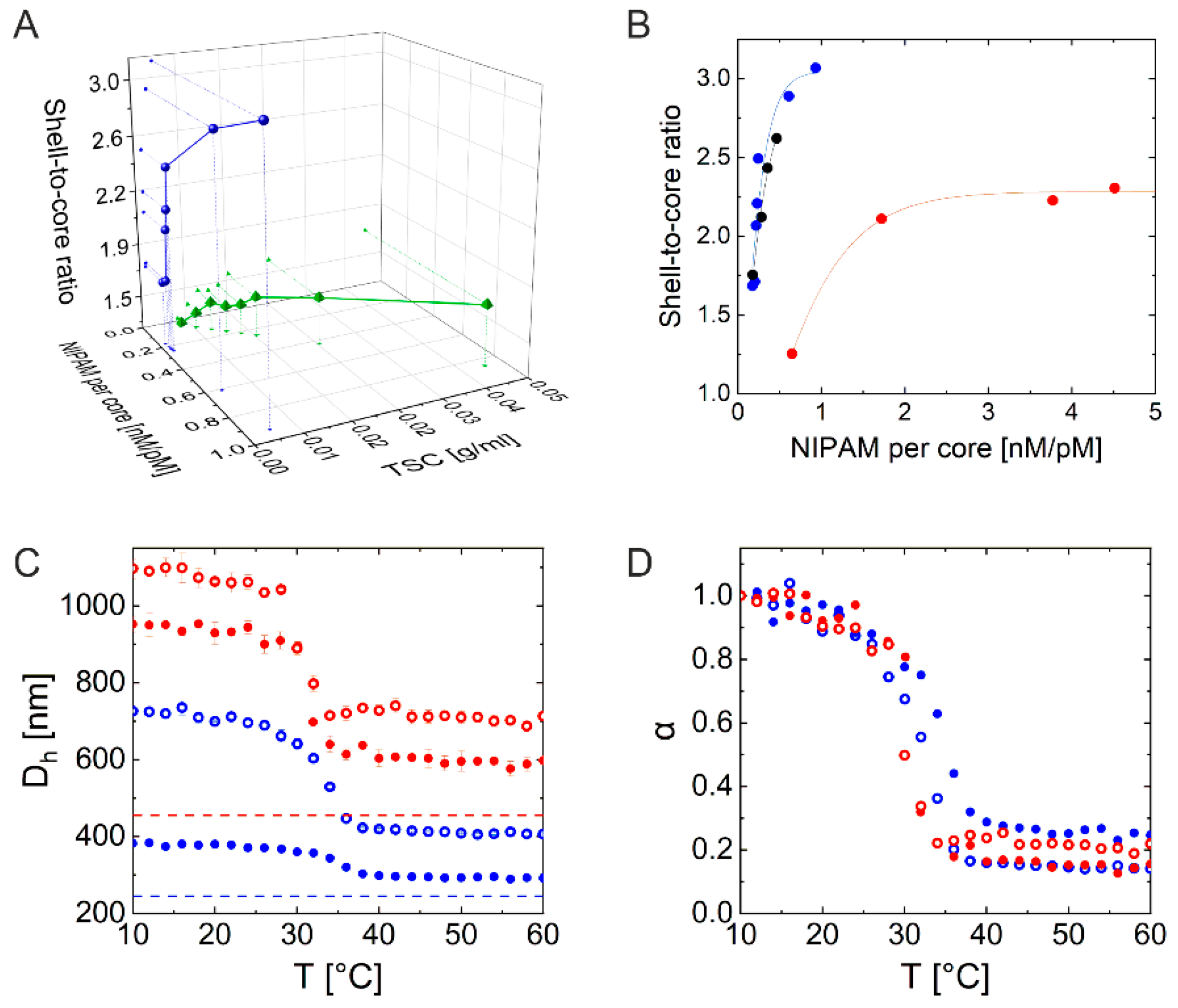
2.1. Synthesis and Characterization
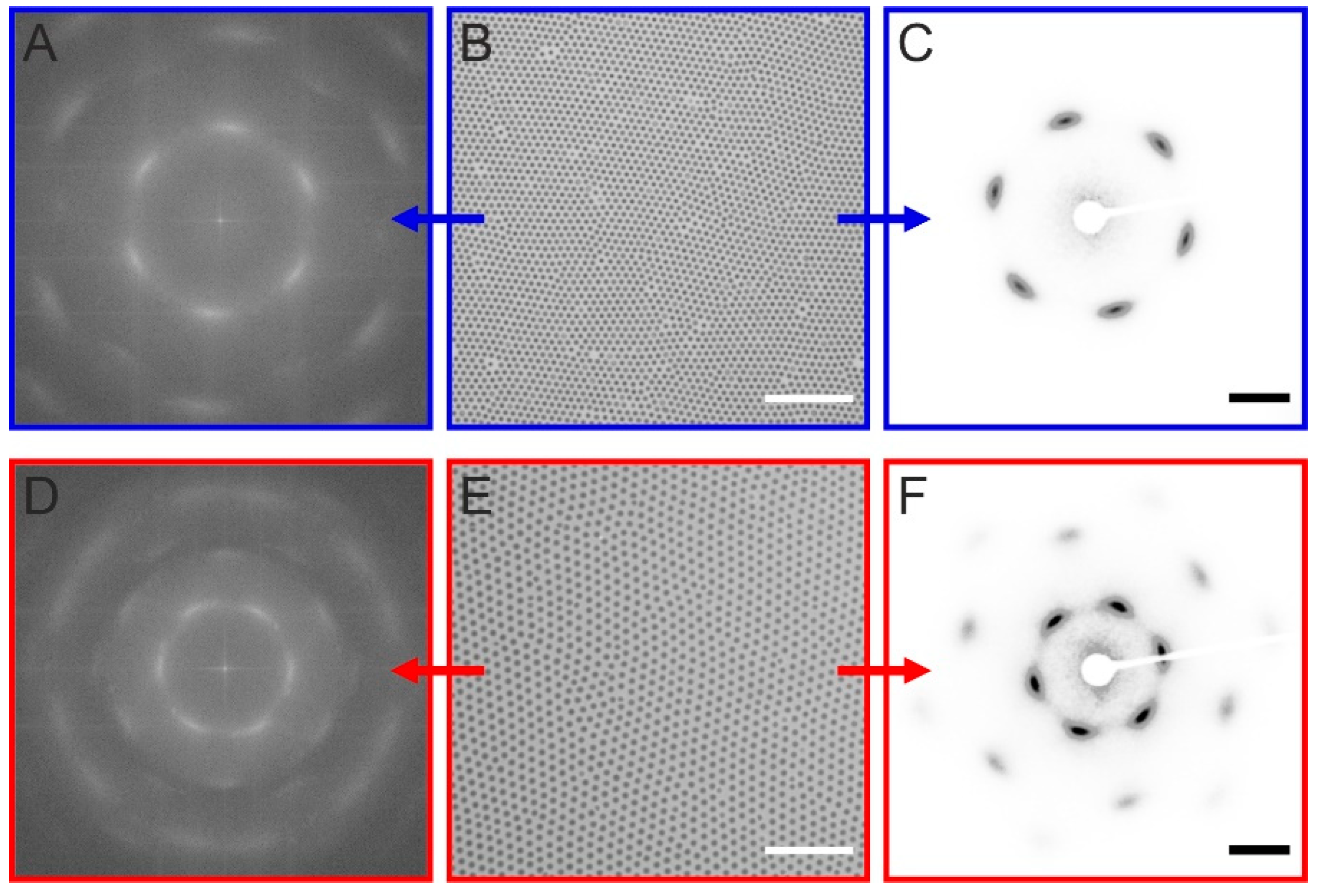
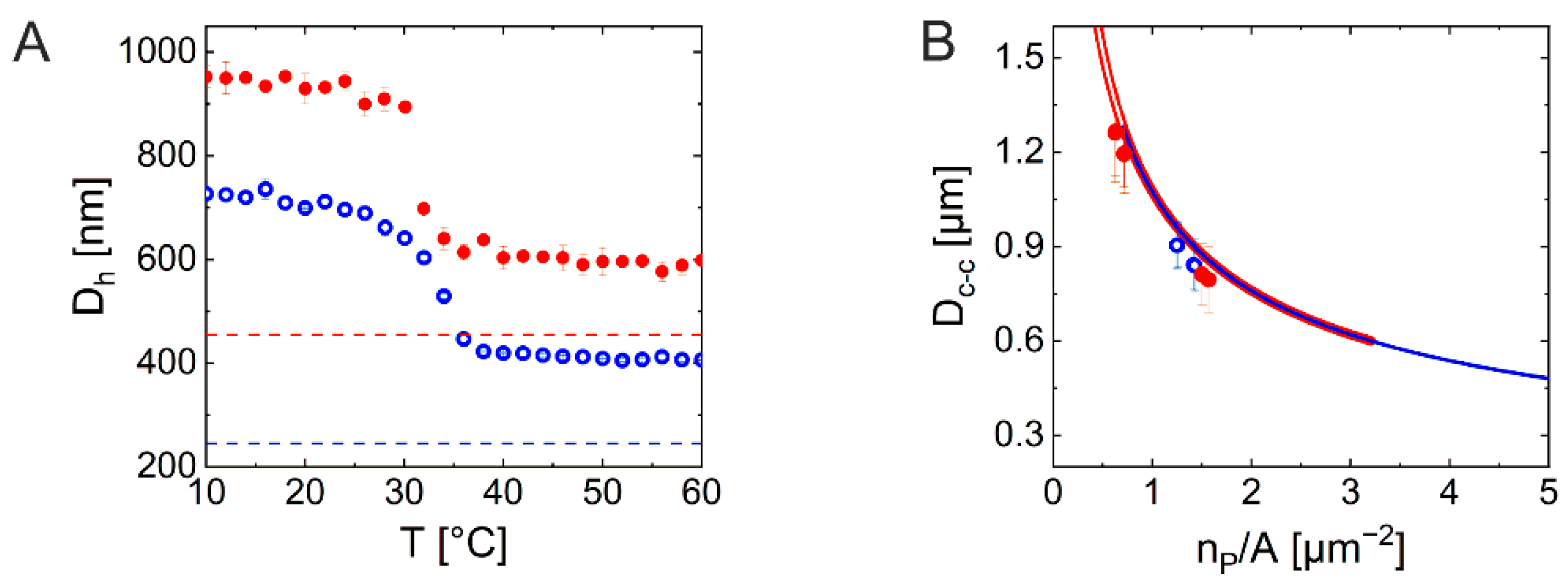
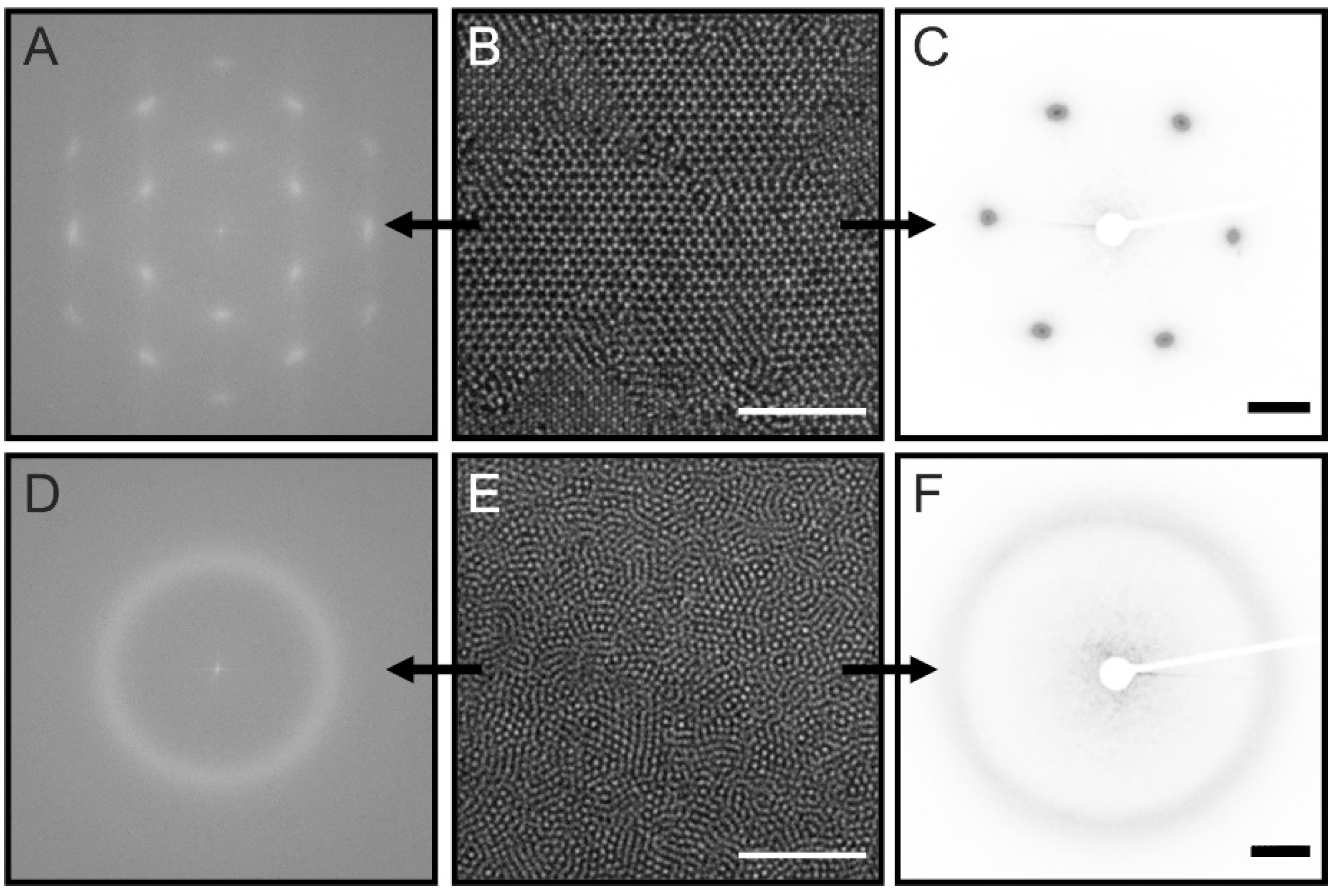
2.2. Investigation of 2D Assemblies Using Optical Microscopy and SALS
2.3. Investigation of 3D Assemblies Using Confocal Microscopy and SALS
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis
4.2.1. Synthesis and Surface Modification of Colloidal Silica Cores
4.2.2. Synthesis of Silica-PNIPAM CS Microgels at Fixed Temperature
4.2.3. Synthesis of Silica-PNIPAM CS Microgels Using a Temperature Ramp
4.3. Methods
4.3.1. Monolayer Preparation
4.3.2. Preparation of 3D Assemblies
4.3.3. Dynamic Light Scattering (DLS)
4.3.4. Small-Angle Light Scattering (SALS)
4.3.5. Transmission Electron Microscopy (TEM)
4.3.6. Optical Light Microscopy
4.3.7. Confocal Laser Scanning Microscopy (CLSM)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and Microgels: From Model Colloids to Applications, Recent Developments, and Future Trends. Langmuir 2019, 35, 6231–6255. [Google Scholar] [CrossRef] [PubMed]
- Plamper, F.A.; Richtering, W. Functional Microgels and Microgel Systems. Acc. Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Dubbert, J.; Nothdurft, K.; Karg, M.; Richtering, W. Core-Shell-Shell and Hollow Double-Shell Microgels with Advanced Temperature Responsiveness. Macromol. Rapid Commun. 2015, 36, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Pastoriza-Santos, I.; Rodriguez-González, B.; Von Klitzing, R.; Wellert, S.; Hellweg, T. Temperature, pH, and Ionic Strength Induced Changes of the Swelling Behavior of PNIPAM−Poly(allylacetic acid) Copolymer Microgels. Langmuir 2008, 24, 6300–6306. [Google Scholar] [CrossRef] [PubMed]
- Acciaro, R.; Gilányi, T.; Varga, I. Preparation of Monodisperse Poly(N-isopropylacrylamide) Microgel Particles with Homogenous Cross-Link Density Distribution. Langmuir 2011, 27, 7917–7925. [Google Scholar] [CrossRef]
- Schmidt, S.; Liu, T.; Rütten, S.; Phan, K.-H.; Möller, M.; Richtering, W. Influence of Microgel Architecture and Oil Polarity on Stabilization of Emulsions by Stimuli-Sensitive Core-Shell Poly(N-isopropylacrylamide-co-methacrylic acid) Microgels: Mickering versus Pickering Behavior? Langmuir 2011, 27, 9801–9806. [Google Scholar] [CrossRef]
- Scotti, A.; Schulte, M.F.; Lopez, C.G.; Crassous, J.J.; Bochenek, S.; Richtering, W. How Softness Matters in Soft Nanogels and Nanogel Assemblies. Chem. Rev. 2022. [Google Scholar] [CrossRef]
- Karg, M. Functional Materials Design through Hydrogel Encapsulation of Inorganic Nanoparticles: Recent Developments and Challenges. Macromol. Chem. Phys. 2016, 217, 242–255. [Google Scholar] [CrossRef]
- Karg, M. Multifunctional inorganic/organic hybrid microgels. Colloid Polym. Sci. 2012, 290, 673–688. [Google Scholar] [CrossRef]
- Karg, M.; Hellweg, T.; Mulvaney, P. Self-Assembly of Tunable Nanocrystal Superlattices Using Poly-(NIPAM) Spacers. Adv. Funct. Mater. 2011, 21, 4668–4676. [Google Scholar] [CrossRef]
- Somerville, W.R.C.; Law, A.D.; Rey, M.; Vogel, N.; Archer, A.J.; Buzza, D.M.A. Pattern formation in two-dimensional hard-core/soft-shell systems with variable soft shell profiles. Soft Matter 2020, 16, 3564–3573. [Google Scholar] [CrossRef] [PubMed]
- Menath, J.; Eatson, J.; Brilmayer, R.; Andrieu-Brunsen, A.; Buzza, D.M.A.; Vogel, N. Defined core–shell particles as the key to complex interfacial self-assembly. Proc. Natl. Acad. Sci. USA 2021, 118, e2113394118. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.A.; Prescott, S.W.; Murdoch, T.J.; Nelson, A.; Gilbert, E.P.; Webber, G.B.; Wanless, E.J. Influence of molecular weight on PNIPAM brush modified colloidal silica particles. Soft Matter 2019, 15, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Asai, M.; Zhao, D.; Kumar, S.K. Role of Grafting Mechanism on the Polymer Coverage and Self-Assembly of Hairy Nanoparticles. ACS Nano 2017, 11, 7028–7035. [Google Scholar] [CrossRef] [PubMed]
- Cors, M.; Wrede, O.; Genix, A.-C.; Anselmetti, D.; Oberdisse, J.; Hellweg, T. Core–Shell Microgel-Based Surface Coatings with Linear Thermoresponse. Langmuir 2017, 33, 6804–6811. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.J.; Dubbert, J.; Rudov, A.A.; Pedersen, J.S.; Lindner, P.; Karg, M.; Potemkin, I.I.; Richtering, W. Multi-Shell Hollow Nanogels with Responsive Shell Permeability. Sci. Rep. 2016, 6, 22736. [Google Scholar] [CrossRef]
- Meng, Z.; Smith, M.H.; Lyon, L.A. Temperature-programmed synthesis of micron-sized multi-responsive microgels. Colloid Polym. Sci. 2009, 287, 277–285. [Google Scholar] [CrossRef]
- Tang, J.S.J.; Bader, R.S.; Goerlitzer, E.S.A.; Wendisch, J.F.; Bourret, G.R.; Rey, M.; Vogel, N. Surface Patterning with SiO2@PNiPAm Core–Shell Particles. ACS Omega 2018, 3, 12089–12098. [Google Scholar] [CrossRef]
- Vasudevan, S.A.; Rauh, A.; Barbera, L.; Karg, M.; Isa, L. Stable in Bulk and Aggregating at the Interface: Comparing Core–Shell Nanoparticles in Suspension and at Fluid Interfaces. Langmuir 2018, 34, 886–895. [Google Scholar] [CrossRef]
- Büchel, G.; Grün, M.; Unger, K.K.; Matsumoto, A.; Kazuo, T. Tailored syntheses of nanostructured silicas: Control of particle morphology, particle size and pore size. Supramol. Sci. 1998, 5, 253–259. [Google Scholar] [CrossRef]
- Kurka, D.W.; Niehues, M.; Kudruk, S.; Gerke, V.; Ravoo, B.J. Polythiolactone—Decorated Silica Particles: A Versatile Approach for Surface Functionalization, Catalysis and Encapsulation. Chem. Eur. J. 2021, 27, 7667–7676. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Wellert, S.; Prevost, S.; Schweins, R.; Dewhurst, C.; Liz-Marzán, L.M.; Hellweg, T. Well defined hybrid PNIPAM core-shell microgels: Size variation of the silica nanoparticle core. Colloid Polym. Sci. 2011, 289, 699–709. [Google Scholar] [CrossRef]
- Vasudevan, S.A.; Rauh, A.; Kröger, M.; Karg, M.; Isa, L. Dynamics and Wetting Behavior of Core–Shell Soft Particles at a Fluid–Fluid Interface. Langmuir 2018, 34, 15370–15382. [Google Scholar] [CrossRef] [PubMed]
- Rauh, A.; Rey, M.; Barbera, L.; Zanini, M.; Karg, M.; Isa, L. Compression of hard core–soft shell nanoparticles at liquid–liquid interfaces: Influence of the shell thickness. Soft Matter 2017, 13, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Ruseva, V.; Lyons, M.; Powell, J.; Austin, J.; Malm, A.; Corbett, J. Capillary dynamic light scattering: Continuous hydrodynamic particle size from the nano to the micro-scale. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 504–511. [Google Scholar] [CrossRef]
- Ruseva, V.; Jankevics, H.; Corbett, J. An optimized filling method for capillary DLS. MethodsX 2019, 6, 606–614. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Bogush, G.; Tracy, M.; Zukoski Iv, C. Preparation of monodisperse silica particles: Control of size and mass fraction. J. Non-Cryst. Solids 1988, 104, 95–106. [Google Scholar] [CrossRef]
- Kern, W. Cleaning solution based on hydrogen peroxide for use in silicon semiconductor technology. RCA Rev. 1970, 31, 187–205. [Google Scholar]
- Brasse, Y.; Müller, M.B.; Karg, M.; Kuttner, C.; König, T.A.F.; Fery, A. Magnetic and Electric Resonances in Particle-to-Film-Coupled Functional Nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 3133–3141. [Google Scholar] [CrossRef]
- Wu, X.; Pelton, R.H.; Hamielec, A.E.; Woods, D.R.; McPhee, W. The kinetics of poly(N-isopropylacrylamide) microgel latex formation. Colloid Polym. Sci. 1994, 272, 467–477. [Google Scholar] [CrossRef]
- Andersson, M.; Maunu, S.L. Structural studies of poly(N-isopropylacrylamide) microgels: Effect of SDS surfactant concentration in the microgel synthesis. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 3305–3314. [Google Scholar] [CrossRef]
- Andersson, M.; Maunu, S.L. Volume phase transition and structure of poly(N-isopropylacrylamide) microgels studied with 1H-NMR spectroscopy in D2O. Colloid Polym. Sci. 2006, 285, 293–303. [Google Scholar] [CrossRef]
- Still, T.; Chen, K.; Alsayed, A.M.; Aptowicz, K.B.; Yodh, A. Synthesis of micrometer-size poly(N-isopropylacrylamide) microgel particles with homogeneous crosslinker density and diameter control. J. Colloid Interface Sci. 2013, 405, 96–102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuk, K.; Gregel, L.; Abgarjan, V.; Croonenbrock, C.; Hänsch, S.; Karg, M. Micron-Sized Silica-PNIPAM Core-Shell Microgels with Tunable Shell-To-Core Ratio. Gels 2022, 8, 516. https://doi.org/10.3390/gels8080516
Kuk K, Gregel L, Abgarjan V, Croonenbrock C, Hänsch S, Karg M. Micron-Sized Silica-PNIPAM Core-Shell Microgels with Tunable Shell-To-Core Ratio. Gels. 2022; 8(8):516. https://doi.org/10.3390/gels8080516
Chicago/Turabian StyleKuk, Keumkyung, Lukas Gregel, Vahan Abgarjan, Caspar Croonenbrock, Sebastian Hänsch, and Matthias Karg. 2022. "Micron-Sized Silica-PNIPAM Core-Shell Microgels with Tunable Shell-To-Core Ratio" Gels 8, no. 8: 516. https://doi.org/10.3390/gels8080516
APA StyleKuk, K., Gregel, L., Abgarjan, V., Croonenbrock, C., Hänsch, S., & Karg, M. (2022). Micron-Sized Silica-PNIPAM Core-Shell Microgels with Tunable Shell-To-Core Ratio. Gels, 8(8), 516. https://doi.org/10.3390/gels8080516