A Comprehensive Review of Cross-Linked Gels as Vehicles for Drug Delivery to Treat Central Nervous System Disorders
Abstract
:1. Introduction
2. Challenges in CNS Drug Delivery
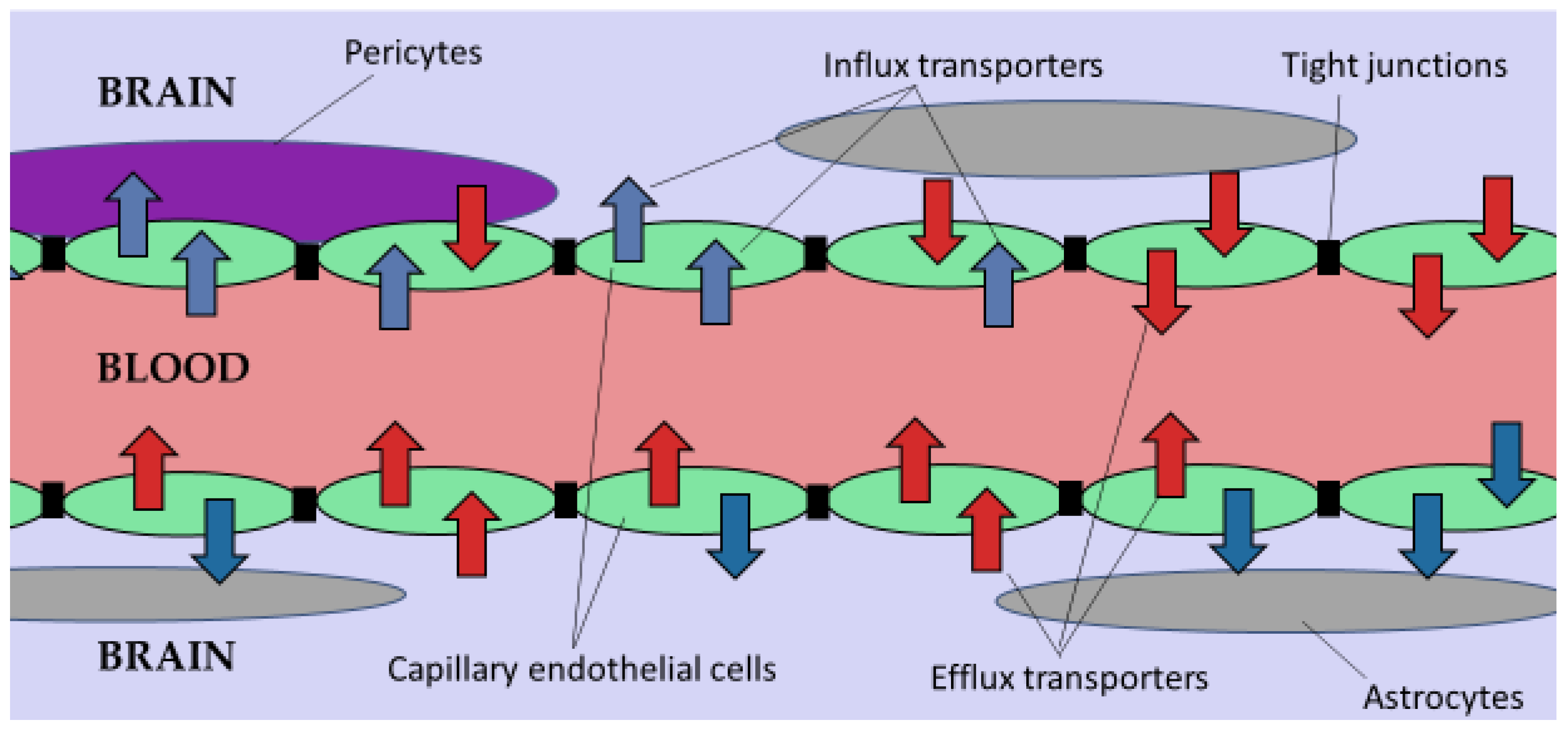
2.1. The Blood-Brain Barrier (BBB)
2.2. The Blood-Brain Cerebrospinal Fluid Barrier (BCSFB)
2.3. Efflux Transporters
2.4. Effects of CNS Diseases on the BBB and Drug Delivery
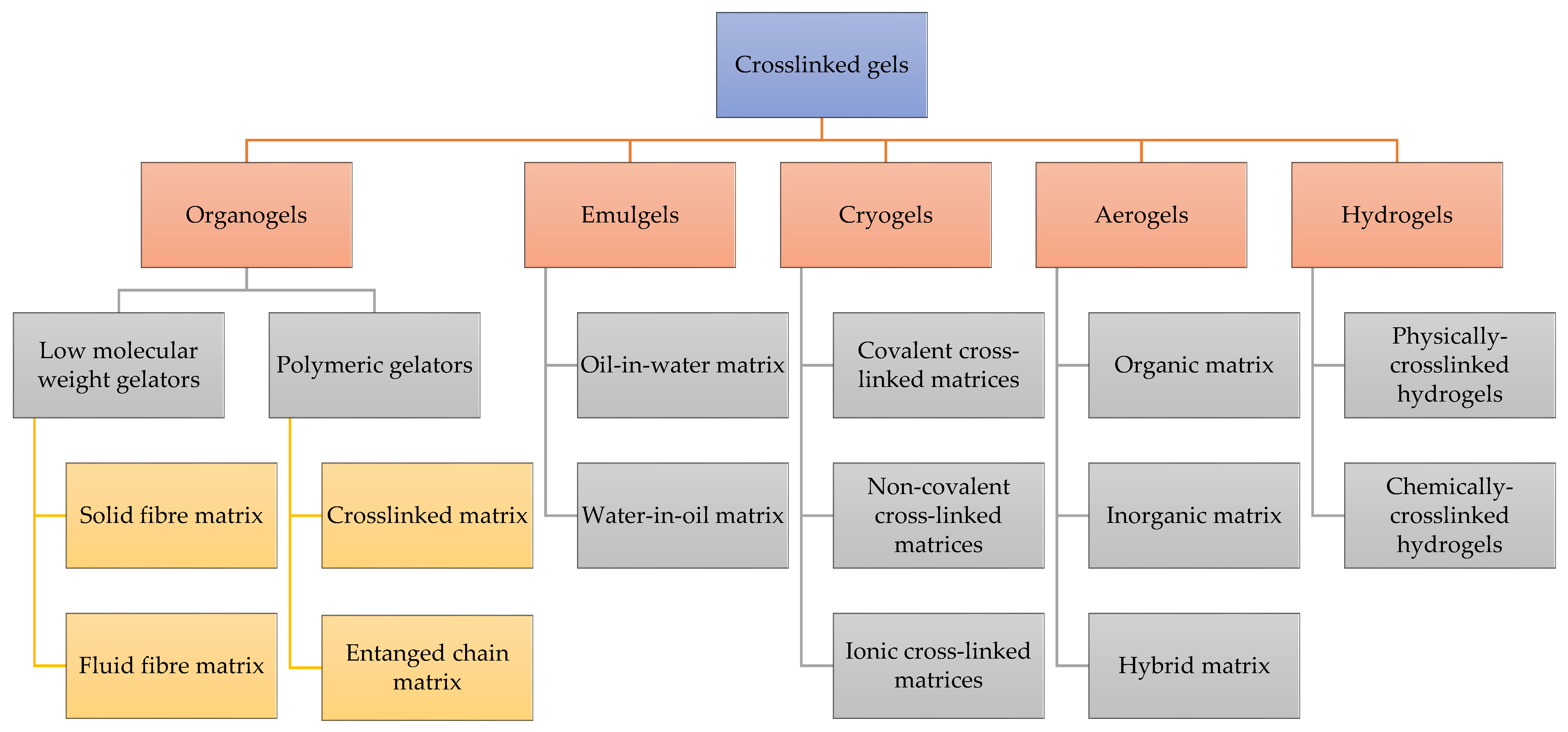
3. Classifications of Cross-Linked Gels
3.1. Emulgels
3.2. Organogels
3.3. Cryogels
3.4. Aerogels
3.5. Hydrogels
4. Polymers and Cross-Linking Agents
4.1. Natural Polymers
4.1.1. Chitosan
4.1.2. Gelatin
4.1.3. Sodium Alginate
4.1.4. Collagen
4.1.5. Cellulose
4.1.6. Hyaluronic Acid
4.1.7. Fibrin
4.2. Synthetic Polymers
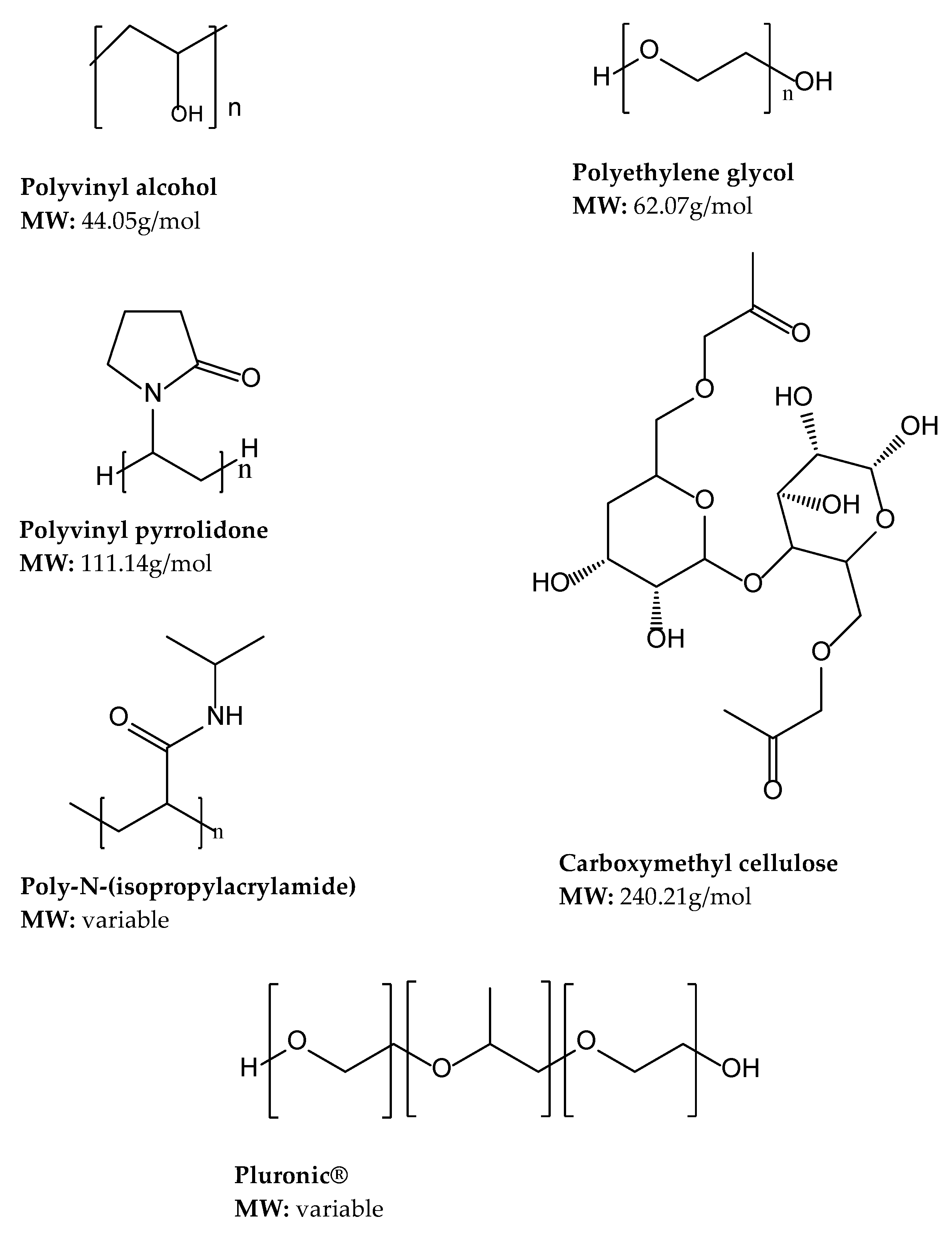
4.2.1. Polyethylene Glycol (PEG)
4.2.2. Polyvinyl Alcohol
4.2.3. Polyvinyl Pyrrolidone (PVP)
4.2.4. Carboxymethyl Cellulose (CMC)
4.2.5. Poly (N-isopropyl acrylamide)
4.2.6. Pluronics®
4.3. Crosslinkers
4.3.1. Natural Crosslinkers
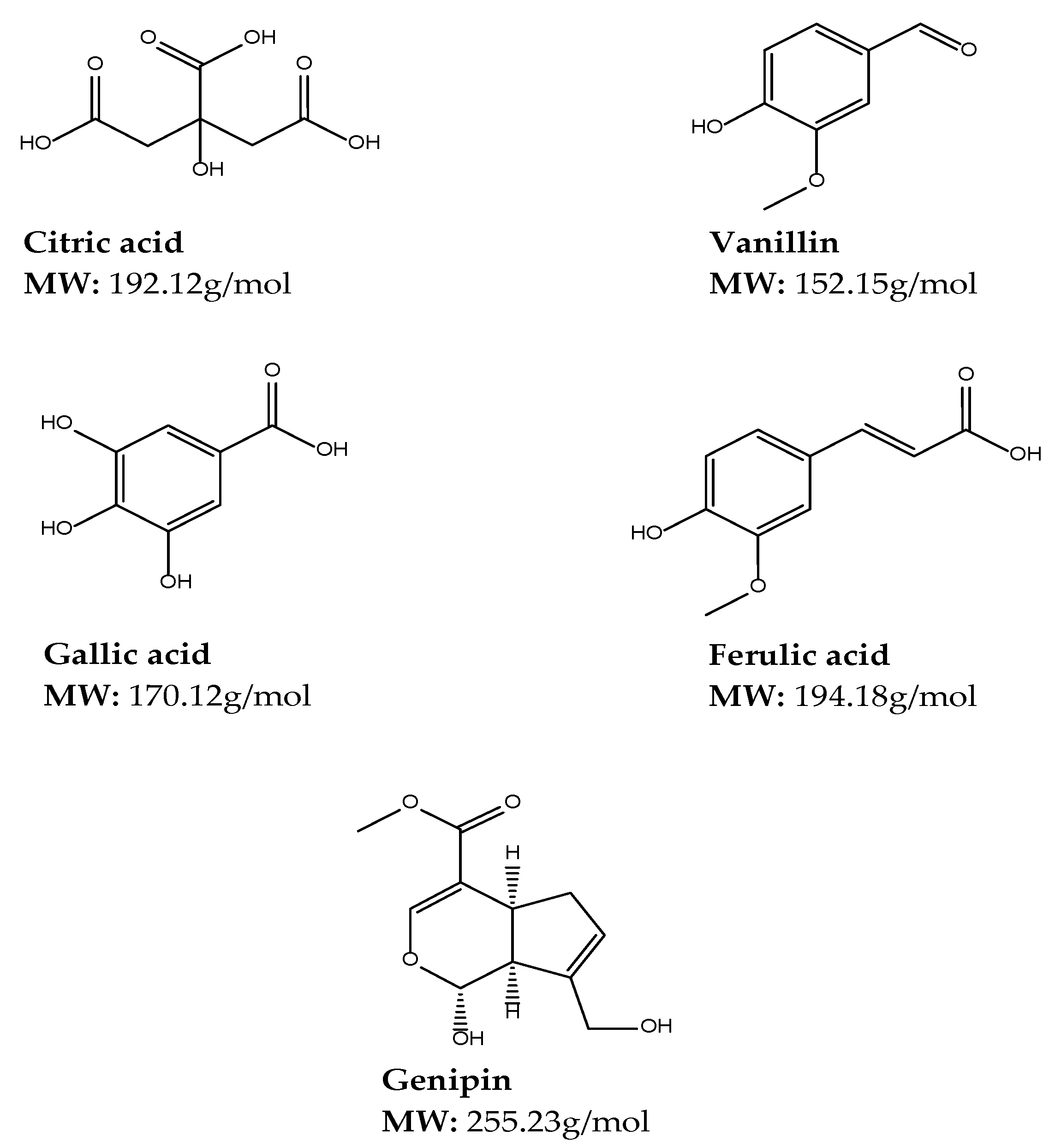
Citric Acid
Vanillin
Gallic Acid
Ferulic Acid
Genipin
4.3.2. Synthetic Crosslinkers
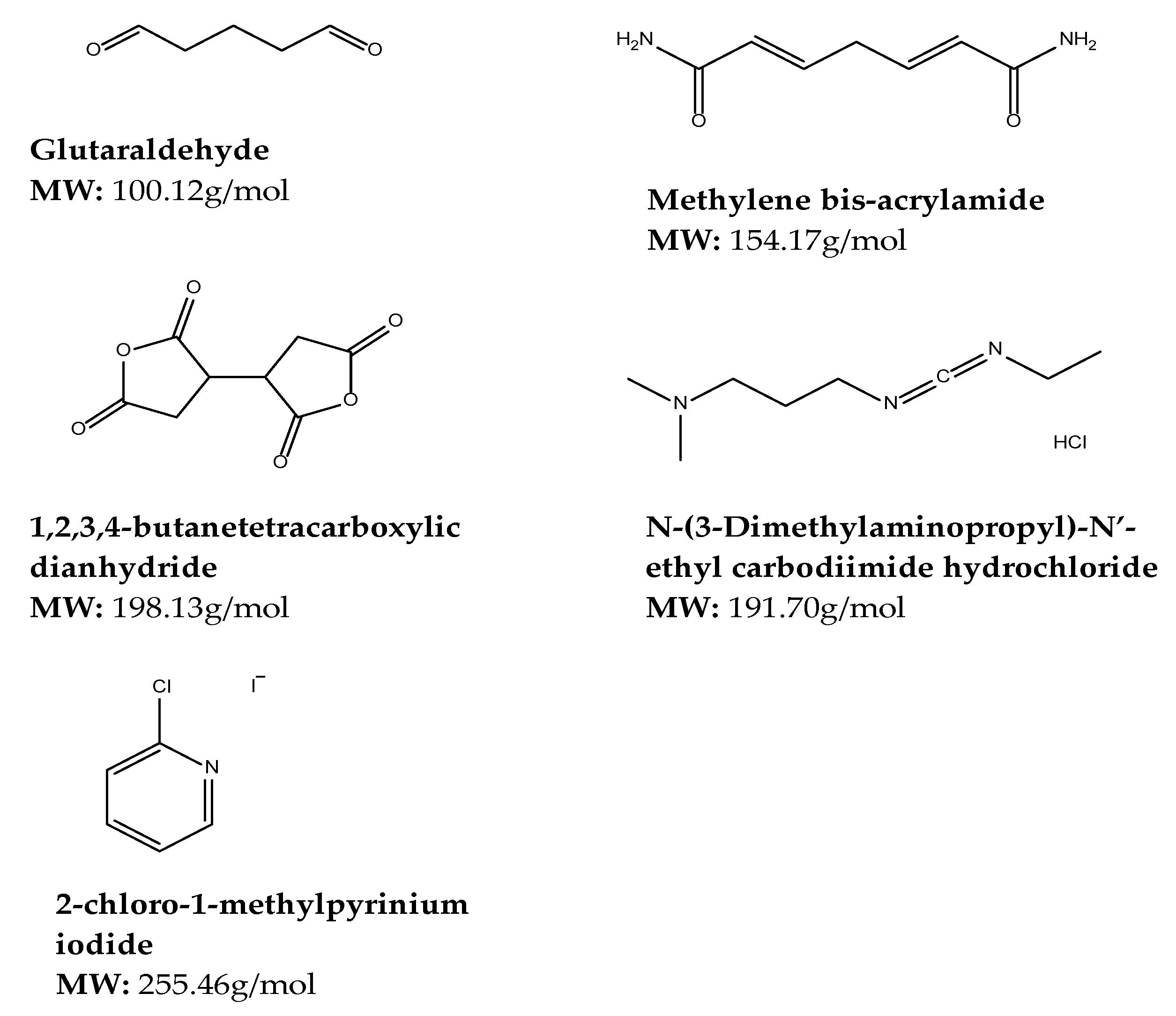
Glutaraldehyde
Methylene-bis-acrylamide
Polymerizable Polyphosphate
1,2,3,4-butanetetracarboxylic Dianhydride (BTCA)
N-(3-Dimethylaminopropyl)-N′-ethyl Carbodiimide Hydrochloride
2-chloro-1-methylpyrinium Iodide
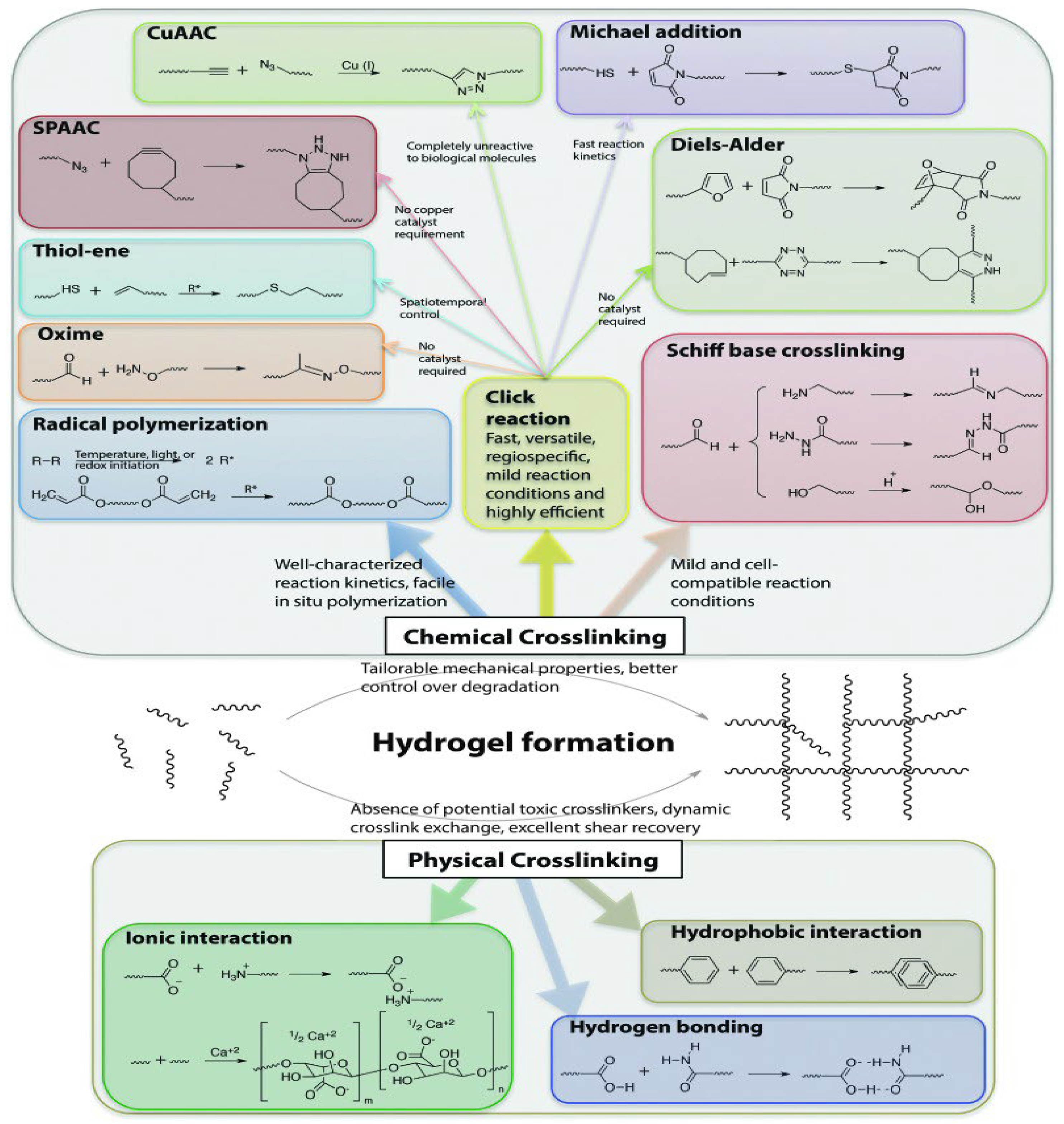
5. Mechanisms of Gelation
6. Opportunities for Cross-Linked Gels in CNS Drug Delivery
6.1. Non-Standard Routes of Administration for CNS Drug Delivery
6.1.1. Intraparenchymal Drug Delivery
6.1.2. Intrathecal Drug Delivery
6.1.3. Nose-to-Brain Drug Delivery
6.1.4. Eye-to-Brain Drug Delivery
6.2. Nanotechnological Interventions
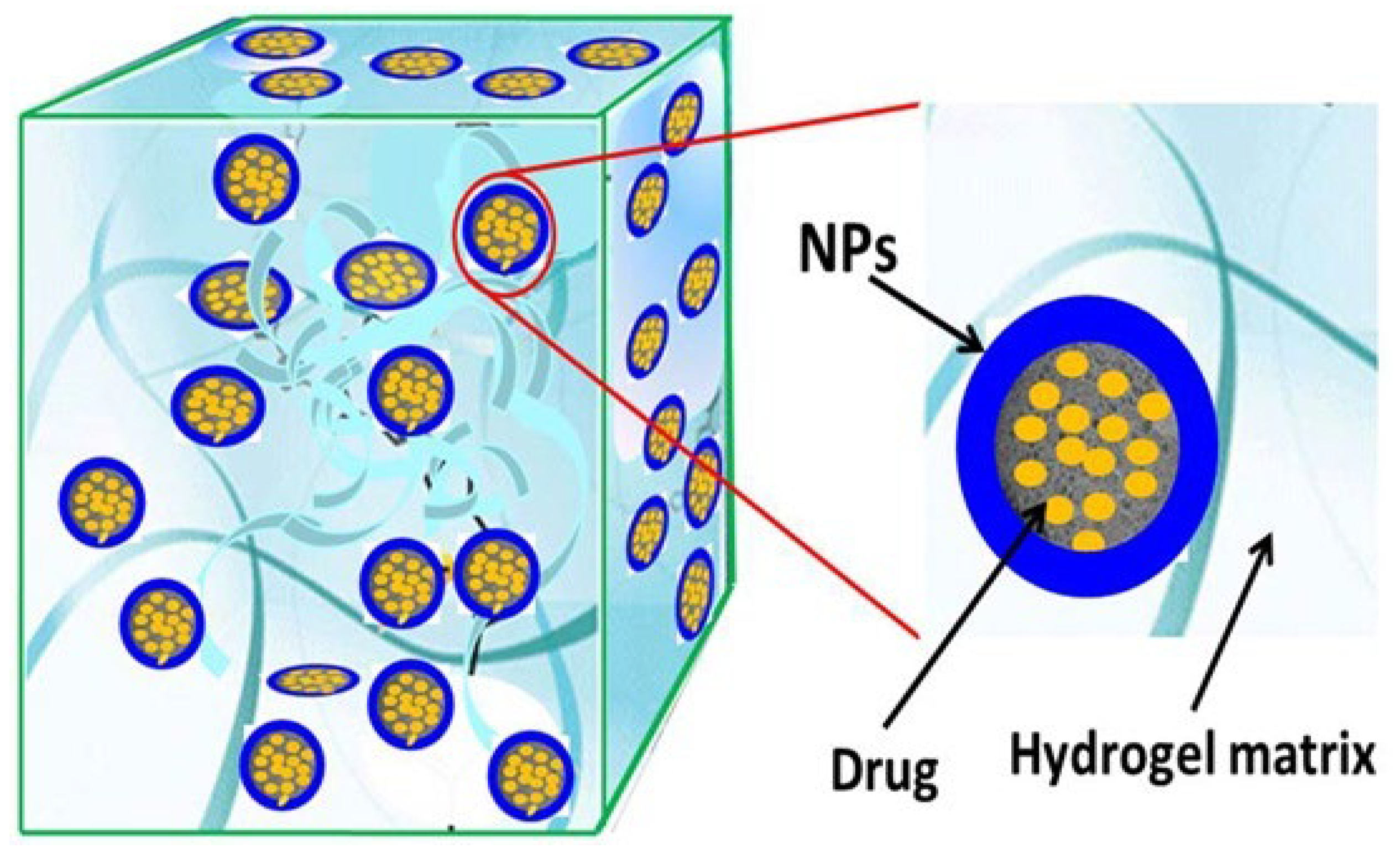
6.2.1. Nanocomposite Cross-Linked Gels
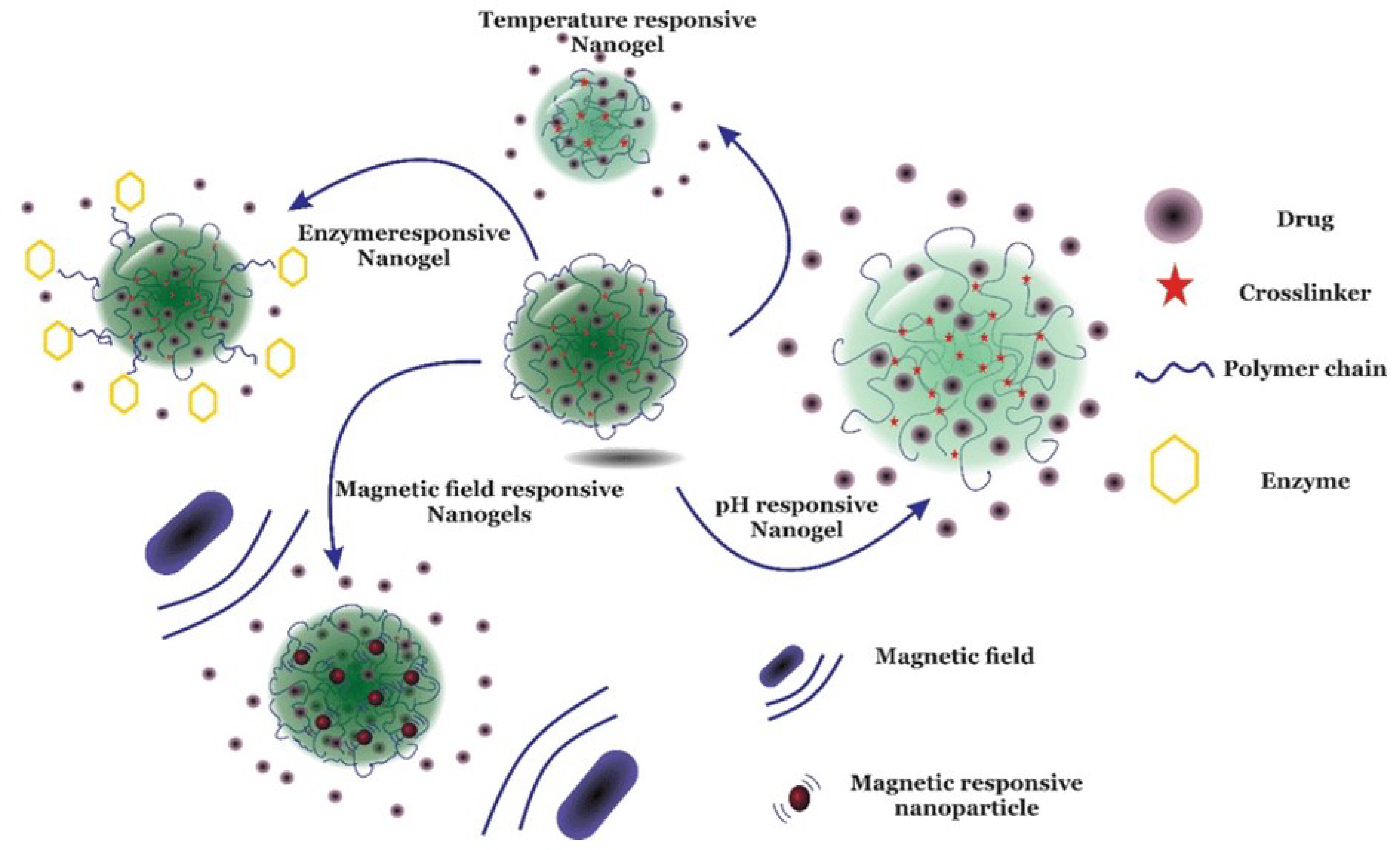
6.2.2. Nano-Sized Cross-Linked Gels
7. Applications of Cross-Linked Gels in CNS Drug Delivery
7.1. Injectable Cross-Linked Gels
7.2. Non-Injectable Cross-Linked Gels
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Loinsigh, E.; Bose, A. Regulatory Considerations for the Use of Biomarkers and Personalized Medicine in CNS Drug Development: A European Perspective. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2019; Volume 29, pp. 259–275. [Google Scholar]
- Li, G.; Shao, K.; Umeshappa, C.S. Recent Progress in Blood-Brain Barrier Transportation Research. In Brain Targeted Drug Delivery System; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–51. [Google Scholar]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative Disease: Models, Mechanisms, and a New Hope. Dis. Model. Mech. 2017, 10, 499. [Google Scholar] [CrossRef]
- Vieira, A.; Filho, M.; Chaves, S.N.; Martins, W.R.; Tolentino, G.P.; De, R.; Pereira, C.; Homem, P.; De Farias, G.L.; Fischer, B.L.; et al. Progressive Resistance Training Improves Bradykinesia, Motor Symptoms and Functional Performance in Patients with Parkinson’s Disease. Clin. Interv. Aging 2020, 15, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Witika, B.A.; Poka, M.S.; Demana, P.H.; Matafwali, S.K.; Melamane, S.; Malungelo Khamanga, S.M.; Makoni, P.A. Lipid-Based Nanocarriers for Neurological Disorders: A Review of the State-of-the-Art and Therapeutic Success to Date. Pharmaceutics 2022, 14, 836. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.P.; Brown, R.H.; Cleveland, D.W. Decoding ALS: From Genes to Mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef]
- National Department of Health South Africa Essential Drugs Programme. Hospital Level (Adults) Standard Treatment Guidelines and Essential Medicines List, 5th ed.; The National Department of Health: Pretoria, South Africa, 2019; ISBN 9781990938498. [Google Scholar]
- Alahmari, A. Blood-Brain Barrier Overview: Structural and Functional Correlation. Neural Plast. 2021, 2021, 6564585. [Google Scholar] [CrossRef]
- Rathod, H.; Mehta, D.; Author, C.; Rathod, H.J.; Mehta, D.P. A Review on Pharmaceutical Gel. Int. J. Pharm. Sci. 2015, 1, 33–47. [Google Scholar]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An Overview of Properties, Biomedical Applications and Obstacles to Clinical Translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D.; Zu, G.; Forson, A.M.; Tromp, L.; Sjollema, J.; van Rijn, P. Nanogels: A Novel Approach in Antimicrobial Delivery Systems and Antimicrobial Coatings. Bioact. Mater. 2021, 6, 3634–3657. [Google Scholar] [CrossRef] [PubMed]
- Mauri, E.; Giannitelli, S.M.; Trombetta, M.; Rainer, A. Synthesis of Nanogels: Current Trends and Future Outlook. Gels 2021, 7, 36. [Google Scholar] [CrossRef]
- Gawdi, R.; Shumway, K.R.; Emmady, P.D. Physiology, Blood Brain Barrier. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32491653 (accessed on 20 July 2022).
- Ndemazie, N.B.; Inkoom, A.; Morfaw, E.F.; Smith, T.; Aghimien, M.; Ebesoh, D.; Agyare, E. Multi-Disciplinary Approach for Drug and Gene Delivery Systems to the Brain. AAPS PharmSciTech 2022, 23, 11. [Google Scholar] [CrossRef]
- Begley, D.J. Delivery of Therapeutic Agents to the Central Nervous System: The Problems and the Possibilities. Pharmacol. Ther. 2004, 104, 29–45. [Google Scholar] [CrossRef]
- Upton, D.H.; Ung, C.; George, S.M.; Tsoli, M.; Kavallaris, M.; Ziegler, D.S. Challenges and Opportunities to Penetrate the Blood-Brain Barrier for Brain Cancer Therapy. Theranostics 2022, 12, 4734–4752. [Google Scholar] [CrossRef]
- Girardin, F. Membrane Transporter Proteins: A Challenge for CNS Drug Development. Dialogues Clin. Neurosci. 2006, 8, 311. [Google Scholar] [CrossRef]
- Tumani, H.; Huss, A.; Bachhuber, F. The Cerebrospinal Fluid and Barriers—Anatomic and Physiologic Considerations. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 146, pp. 21–32. [Google Scholar]
- Engelhardt, B.; Sorokin, L. The Blood-Brain and the Blood-Cerebrospinal Fluid Barriers: Function and Dysfunction. Semin. Immunopathol. 2009, 31, 497–511. [Google Scholar] [CrossRef]
- Strazielle, N.; Ghersi-Egea, J.F. Efflux Transporters in Blood-Brain Interfaces of the Developing Brain. Front. Neurosci. 2015, 9, 21. [Google Scholar] [CrossRef]
- Agarwal, S.; Hartz, A.M.S.; Elmquist, W.F.; Bauer, B. Breast Cancer Resistance Protein and P-Glycoprotein in Brain Cancer: Two Gatekeepers Team Up. Curr. Pharm. Des. 2011, 17, 2793–2802. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacol. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef]
- Tishler, D.M.; Weinberg, K.I.; Hinton, D.R.; Barbaro, N.; Annett, G.M.; Raffel, C. MDR1 Gene Expression in Brain of Patients with Medically Intractable Epilepsy. Epilepsia 1995, 36, 1–6. [Google Scholar] [CrossRef]
- Sakata, S.; Fujiwara, M.; Ohtsuka, K.; Kamma, H.; Nagane, M.; Sakamoto, A.; Fujioka, Y. ATP-Binding Cassette Transporters in Primary Central Nervous System Lymphoma: Decreased Expression of MDR1 P-Glycoprotein and Breast Cancer Resistance Protein in Tumor Capillary Endothelial Cells. Oncol. Rep. 2011, 25, 333–339. [Google Scholar] [CrossRef]
- Ginguené, C.; Champier, J.; Maallem, S.; Strazielle, N.; Jouvet, A.; Fèvre-Montange, M.; Ghersi-Egea, J.F. P-glycoprotein (ABCB1) and Breast Cancer Resistance Protein (ABCG2) Localize in the Microvessels Forming the Blood-Tumor Barrier in Ependymomas. Brain Pathol. 2010, 20, 926. [Google Scholar] [CrossRef]
- Vendel, E.; Rottschäfer, V.; de Lange, E.C.M. The 3D Brain Unit Network Model to Study Spatial Brain Drug Exposure under Healthy and Pathological Conditions. Pharm. Res. 2020, 37, 137. [Google Scholar] [CrossRef] [PubMed]
- Drouin-Ouellet, J.; Sawiak, S.J.; Cisbani, G.; Lagacé, M.; Kuan, W.-L.; Saint-Pierre, M.; Dury, R.J.; Alata, W.; St-Amour, I.; Mason, S.L.; et al. Cerebrovascular and Blood-Brain Barrier Impairments in Huntington’s Disease: Potential Implications for Its Pathophysiology. Ann. Neurol. 2015, 78, 160–177. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The Blood–Brain Barrier and Blood–Tumour Barrier in Brain Tumours and Metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Mayhan, W.G. VEGF Increases Permeability of the Blood-Brain Barrier via a Nitric Oxide Synthase/CGMP-Dependent Pathway. Am. J. Physiol. Physiol. 1999, 276, C1148–C1153. [Google Scholar] [CrossRef]
- Fukumura, D.; Jain, R.K. Role of Nitric Oxide in Angiogenesis and Microcirculation in Tumors. Cancer Metastasis Rev. 1998, 17, 77–89. [Google Scholar] [CrossRef]
- Jablonski, M.R.; Markandaiah, S.S.; Jacob, D.; Meng, N.J.; Li, K.; Gennaro, V.; Lepore, A.C.; Trotti, D.; Pasinelli, P. Inhibiting Drug Efflux Transporters Improves Efficacy of ALS Therapeutics. Ann. Clin. Transl. Neurol. 2014, 1, 996–1005. [Google Scholar] [CrossRef]
- Li, J.; Zheng, M.; Shimoni, O.; Banks, W.A.; Bush, A.I.; Gamble, J.R.; Shi, B. Development of Novel Therapeutics Targeting the Blood-Brain Barrier: From Barrier to Carrier. Adv. Sci. 2021, 8, e2101090. [Google Scholar] [CrossRef]
- Nance, E.; Pun, S.H.; Saigal, R.; Sellers, D.L. Drug Delivery to the Central Nervous System. Nat. Rev. Mater. 2022, 7, 314–331. [Google Scholar] [CrossRef]
- Sabalingam, S.; Siriwardhene, M.A. A Review on Emerging Applications of Emulgel as Topical Drug Delivery System. World J. Adv. Res. Rev. 2022, 13, 452–463. [Google Scholar] [CrossRef]
- Sharma, V.; Nayak, S.K.; Paul, S.R.; Choudhary, B.; Ray, S.S.; Pal, K. Emulgels. Polym. Gels 2018, 9, 251–264. [Google Scholar] [CrossRef]
- Talat, M.; Zaman, M.; Khan, R.; Jamshaid, M.; Akhtar, M.; Mirza, A.Z. Emulgel: An Effective Drug Delivery System. Drug Dev. Ind. Pharm. 2021, 47, 1193–1199. [Google Scholar] [CrossRef]
- Verma, A.; Jain, A.; Tiwari, A.; Jain, S.K. Emulgels: Application Potential in Drug Delivery. In Functional Biopolymers; Springer: Cham, Switzerland, 2018; pp. 343–371. [Google Scholar] [CrossRef]
- Redkar, M.R.; Patil, S.V.; Rukari, T.G. (PDF) Emulgel: A Modern Tool For Topical Drug Delivery. World J. Pharm. Res. 2019, 8, 586–587. [Google Scholar]
- Sreevidya, V.S. An Overview on Emulgel. Int. J. Pharm. Phytopharm. Res. 2019, 9, 92–97. [Google Scholar]
- Ashara, K.; Soniwala, M.; Shah, K. Review Article Emulgel: A Novel Drug Delivery System. J. Pakistan Assoc. Dermatol. 2016, 26, 244–249. [Google Scholar]
- Lampp, L.; Rogozhnikova, O.Y.; Trukhin, D.V.; Tormyshev, V.M.; Bowman, M.K.; Devasahayam, N.; Krishna, M.C.; Mäder, K.; Imming, P. A Radical Containing Injectable In-Situ-Oleogel and Emulgel for Prolonged in-Vivo Oxygen Measurements with CW EPR. Free Radic. Biol. Med. 2019, 130, 120–127. [Google Scholar] [CrossRef]
- Vintiloiu, A.; Leroux, J.C. Organogels and Their Use in Drug Delivery—A Review. J. Control. Release 2008, 125, 179–192. [Google Scholar] [CrossRef]
- Mujawar, N.K.; Ghatage, S.L.; Yeligar, V.C. Organogel: Factors And Its Importance. Int. J. Biol. Chem. Sci. 2014, 4, 758–773. [Google Scholar]
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, Promising Drug Delivery Systems: An Update of State-of-the-Art and Recent Applications. J. Control. Release 2018, 271, 1–20. [Google Scholar] [CrossRef]
- Das, J.; Bhattacharjee, B.; Dutta, J.J. Tirna Paul ORGANOGEL: An Ideal Drug Delivery Carrier. World J. Pharm. Res. 2021, 10, 446–465. [Google Scholar]
- Wang, D.; Zhao, J.; Liu, X.; Sun, F.; Zhou, Y.; Teng, L.; Li, Y. Parenteral Thermo-Sensitive Organogel for Schizophrenia Therapy, in Vitro and in Vivo Evaluation. Eur. J. Pharm. Sci. 2014, 60, 40–48. [Google Scholar] [CrossRef]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Joshi Navare, K.; Mohammed, H.S.; Bencherif, S.A.; Memic, A.; et al. Latest Advances in Cryogel Technology for Biomedical Applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef]
- Eggermont, L.J.; Rogers, Z.J.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable Cryogels for Biomedical Applications. Trends Biotechnol. 2020, 38, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Qiao, Y.; Thakor, A.S. Three-Dimensional Cryogels for Biomedical Applications. J. Biomed. Mater. Res. A 2019, 107, 2736–2755. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 50. Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef]
- Savina, I.N.; Zoughaib, M.; Yergeshov, A.A. Design and Assessment of Biodegradable Macroporous Cryogels as Advanced Tissue Engineering and Drug Carrying Materials. Gels 2021, 7, 79. [Google Scholar] [CrossRef]
- Jones, L.O.; Williams, L.; Boam, T.; Kalmet, M.; Oguike, C.; Hatton, F.L. Cryogels: Recent Applications in 3D-Bioprinting, Injectable Cryogels, Drug Delivery, and Wound Healing. Beilstein J. Org. Chem. 2021, 17, 2553. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, K.; Liu, J.; Ding, Y.; Li, W.; Zhang, A. Thermoresponsive Cryogels from Dendronized Interpenetrating Polymer Network Showing Dual-Shape Memory. Eur. Polym. J. 2020, 141, 110092. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive Hydrogels in Biomedical Applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef]
- Pasparakis, G.; Vamvakaki, M. Multiresponsive Polymers: Nano-Sized Assemblies, Stimuli-Sensitive Gels and Smart Surfaces. Polym. Chem. 2011, 2, 1234–1248. [Google Scholar] [CrossRef]
- Bilici, C.; Karayel, S.; Demir, T.T.; Okay, O. Self-Oscillating PH-Responsive Cryogels as Possible Candidates of Soft Materials for Generating Mechanical Energy. J. Appl. Polym. Sci. 2010, 118, 2981–2988. [Google Scholar] [CrossRef]
- García-González, C.A.; Sosnik, A.; Kalmár, J.; De Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in Drug Delivery: From Design to Application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An Opinion Paper on Aerogels for Biomedical and Environmental Applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and Biomedical Applications of Aerogels: Possibilities and Challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Michel, A.; Matthias, K.; Nicholas, L. Aerogels Handbook; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2011; Volume 1107. [Google Scholar]
- Ferreira-Gonçalves, T.; Constantin, C.; Neagu, M.; Reis, C.P.; Sabri, F.; Simón-Vázquez, R. Safety and Efficacy Assessment of Aerogels for Biomedical Applications. Biomed. Pharmacother. 2021, 144, 112356. [Google Scholar] [CrossRef]
- Wang, W.; Narain, R.; Zeng, H. Hydrogels. In Polymer Science and Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–244. [Google Scholar]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A Mini Review on Hydrogels Classification and Recent Developments in Miscellaneous Applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, S. A Review on Biomacromolecular Hydrogel Classification and Its Applications. Int. J. Biol. Macromol. 2020, 162, 737–747. [Google Scholar] [CrossRef]
- Garg, S.; Garg, A. Hydrogel: Classification, Properties, Preparation and Technical Features. Asian J. Biomater. Res. 2016, 2, 163–170. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as Drug Delivery Systems; Pros and Cons. Trends Pharm. Sci. 2019, 5, 7–24. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hoffman, A.S. Hydrogels. In Biomaterials Science; Academic Press: Cambridge, MA, USA, 2020; pp. 153–166. [Google Scholar]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Jalalvandi, E.; Cabral, J.; Hanton, L.R.; Moratti, S.C. Cyclodextrin-Polyhydrazine Degradable Gels for Hydrophobic Drug Delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, J.; Cai, Y.; Zhan, J.; Gao, J.; Song, M.; Shi, Y.; Yang, Z. A Glycyrrhetinic Acid-Modified Curcumin Supramolecular Hydrogel for Liver Tumor Targeting Therapy. Sci. Rep. 2017, 7, 44210. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, K.; Taniguchi, I.; Fukui, H.; Sunamoto, J. Hydrogel Nanoparticle Formed by Self-Assembly of Hydrophobized Polysaccharide. Stabilization of Adriamycin by Complexation. Eur. J. Pharm. Biopharm. 1996, 42, 286–290. [Google Scholar]
- Gong, Z.; Zhang, G.; Zeng, X.; Li, J.; Li, G.; Huang, W.; Sun, R.; Wong, C. High-Strength, Tough, Fatigue Resistant, and Self-Healing Hydrogel Based on Dual Physically Cross-Linked Network. ACS Appl. Mater. Interfaces 2016, 8, 24030–24037. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent Crosslinked Carboxymethyl Cellulose-PEG Hydrogels for Potential Wound Dressing Applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Li, C.; Sun, X.; Meng, Q.; Ma, Y.; Wei, Z. Chemically Crosslinked Hydrogel Film Leads to Integrated Flexible Supercapacitors with Superior Performance. Adv. Mater. 2015, 27, 7451–7457. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B.; Ahmad, Z.; et al. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Onaciu, A.; Munteanu, R.A.; Moldovan, A.I.; Moldovan, C.S.; Berindan-Neagoe, I. Hydrogels Based Drug Delivery Synthesis, Characterization and Administration. Pharmaceutics 2019, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Caillol, S. Molecules Special Issue “Natural Polymers and Biopolymers II”. Molecules 2020, 26, 112. [Google Scholar] [CrossRef]
- Singhal, R.; Gupta, K. A Review: Tailor-Made Hydrogel Structures (Classifications and Synthesis Parameters). Polym. Plast. Technol. Eng. 2016, 55, 54–70. [Google Scholar] [CrossRef]
- Do, N.H.N.; Truong, Q.T.; Le, P.K.; Ha, A.C. Journal Pre-Proof Recent Developments in Chitosan Hydrogels Carrying Natural Bioactive Compounds. Carbohydr. Polym. 2021, 294, 118159. [Google Scholar] [CrossRef]
- Radulescu, D.M.; Neacsu, I.A.; Grumezescu, A.M.; Andronescu, E. New Insights of Scaffolds Based on Hydrogels in Tissue Engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.; Alonso, J.M.; Sáez, V.; Cid, S.B.; Moreno-benítez, I.; Larrauri, B.; González, R.P.; Vilas-vilela, J.L.; Pérez-álvarez, L. Self-Healing, Antibacterial and Anti-Inflammatory Chitosan-PEG Hydrogels for Ulcerated Skin Wound Healing and Drug Delivery. Biomater. Adv. 2022, 139, 212–992. [Google Scholar] [CrossRef] [PubMed]
- Ramdhan, T.; Ching, S.H.; Prakash, S.; Bhandari, B. Physical and Mechanical Properties of Alginate Based Composite Gels. Trends Food Sci. Technol. 2020, 106, 150–159. [Google Scholar] [CrossRef]
- Phatchayawat, P.P.; Khamkeaw, A.; Yodmuang, S.; Phisalaphong, M. 3D Bacterial Cellulose-Chitosan-Alginate-Gelatin Hydrogel Scaffold for Cartilage Tissue Engineering. Biochem. Eng. J. 2022, 184, 108476. [Google Scholar] [CrossRef]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Biomaterials for 3D Cell Biology Prospective Article. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Carmona, S.; Mellado, C.; Mel, M.; Aguayo, C.; Fern, K. Biomaterials Advances Novel and Effective Hemostats Based on Graphene Oxide-Polymer Aerogels: In Vitro and in Vivo Evaluation. Biomater. Adv. 2022, 139, 213007. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing Degradable Hydrogels for Orthogonal Control of Cell Microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef] [PubMed]
- Bulut, E.; Şanlı, O. Novel Ionically Crosslinked Acrylamide-Grafted Poly(Vinyl Alcohol)/Sodium Alginate/Sodium Carboxymethyl Cellulose PH-Sensitive Microspheres for Delivery of Alzheimer’s Drug Donepezil Hydrochloride: Preparation and Optimization of Release Conditions. Artif. Cells Nanomed. Biotechnol. 2016, 44, 431–442. [Google Scholar] [CrossRef]
- Bogdanova, L.R.; Zelenikhin, P.V.; Makarova, A.O.; Zueva, O.S.; Salnikov, V.V.; Zuev, Y.F.; Ilinskaya, O.N. Alginate-Based Hydrogel as Delivery System for Therapeutic Bacterial RNase. Polymers 2022, 14, 2461. [Google Scholar] [CrossRef]
- Liu, K.; Wiendels, M.; Yuan, H.; Ruan, C.; Kouwer, P.H.J. Cell-Matrix Reciprocity in 3D Culture Models with Nonlinear Elasticity. Bioact. Mater. 2022, 9, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Promoteur, E. In Silico Model for Gel Aspiration-Ejection (GAE) Process in the Context of Clinical Peripheral Nerve Repair. Master’s Thesis, University of Liège, Liège, Belgium, 2022; p. 6. [Google Scholar]
- Kang, H.; Liu, R.; Huang, Y. Cellulose-Based Gels. Macromol. Chem. Phys. 2016, 217, 1322–1334. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Chen, W.; Chen, Y. Thiol-Ene Crosslinked Cellulose-Based Gel Polymer Electrolyte with Good Structural Integrity for High Cycling Performance Lithium-Metal Battery. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Ullah, H.; Santos, H.A.; Khan, T. Applications of Bacterial Cellulose in Food, Cosmetics and Drug Delivery. Cellulose 2016, 23, 2291–2314. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, B.; Sarkar, K. Nanocellulose as Sustainable Biomaterials for Drug Delivery. Sens. Int. 2022, 3, 100–135. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic Acid and Its Biomedical Applications: A Review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Nikjoo, D.; van der Zwaan, I.; Brülls, M.; Tehler, U.; Frenning, G. Hyaluronic Acid Hydrogels for Controlled Pulmonary Drug Delivery—A Particle Engineering Approach. Pharmaceutics 2021, 13, 1878. [Google Scholar] [CrossRef]
- Schneider-Barthold, C.; Baganz, S.; Wilhelmi, M.; Scheper, T.; Pepelanova, I. Hydrogels Based on Collagen and Fibrin—Frontiers and Applications. BioNanoMaterials 2016, 17, 3–12. [Google Scholar] [CrossRef]
- Murphy, K.C.; Whitehead, J.; Zhou, D.; Ho, S.S.; Leach, J.K. Engineering Fibrin Hydrogels to Promote the Wound Healing Potential of Mesenchymal Stem Cell Spheroids. Acta Biomater. 2017, 64, 176. [Google Scholar] [CrossRef]
- Ahearne, M.; Buckley, C.T.; Kelly, D.J. A Growth Factor Delivery System for Chondrogenic Induction of Infrapatellar Fat Pad-Derived Stem Cells in Fibrin Hydrogels. Biotechnol. Appl. Biochem. 2011, 58, 345–352. [Google Scholar] [CrossRef]
- Gandhi, J.K.; Manzar, Z.; Bachman, L.A.; Andrews-Pfannkoch, C.; Knudsen, T.; Hill, M.; Schmidt, H.; Iezzi, R.; Pulido, J.S.; Marmorstein, A.D. Fibrin Hydrogels as a Xenofree and Rapidly Degradable Support for Transplantation of Retinal Pigment Epithelium Monolayers. Acta Biomater. 2018, 67, 134–146. [Google Scholar] [CrossRef]
- Tanaka, R.; Saito, Y.; Fujiwara, Y.; Jo, J.; Tabata, Y. Preparation of Fibrin Hydrogels to Promote the Recruitment of Anti-Inflammatory Macrophages. Acta Biomater. 2019, 89, 152–165. [Google Scholar] [CrossRef]
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial Hydrogels for Biomedical Applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef]
- Hassan, T.; Zhou, C.; Saeed, S. Polymers, An Infrangible Part of Our Life. J. Islam. Med. Dent. Coll. 2021, 10, 131–132. [Google Scholar] [CrossRef]
- Bi, X.; Liang, A. In Situ-Forming Cross-linking Hydrogel Systems: Chemistry and Biomedical Applications. Emerg. Concepts Anal. Appl. Hydrogels 2016, 86, 541–547. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-Linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Priya, V.S.V.; Roy, H.K.; Jyothi, N.; Prasanthi, N.L. Polymers in Drug Delivery Technology, Types of Polymers and Applications. Sch. Acad. J. Pharm. 2016, 5, 305–308. [Google Scholar] [CrossRef]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Functional Biomaterials Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef]
- Kfoury, M.; Landy, D.; Fourmentin, S. Combination of DES and Macrocyclic Host Molecules: Review and Perspectives. Curr. Opin. Green Sustain. Chem. 2022, 36, 100630. [Google Scholar] [CrossRef]
- Nafo Id, W.; Al-Mayah, A. Mechanical Characterization of PVA Hydrogels’ Rate-Dependent Response Using Multi-Axial Loading. PLoS ONE 2020, 15, e0233021. [Google Scholar] [CrossRef]
- Fatima, F.; Singh, V. Materials Today: Proceedings Assessment of Antibacterial Properties of Electrospun Fish Collagen/Poly (Vinyl) Alcohol Nanofibers with/Biosurfactant Rhamnolipid. Mater. Today Proc. 2022, 06, 2214–7853. [Google Scholar] [CrossRef]
- Husain, M.S.B.; Gupta, A.; Alashwal, B.Y.; Sharma, S. Synthesis of PVA/PVP Based Hydrogel for Biomedical Applications: A Review. Energy Sources, Part A Recover. Util. Environ. Eff. 2018, 40, 2388–2393. [Google Scholar] [CrossRef]
- Contardi, M.; Kossyvaki, D.; Picone, P.; Summa, M.; Guo, X.; Heredia-Guerrero, J.A.; Giacomazza, D.; Carzino, R.; Goldoni, L.; Scoponi, G.; et al. Electrospun Polyvinylpyrrolidone (PVP) Hydrogels Containing Hydroxycinnamic Acid Derivatives as Potential Wound Dressings. Chem. Eng. J. 2021, 409, 128144. [Google Scholar] [CrossRef]
- Awasthi, R.; Manchanda, S.; Das, P.; Velu, V.; Malipeddi, H.; Pabreja, K.; Pinto, T.D.J.A.; Gupta, G.; Dua, K. Poly(Vinylpyrrolidone). In Engineering of Biomaterials for Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 255–272. [Google Scholar]
- Gibas, I.; Janik, H. Review: Synthetic Polymer Hydrogels for Biomedical Applications. Chem. Chem. Technol. 2010, 4, 297–304. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Qin, Z.; Sun, X.; Zhang, H.; Yu, Q.; Yao, M.; He, S.; Dong, X.; Yao, F.; et al. Dual Physically Cross-Linked Carboxymethyl Cellulose-Based Hydrogel with High Stretchability and Toughness as Sensitive Strain Sensors. Cellulose 2020, 27, 9975–9989. [Google Scholar] [CrossRef]
- Long, L.; Li, F.; Shu, M.; Zhang, C.; Weng, Y. Materials Fabrication and Application of Carboxymethyl Cellulose-Carbon Nanotube Aerogels. Materials 2019, 12, 1867. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.; Yang, D.; Zhang, T.; Yue, X.; Qiu, F. Thermal-Responsive PNIPAm-Acrylic/Ag NRs Hybrid Hydrogel with Atmospheric Window Full-Wavelength Thermal Management for Smart Windows. Sol. Energy Mater. Sol. Cells 2020, 206, 110336. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Hua, L.; Xie, M.; Jian, Y.; Wu, B.; Chen, C.; Zhao, C. Multiple-Responsive and Amphibious Hydrogel Actuator Based on Asymmetric UCST-Type Volume Phase Transition. ACS Appl. Mater. Interfaces 2019, 11, 43641–43648. [Google Scholar] [CrossRef]
- Mourey, T.H.; Schunk, T.C. Synthetic Polymers; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 51, ISBN 9780128098806. [Google Scholar]
- Yu, J.; Qiu, H.; Yin, S.; Wang, H.; Li, Y. Polymeric Drug Delivery System Based on Pluronics for Cancer Treatment. Molecules 2021, 26, 3610. [Google Scholar] [CrossRef]
- Mahajan, H.S.; Jadhao, V.D. Pullulan and Pluronic F-127 Based in Situ Gel System for Intranasal Delivery: Development, in Vitro and in Vivo Evaluation. J. Bioact. Compat. Polym. 2022, 37, 406–418. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Krishna, S.; Hu, Q.; Xuan, M.; Xie, H. Environment-Sensitive Hydrogels as Potential Drug Delivery Systems for the Treatment of Periodontitis. Mater. Express 2020, 10, 975–985. [Google Scholar] [CrossRef]
- Gegel, N.O.; Shipovskaya, A.B.; Khaptsev, Z.Y.; Radionov, R.V.; Belyaeva, A.A.; Kharlamov, V.N.; Gegel, N.O.; Shipovskaya, A.B.; Khaptsev, Z.Y.; Radionov, R.V.; et al. Thermosensitive Chitosan-Containing Hydrogels: Their Formation, Properties, Antibacterial Activity, and Veterinary Usage. Gels 2022, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Boonlai, W.; Tantishaiyakul, V.; Hirun, N.; Sangfai, T.; Suknuntha, K. Thermosensitive Poloxamer 407/Poly(Acrylic Acid) Hydrogels with Potential Application as Injectable Drug Delivery System. AAPS PharmSciTech 2018, 19, 2103–2117. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Bahrani, S.; Aslani, R.; Hashemi, S.A.; Mousavi, S.M.; Ghaedi, M. Introduction to Molecularly Imprinted Polymer. Interface Sci. Technol. 2021, 33, 511–556. [Google Scholar] [CrossRef]
- Alavarse, A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for Polysaccharides and Proteins: Synthesis Conditions, Mechanisms, and Crosslinking Efficiency, a Review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Hanif, M.; Azeem, M.; Mahmood, K.; Gondal, S.A. Role of Crosslinkers for Synthesizing Biocompatible, Biodegradable and Mechanically Strong Hydrogels with Desired Release Profile. Polym. Bull. 2021. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the Barrier and Mechanical Properties of Corn Starch-Based Edible Films: Effect of Citric Acid and Carboxymethyl Cellulose. Ind. Crops Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Simões, B.M.; Cagnin, C.; Yamashita, F.; Olivato, J.B.; Garcia, P.S.; de Oliveira, S.M.; Eiras Grossmann, M.V. Citric Acid as Crosslinking Agent in Starch/Xanthan Gum Hydrogels Produced by Extrusion and Thermopressing. Lwt 2020, 125, 108950. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Chen, L.; Zhou, X.; Fan, X.; Hu, Y.; Niu, X.; Xu, X.; Zhou, G.; Ullah, N.; et al. Antibacterial Aerogels with Nano-silver Reduced in Situ by Carboxymethyl Cellulose for Fresh Meat Preservation. Int. J. Biol. Macromol. 2022, 213, 621–630. [Google Scholar] [CrossRef]
- Künne, S.; Püttmann, F.; Linhorst, M.; Moerschbacher, B.M.; Winter, M.; Li, J.; Placke, T. Comparative Study on Chitosans as Green Binder Materials for LiMn2O4 Positive Electrodes in Lithium Ion Batteries. ChemElectroChem 2022, e202200600. [Google Scholar] [CrossRef]
- Tomadoni, B.; Ponce, A.; Pereda, M.; Ansorena, M.R. Vanillin as a Natural Cross-Linking Agent in Chitosan-Based Films: Optimizing Formulation by Response Surface Methodology. Polym. Test. 2019, 78, 105935. [Google Scholar] [CrossRef]
- Brito, G.B.; Peixoto, V.O.D.S.; Martins, M.T.; Rosário, D.K.A.; Ract, J.N.; Conte-Júnior, C.A.; Torres, A.G.; Castelo-Branco, V.N. Development of Chitosan-Based Oleogels via Crosslinking with Vanillin Using an Emulsion Templated Approach: Structural Characterization and Their Application as Fat-Replacer. Food Struct. 2022, 32, 100264. [Google Scholar] [CrossRef]
- Xie, M.; Hu, B.; Wang, Y.; Zeng, X. Grafting of Gallic Acid onto Chitosan Enhances Antioxidant Activities and Alters Rheological Properties of the Copolymer. Agric. Food Chem. 2014, 62, 9128–9136. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Koivisto, J.T.; Kellomäki, M.; Kellomaki, M.; And, B. Injectable and Self-Healing Biobased Composite Hydrogels as Future Anticancer Therapeutic Biomaterials. Nano Sel. 2022, 3, 1213–1222. [Google Scholar] [CrossRef]
- Haute, G.V.; Caberlon, E.; Squizani, E.; de Mesquita, F.C.; Pedrazza, L.; Martha, B.A.; da Silva Melo, D.A.; Cassel, E.; Czepielewski, R.S.; Bitencourt, S.; et al. Gallic Acid Reduces the Effect of LPS on Apoptosis and Inhibits the Formation of Neutrophil Extracellular Traps. Toxicol. In Vitro 2015, 30, 309–317. [Google Scholar] [CrossRef]
- Noel, A.; Borguet, Y.P.; Raymond, J.E.; Wooley, K.L. Poly(Carbonate−amide)s Derived from Bio-Based Resources: Poly(Ferulic Acid-Co-Tyrosine). Macromolecules 2014, 47, 2974–2983. [Google Scholar] [CrossRef]
- Saletti, M.; Paolino, M.; Ballerini, L.; Giuliani, G.; Leone, G.; Lamponi, S.; Andreassi, M.; Bonechi, C.; Donati, A.; Piovani, D.; et al. Click-Chemistry Cross-Linking of Hyaluronan Graft Copolymers. Pharmaceutics 2022, 14, 1041. [Google Scholar] [CrossRef]
- Ouimet, M.A.; Griffin, J.; Carbone-Howell, A.L.; Wu, W.H.; Stebbins, N.D.; Di, R.; Uhrich, K.E. Biodegradable Ferulic Acid-Containing Poly(Anhydride-Ester): Degradation Products with Controlled Release and Sustained Antioxidant Activity. Biomacromolecules 2013, 14, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Cassimjee, H.; Kumar, P.; Ubanako, P.; Choonara, Y.E. Genipin-Crosslinked, Proteosaccharide Scaffolds for Potential Neural Tissue Engineering Applications. Pharmaceutics 2022, 14, 441. [Google Scholar] [CrossRef] [PubMed]
- Du, J.R.; Hsu, L.H.; Xiao, E.S.; Guo, X.; Zhang, Y.; Feng, X. Using Genipin as a “Green” Crosslinker to Fabricate Chitosan Membranes for Pervaporative Dehydration of Isopropanol. Sep. Purif. Technol. 2020, 244, 116843. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Ahearne, M. Significance of Crosslinking Approaches in the Development of next Generation Hydrogels for Corneal Tissue Engineering. Pharmaceutics 2021, 13, 319. [Google Scholar] [CrossRef]
- Pal, K.; Paulson, A.T.; Rousseau, D. Biopolymers in Controlled-Release Delivery Systems. In Handbook of Biopolymers and Biodegradable Plastics; Ebnesajjad, S., Ed.; William Andrew Publishing: Boston, MA, USA, 2013; pp. 329–363. [Google Scholar] [CrossRef]
- Pich, A.; Richtering, W. Polymer Nanogels and Microgels. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; Volume 6, pp. 309–350. [Google Scholar]
- Kari, F.W. National Toxicology Program Toxicity Report Series Number 25 NTP Technical Report on Toxicity Studies of Glutaraldehyde Administered by Inhalation to F344/N Rats and B6C3F 1 Mice. Toxic Rep. Ser. 1993, 25, E1–E10. [Google Scholar]
- Mohamed, R.R.; Fahim, M.E.; Soliman, S.M.A. Development of Hydrogel Based on Carboxymethyl Cellulose/Poly (4—Vinylpyridine) for Controlled Releasing of Fertilizers. BMC Chem. 2022, 16, 52. [Google Scholar] [CrossRef]
- Serhan, M.; Sprowls, M.; Jackemeyer, D.; Long, M.; Perez, I.D.; Maret, W.; Tao, N.; Forzani, E. Total Iron Measurement in Human Serum with a Smartphone. R. Soc. Chem. 2012, 3, 1–3. [Google Scholar] [CrossRef]
- Mohana Raju, K.; Padmanabha Raju, M.; Murali Mohan, Y. Synthesis and Water Absorbency of Crosslinked Superabsorbent Polymers. J. Appl. Polym. Sci. 2002, 85, 1795–1801. [Google Scholar] [CrossRef]
- Kono, H.; Nakamura, T. Polymerization of β-Cyclodextrin with 1,2,3,4-Butanetetracarboxylic Dianhydride: Synthesis, Structural Characterization, and Bisphenol A Adsorption Capacity. React. Funct. Polym. 2013, 73, 1096–1102. [Google Scholar] [CrossRef]
- Zheng, P.; Lin, Q.; Li, F.; Ou, Y.; Chen, N. Development and Characterization of a Defatted Soy Flour-Based Bio-Adhesive Crosslinked by 1,2,3,4-Butanetetracarboxylic Acid. Int. J. Adhes. Adhes. 2017, 78, 148–154. [Google Scholar] [CrossRef]
- Lam, Y.; Kan, C.; Yuen, C. Wrinkle-Resistant Finishing of Cotton Fabric with BTCA—The Effect of Co-Catalyst. Text. Res. J. 2011, 81, 482–493. [Google Scholar] [CrossRef]
- Portocarrero Huang, G.; Shanmugasundaram, S.; Masih, P.; Pandya, D.; Amara, S.; Collins, G.; Livingston Arinzeh, T. An Investigation of Common Crosslinking Agents on the Stability of Electrospun Collagen Scaffolds. J. Biomed. Mater. Res. 2015, 103A, 762–771. [Google Scholar] [CrossRef]
- Yeh, M.K.; Liang, Y.M.; Cheng, K.M.; Dai, N.T.; Liu, C.C.; Young, J.J. A Novel Cell Support Membrane for Skin Tissue Engineering: Gelatin Film Cross-Linked with 2-Chloro-1-Methylpyridinium Iodide. Polymer 2011, 52, 996–1003. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, X.; Yu, Y.; Zhao, S.; Song, H.; Chen, A.; Shang, Z. Preparation and Characterization of Vanillin Cross-Linked Chitosan Microspheres of Pterostilbene. Int. J. Polym. Anal. Charact. 2014, 19, 83–93. [Google Scholar] [CrossRef]
- Ismael, M.N.M.; El Nemr, A.; El Ashry, E.S.H.; Abdel Hamid, H. Removal of Hexavalent Chromium by Cross-Linking Chitosan and N,N′-Methylene Bis-Acrylamide. Environ. Process. 2020, 7, 911–930. [Google Scholar] [CrossRef]
- Shen, S.; Chen, X.; Shen, Z.; Chen, H. Pharmaceutics Marine Polysaccharides for Wound Dressings Application: An Overview. Pharmaceutics 2021, 13, 1666. [Google Scholar] [CrossRef]
- Orhan, B.; Kaygusuz, H.; Erim, F.B. Sustainable Alginate-Carboxymethyl Cellulose Superabsorbents Prepared by a Novel Quasi-Cryogelation Method. J. Polym. Res. 2022, 29, 333. [Google Scholar] [CrossRef]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef]
- Meftahi, A.; Khajavi, R.; Rashidi, A.; Rahimi, M.K.; Bahador, A. Preventing the Collapse of 3D Bacterial Cellulose Network via Citric Acid. J. Nanostruct. Chem. 2018, 8, 311–320. [Google Scholar] [CrossRef]
- Poole, L.G.; Kopec, A.K.; Flick, M.J.; Luyendyk, J.P.; Liaw, P.; James Luyendyk, C.P. Cross-Linking by Tissue Transglutaminase-2 Alters Fibrinogen-Directed Macrophage Proinflammatory Activity. J. Thromb. Haemost. 2022, 20, 1183–1192. [Google Scholar] [CrossRef]
- Matveeva, V.G.; Senokosova, E.A.; Sevostianova, V.V.; Khanova, M.Y.; Glushkova, T.V.; Akentieva, T.N.; Antonova, L.V.; Barbarash, L.S. Advantages of Fibrin Polymerization Method without the Use of Exogenous Thrombin for Vascular Tissue Engineering Applications. Biomedicines 2022, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.X.Q.; Chen, S.S.; Chang, H.M.; Cao, N.D.T.; Singh, R. Effects of Polyethylene Glycol and Glutaraldehyde Cross-Linker on TFC-FO Membrane Performance. Environ. Technol. Innov. 2020, 20, 101059. [Google Scholar] [CrossRef]
- Nascimento, F.C.D.; de Aguiar, L.C.V.; Costa, L.A.T.; Fernandes, M.T.; Marassi, R.J.; Gomes, A.D.S.; de Castro, J.A. Formulation and Characterization of Crosslinked Polyvinyl Alcohol (PVA) Membranes: Effects of the Crosslinking Agents. Polym. Bull. 2021, 78, 917–929. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, Processing and Application of Hydrogels: A Review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Jyoti Bharali, D.; Maitra, A.; Mitra, S. Hydrogel Nanoparticles: Cross-Linked Polyvinylpyrrolidone. In Dekker Encyclopedia of Nanoscience and Nanotechnology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2014; Volume 6, pp. 1835–1845. [Google Scholar]
- Jeon, J.G.; Kim, H.C.; Palem, R.R.; Kim, J.; Kang, T.J. Cross-Linking of Cellulose Nanofiber Films with Glutaraldehyde for Improved Mechanical Properties. Mater. Lett. 2019, 250, 99–102. [Google Scholar] [CrossRef]
- Liang, L.; Rieke, P.C.; Liu, J.; Fryxell, G.E.; Young, J.S.; Engelhard, M.H.; Alford, K.L. Surfaces with Reversible Hydrophilic/Hydrophobic Characteristics on Cross-Linked Poly(N-Isopropylacrylamide) Hydrogels. Langmuir 2000, 16, 8016–8023. [Google Scholar] [CrossRef]
- Ullah Khan, K.; Akhtar, N.; Usman Minhas, M. Poloxamer-407-Co-Poly (2-Acrylamido-2-Methylpropane Sulfonic Acid) Cross-Linked Nanogels for Solubility Enhancement of Olanzapine: Synthesis, Characterization, and Toxicity Evaluation. AAPS 2020, 21, 141. [Google Scholar] [CrossRef]
- Alshaikh, R.A.; Waeber, C.; Ryan, K.B. Polymer Based Sustained Drug Delivery to the Ocular Posterior Segment: Barriers and Future Opportunities for the Treatment of Neovascular Pathologies. Adv. Drug Deliv. Rev. 2022, 187, 114342. [Google Scholar] [CrossRef]
- Wang, M.; Guo, L.; Sun, H. Manufacture of Biomaterials. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; Volumes 1–3, pp. 116–134. [Google Scholar]
- Badali, E.; Hosseini, M.; Mohajer, M.; Hassanzadeh, S.; Saghati, S.; Hilborn, J.; Khanmohammadi, M. Enzymatic Crosslinked Hydrogels for Biomedical Application. Polym. Sci. Ser. A 2021, 63, S1–S22. [Google Scholar] [CrossRef]
- Silvestro, I.; Francolini, I.; Di Lisio, V.; Martinelli, A.; Pietrelli, L.; Scotto d’Abusco, A.; Scoppio, A.; Piozzi, A. Preparation and Characterization of TPP-Chitosan Crosslinked Scaffolds for Tissue Engineering. Materials 2020, 13, 3577. [Google Scholar] [CrossRef]
- Cruz, A.; Couto, L.; Esplugas, S.; Sans, C. Study of the Contribution of Homogeneous Catalysis on Heterogeneous Fe(III)/Alginate Mediated Photo-Fenton Process. Chem. Eng. J. 2017, 318, 272–280. [Google Scholar] [CrossRef]
- Wahab, A.H.A.; Saad, A.P.M.; Harun, M.N.; Syahrom, A.; Ramlee, M.H.; Sulong, M.A.; Kadir, M.R.A. Developing Functionally Graded PVA Hydrogel Using Simple Freeze-Thaw Method for Artificial Glenoid Labrum. J. Mech. Behav. Biomed. Mater. 2019, 91, 406–415. [Google Scholar] [CrossRef]
- Pearce, H.A.; Kim, Y.S.; Diaz-Gomez, L.; Mikos, A.G. Tissue Engineering Scaffolds. Biomater. Sci. 2020, 1317–1334. [Google Scholar] [CrossRef]
- Kirtania, M.D.; Kahali, N.; Maity, A. Inulin-Based Hydrogel. In Plant and Algal Hydrogels for Drug Delivery and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2021; pp. 261–292. [Google Scholar]
- Li, Y.; Wang, X.; Han, Y.; Sun, H.Y.; Hilborn, J.; Shi, L. Click Chemistry-Based Biopolymeric Hydrogels for Regenerative Medicine. Biomed. Mater. 2021, 16, 022003. [Google Scholar] [CrossRef]
- Mohabatpour, F.; Yazdanpanah, Z.; Papagerakis, S.; Chen, X.; Papagerakis, P. Self-Crosslinkable Oxidized Alginate-Carboxymethyl Chitosan Hydrogels as an Injectable Cell Carrier for In Vitro Dental Enamel Regeneration. J. Funct. Biomater. 2022, 13, 71. [Google Scholar] [CrossRef]
- Astudillo-Ortiz, E.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Evaluation of Injectable Hyaluronic Acid-Based Hydrogels for Endodontic Tissue Regeneration. Materials 2021, 14, 7325. [Google Scholar] [CrossRef]
- Sellers, D.L.; Kim, T.H.; Mount, C.W.; Pun, S.H.; Horner, P.J. Poly(Lactic-Co-Glycolic) Acid Microspheres Encapsulated in Pluronic F-127 Prolong Hirudin Delivery and Improve Functional Recovery from a Demyelination Lesion. Biomaterials 2014, 35, 8895–8902. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.H.; Gao, M.; Lin, J.; Wu, W.; Wang, J.; Chew, S.Y. Three-Dimensional Aligned Nanofibers-Hydrogel Scaffold for Controlled Non-Viral Drug/Gene Delivery to Direct Axon Regeneration in Spinal Cord Injury Treatment. Sci. Rep. 2017, 7, 42212. [Google Scholar] [CrossRef]
- Calias, P.; Banks, W.A.; Begley, D.; Scarpa, M.; Dickson, P. Intrathecal Delivery of Protein Therapeutics to the Brain: A Critical Reassessment. Pharmacol. Ther. 2014, 144, 114–122. [Google Scholar] [CrossRef]
- Fowler, M.J.; Cotter, J.D.; Knight, B.E.; Sevick-Muraca, E.M.; Sandberg, D.I.; Sirianni, R.W. Intrathecal Drug Delivery in the Era of Nanomedicine. Adv. Drug Deliv. Rev. 2020, 165–166, 77–95. [Google Scholar] [CrossRef]
- LeBel, C.; Bourdeau, A.; Lau, D.; Hunt, P. Biologic Response to Peripheral and Central Administration of Recombinant Human Leptin in Dogs. Obes. Res. 1999, 7, 577–585. [Google Scholar] [CrossRef]
- Yousfan, A.; Rubio, N.; Natouf, A.H.; Daher, A.; Al-Kafry, N.; Venner, K.; Kafa, H. Preparation and Characterization of PHT-Loaded Chitosan Lecithin Nanoparticles for Intranasal Drug Delivery to the Brain. RSC Adv. 2020, 10, 28992–29009. [Google Scholar] [CrossRef]
- Keller, L.-A.; Merkel, O.; Popp, A. Intranasal Drug Delivery: Opportunities and Toxicologic Challenges during Drug Development. Drug Deliv. Transl. Res. 2022, 12, 735–757. [Google Scholar] [CrossRef]
- Liu, S.; Yang, S.; Ho, P.C. Intranasal Administration of Carbamazepine-Loaded Carboxymethyl Chitosan Nanoparticles for Drug Delivery to the Brain. Asian J. Pharm. Sci. 2018, 13, 72–81. [Google Scholar] [CrossRef]
- Melamane, S.; Walker, R.B.; Khamanga, S.M.M. Formulation Optimization of Smart Thermosetting Lamotrigine Loaded Hydrogels Using Response Surface Methodology, Box Benhken Design and Artificial Neural Networks. Drug Dev. Ind. Pharm. 2020, 46, 1402–1415. [Google Scholar] [CrossRef]
- Karna, S. Commentary: Eye as a Window to the Brain. Indian J. Ophthalmol. 2020, 68, 563. [Google Scholar] [CrossRef]
- Majeed, A.; Khan, N.A. Ocular in Situ Gel: An Overview. J. Drug Deliv. Ther. 2019, 9, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S. Carbopol/Chitosan Based PH Triggered In Situ Gelling System for Ocular Delivery of Timolol Maleate. Sci. Pharm. 2010, 78, 959–976. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Bai, L.; Zhang, H.; Ma, Q.; Luo, R.; Lei, F.; Fei, Q.; He, N. A Novel Flunarizine Hydrochloride-Loaded Organogel for Intraocular Drug Delivery in Situ: Design, Physicochemical Characteristics and Inspection. Int. J. Pharm. 2020, 576, 119027. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Kaushik, A.; Ghosal, A.; Bala, J.; Nikkhah-Moshaie, R.; Wani, W.A.; Manickam, P.; Nair, M. Nanocomposite Hydrogels: Advances in Nanofillers Used for Nanomedicine. Gels 2018, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite Hydrogels: 3D Polymer-Nanoparticle Synergies for on-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef]
- Bhat, A.H.; Khan, I.; Amil Usmani, M.; Rather, J.A. Bioplastics and Bionanocomposites Based on Nanoclays and Other Nanofillers. In Nanoclay Reinforced Polymer Composites; Springer: Singapore, 2016; pp. 115–139. [Google Scholar] [CrossRef]
- Wu, C.; Liu, J.; Zhai, Z.; Yang, L.; Tang, X.; Zhao, L.; Xu, K.; Zhong, W. Double-Crosslinked Nanocomposite Hydrogels for Temporal Control of Drug Dosing in Combination Therapy. Acta Biomater. 2020, 106, 278–288. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, H.; Qian, Z.; Fan, N.; Choi, W.; Zhao, F.; Lee, B.P. A Moldable Nanocomposite Hydrogel Composed of a Mussel-Inspired Polymer and a Nanosilicate as a Fit-to-Shape Tissue Sealant. Angew. Chem. Int. Ed. 2017, 56, 4224–4228. [Google Scholar] [CrossRef]
- Gong, C.; Lu, C.; Li, B.; Shan, M.; Wu, G. Injectable Dopamine-Modified Poly(α,β-Aspartic Acid) Nanocomposite Hydrogel as Bioadhesive Drug Delivery System. J. Biomed. Mater. Res. Part A 2017, 105, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, J.; Hu, Y.; Yang, Y.; Gou, Z.; Du, T.; Mao, J.; Gou, M. A Conformal Hydrogel Nanocomposite for Local Delivery of Paclitaxel. J. Biomater. Sci. Polym. Ed. 2017, 28, 107–118. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click Hydrogels, Microgels and Nanogels: Emerging Platforms for Drug Delivery and Tissue Engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef]
- Yadav, H.K.; Halabi, A.; Alsalloum, N.A. Nanogels as Novel Drug Delivery Systems—A Review. Available online: https://www.semanticscholar.org/paper/Nanogels-as-Novel-Drug-Delivery-Systems-A-Review-Yadav-Halabi/89312e50c1c0c1dd83d81669295508cce5e7c370 (accessed on 16 June 2022).
- Jain, S.; Ancheria, R.K.; Shrivastava, S.; Soni, S.L.; Sharma, M. An Overview of Nanogel–Novel Drug Delivery System. Asian J. Pharm. Res. Dev. 2019, 7, 47–55. [Google Scholar] [CrossRef]
- Anooj, E.; Charumathy, M.; Sharma, V.; Vibala, B.V.; Gopukumar, S.T.; Jainab, S.I.B.; Vallinayagam, S. Nanogels: An Overview of Properties, Biomedical Applications, Future Research Trends and Developments. J. Mol. Struct. 2021, 1239, 130446. [Google Scholar] [CrossRef]
- Ahmed, S.; Alhareth, K.; Mignet, N. Advancement in Nanogel Formulations Provides Controlled Drug Release. Int. J. Pharm. 2020, 584, 119435. [Google Scholar] [CrossRef]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-Responsive Polymeric Nanogels as Smart Drug Delivery Systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S.V.; Batrakova, E.V.; Kabanov, A.V. Nanogels for Oligonucleotide Delivery to the Brain. Bioconjug. Chem. 2004, 15, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shao, X.; Dai, X.; Guo, Q.; Yuan, B.; Liu, Y.; Jiang, W. Recent Trends in the Development of Hydrogel Therapeutics for the Treatment of Central Nervous System Disorders. NPG Asia Mater. 2022, 14, 14. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Drug Delivery Systems, CNS Protection, and the Blood Brain Barrier. Biomed Res. Int. 2014, 2014, 869269. [Google Scholar] [CrossRef]
- Vashist, A.; Kaushik, A.; Vashist, A.; Bala, J.; Nikkhah-Moshaie, R.; Sagar, V.; Nair, M. Nanogels as Potential Drug Nanocarriers for CNS Drug Delivery. Drug Discov. Today 2018, 23, 1436–1443. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, L.; Cheng, W.; Hao, F.; Zhou, L.; Li, Q. Injectable Hydrogels in Repairing Central Nervous System Injuries. Adv. Mater. Sci. Eng. 2021, 2021, 7381980. [Google Scholar] [CrossRef]
- Correll, C.U.; Kim, E.; Sliwa, J.K.; Hamm, W.; Gopal, S.; Mathews, M.; Venkatasubramanian, R.; Saklad, S.R. Pharmacokinetic Characteristics of Long-Acting Injectable Antipsychotics for Schizophrenia: An Overview. CNS Drugs 2021, 35, 39–59. [Google Scholar] [CrossRef]
- Tobinick, E.L. Perispinal Delivery of CNS Drugs. CNS Drugs 2016, 30, 469–480. [Google Scholar] [CrossRef]
- Shatsberg, Z.; Zhang, X.; Ofek, P.; Malhotra, S.; Krivitsky, A.; Scomparin, A.; Tiram, G.; Calderón, M.; Haag, R.; Satchi-Fainaro, R. Functionalized Nanogels Carrying an Anticancer MicroRNA for Glioblastoma Therapy. J. Control. Release 2016, 239, 159–168. [Google Scholar] [CrossRef]
- She, D.; Huang, H.; Li, J.; Peng, S.; Wang, H.; Yu, X. Hypoxia-Degradable Zwitterionic Phosphorylcholine Drug Nanogel for Enhanced Drug Delivery to Glioblastoma. Chem. Eng. J. 2021, 408, 127359. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, Q.; Mu, K.; Xie, H.; Zhu, Y.; Zhu, W.; Zhao, Y.; Xu, H.; Yang, X. PH/Temperature Sensitive Magnetic Nanogels Conjugated with Cy5.5-Labled Lactoferrin for MR and Fluorescence Imaging of Glioma in Rats. Biomaterials 2013, 34, 7418–7428. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H. Development of Tumor-Targeting Antitumor Agents Based on Polymer Effect. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2020, 140, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Han, Y.; Han, Z.; Zhang, D.; Zhang, L.; Zhao, G.; Li, S.; Jin, G.; Yu, R.; Liu, H. In Situ Implantable, Post-Trauma Microenvironment-Responsive, ROS Depletion Hydrogels for the Treatment of Traumatic Brain Injury. Biomaterials 2021, 270, 120675. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, Z.; Nourbakhsh, M.S.; Kiani, S.; Heydari, Y.; Ashtiani, M.K.; Daemi, H.; Baharvand, H. Co-Delivery of Minocycline and Paclitaxel from Injectable Hydrogel for Treatment of Spinal Cord Injury. J. Control. Release 2020, 321, 145–158. [Google Scholar] [CrossRef]
- Li, J.; Darabi, M.; Gu, J.; Shi, J.; Xue, J.; Huang, L.; Liu, Y.; Zhang, L.; Liu, N.; Zhong, W.; et al. A Drug Delivery Hydrogel System Based on Activin B for Parkinson’s Disease. Biomaterials 2016, 102, 72–86. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Z.; Zhou, J.; Xing, Q.; Ma, S.; Li, Q.; Zhang, Y.; Yao, M.; Wang, X.; Li, Q.; et al. Potential Application of an Injectable Hydrogel Scaffold Loaded with Mesenchymal Stem Cells for Treating Traumatic Brain Injury. J. Mater. Chem. B 2018, 6, 2982–2992. [Google Scholar] [CrossRef]
- Kang, N.-W.; Yoon, S.-Y.; Kim, S.; Yu, N.-Y.; Park, J.-H.; Lee, J.-Y.; Cho, H.-J.; Kim, D.-D. Subcutaneously Injectable Hyaluronic Acid Hydrogel for Sustained Release of Donepezil with Reduced Initial Burst Release: Effect of Hybridization of Microstructured Lipid Carriers and Albumin. Pharmaceutics 2021, 13, 864. [Google Scholar] [CrossRef]
- Gerson, T.; Makarov, E.; Senanayake, T.H.; Gorantla, S.; Poluektova, L.Y.; Vinogradov, S.V. Nano-NRTIs Demonstrate Low Neurotoxicity and High Antiviral Activity against HIV Infection in the Brain. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 177–185. [Google Scholar] [CrossRef]
- Jahromi, L.P.; Mohammadi-samani, S.; Heidari, R.; Azadi, A.; Sciences, P. In Vitro- and in Vivo Evaluation of Methotrexate-Loaded Hydrogel. J. Pharm. Pharm. Sci. 2018, 21, 305–317. [Google Scholar] [CrossRef]
- Baklaushev, V.P.; Nukolova, N.N.; Khalansky, A.S.; Gurina, O.I.; Yusubalieva, G.M.; Grinenko, N.P.; Gubskiy, I.L.; Melnikov, P.A.; Kardashova, K.S.; Kabanov, A.V.; et al. Treatment of Glioma by Cisplatin-Loaded Nanogels Conjugated with Monoclonal Antibodies against Cx43 and BSAT1. Drug Deliv. 2015, 22, 276–285. [Google Scholar] [CrossRef]
- Ikeda, K.; Okada, T.; Sawada, S.; Akiyoshi, K.; Matsuzaki, K. Inhibition of the Formation of Amyloid β-Protein Fibrils Using Biocompatible Nanogels as Artificial Chaperones. FEBS Lett. 2006, 580, 6587–6595. [Google Scholar] [CrossRef]
- Wang, Y.; Ying, X.; Chen, L.; Liu, Y.; Wang, Y.; Liang, J.; Xu, C.; Guo, Y.; Wang, S.; Hu, W.; et al. Electroresponsive Nanoparticles Improve Antiseizure Effect of Phenytoin in Generalized Tonic-Clonic Seizures. Neurotherapeutics 2016, 13, 603–613. [Google Scholar] [CrossRef]
- Qi, X.-J.; Xu, D.; Tian, M.-L.; Zhou, J.-F.; Wang, Q.-S.; Cui, Y.-L. Thermosensitive Hydrogel Designed for Improving the Antidepressant Activities of Genipin via Intranasal Delivery. Mater. Des. 2021, 206, 109816. [Google Scholar] [CrossRef]
- Chen, W.; Zou, Y.; Zhong, Z.; Haag, R. Cyclo(RGD)-Decorated Reduction-Responsive Nanogels Mediate Targeted Chemotherapy of Integrin Overexpressing Human Glioblastoma In Vivo. Small 2017, 13, 1601997. [Google Scholar] [CrossRef]
- Azadi, A.; Hamidi, M.; Rouini, M.-R. Methotrexate-Loaded Chitosan Nanogels as ‘Trojan Horses’ for Drug Delivery to Brain: Preparation and in Vitro/in Vivo Characterization. Int. J. Biol. Macromol. 2013, 62, 523–530. [Google Scholar] [CrossRef]
- Galgatte, U.C.; Kumbhar, A.B.; Chaudhari, P.D. Development of in Situ Gel for Nasal Delivery: Design, Optimization, in Vitro and in Vivo Evaluation. Drug Deliv. 2014, 21, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Qian, S.; Wong, Y.C.; Zuo, Z. Development, Characterization and Application of in Situ Gel Systems for Intranasal Delivery of Tacrine. Int. J. Pharm. 2014, 468, 272–282. [Google Scholar] [CrossRef]
- Abdulla, N.A.; Balata, G.F.; El-ghamry, H.A.; Gomaa, E. Intranasal Delivery of Clozapine Using Nanoemulsion-Based in-Situ Gels: An Approach for Bioavailability Enhancement. Saudi Pharm. J. 2021, 29, 1466–1485. [Google Scholar] [CrossRef]
- Haidary, H.A.; Padhy, R.K. Clozapine. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30571020 (accessed on 2 August 2022).
- McCrorie, P.; Mistry, J.; Taresco, V.; Lovato, T.; Fay, M.; Ward, I.; Ritchie, A.A.; Clarke, P.A.; Smith, S.J.; Marlow, M.; et al. Etoposide and Olaparib Polymer-Coated Nanoparticles within a Bioadhesive Sprayable Hydrogel for Post-Surgical Localised Delivery to Brain Tumours. Eur. J. Pharm. Biopharm. 2020, 157, 108–120. [Google Scholar] [CrossRef]
- Picone, P.; Sabatino, M.A.; Ditta, L.A.; Amato, A.; San Biagio, P.L.; Mulè, F.; Giacomazza, D.; Dispenza, C.; Di Carlo, M. Nose-to-Brain Delivery of Insulin Enhanced by a Nanogel Carrier. J. Control. Release 2018, 270, 23–36. [Google Scholar] [CrossRef]
- Madhav, S.; Dewari, A.; Tyagi, Y. Asian Journal of Nanoscience and Materials An Innovative Approach Delivery of Anticonvulsant via Transcranial Route Using a Smart Bio-Functional Agent Cum Musa Acuminata. Asian J. Nanosci. Mater. 2020, 3, 82–92. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; Abou-Taleb, H.A.; Naguib, D.M. Nanosized Nasal Emulgel of Resveratrol: Preparation, Optimization, in Vitro Evaluation and in Vivo Pharmacokinetic Study. Drug Dev. Ind. Pharm. 2019, 45, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Al Harthi, S.; Alavi, S.E.; Radwan, M.A.; El Khatib, M.M.; AlSarra, I.A. Nasal Delivery of Donepezil HCl-Loaded Hydrogels for the Treatment of Alzheimer’s Disease. Sci. Rep. 2019, 9, 9563. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Class of Cross-Linked Gel | Advantages | Disadvantages | Applications | Ref |
|---|---|---|---|---|
| Emulgel | Thixotropic Easily spreadable Long shelf life Improved loading efficiency Great stability | Allergic reactions Poor permeability Contact dermatitis Not easily absorbed | Topical emulgel of mefenamic acid. Topical emulgel microemulsion | [34,36,37,39,40] |
| Organogel | Ease of preparation May be used for transdermal, oral, and parenteral. Non-irritating Good resistance to microbial contamination. | Lack of biocompatibility formulations. Poor stability to temperature Greasy in nature | Intraocular flunarizine hydrochloride-loaded organogel Biodegradables in-situ forming organogel. | [42,43,44,45] |
| Hydrogel | Capable of retaining a high amount of water Hydrophilicity Biocompatibility potential Controlled drug release Smart drug delivery | It may be difficult to handle It may be difficult to sterilize Usually mechanically weak. | Chemically cross-linked by glutaraldehyde for biomedical applications. Physically cross-linked hydrogel consisting of poly (acrylamide-co-acrylic acid) (PAM-co-PAA) and poly(vinyl alcohol) (PVA). | [43,45,62,63,64,65,66,67,68,69] |
| Aerogel | High porosity Low bulk density Exceptional textural features | Low mechanical strength High environmental and economic costs of operation | Incorporation of niacin/nicotinic acid and ibuprofen in an aerogel | [73,74] |
| Cryogels | Substantial pore size and porosity High water content Great pore connectivity and consistency Flexibility of preparation Economically and environmentally friendly | Insufficient retention at injection site. Injectable cryogels may cause serios side effects. Need for repeated injections Increased costs | Thermoresponsive cryogels containing oligoehylene glycol Polyacrylic acid cryogels as PH oscillatory bromate-sulphite ferrocynide processes. | [63,66,69] |
| Polymer | Advantages | Disadvantages | Cross-Linking Agent or Factor | Effect | Ref |
|---|---|---|---|---|---|
| Chitosan | Antioxidant, Antifungal, Anti-inflammatory, Antibacterial Non-toxic Cost-effective Easy structure modification Thermal and chemical stability Responsive to external stimuli A polycationic character that promotes fast gelling in the basic pH of normal tissues | Relatively poor mechanical and barrier properties Naturally brittle Low lipophilicity for emulsions | Vanillin N,N/-methylene bis-acrylamide (MBA) Poly (N-isopropyl acrylamide | Improves the balance of chitosan between affinity and insolubility in oil due to the hydrophobic methoxyphenyl group in the vanillin aromatic ring. Assisted in the adsorption of Cr6+ ions from its water solution. High antibacterial activity cotton fabrics | [80,82,136,158,159] |
| Gelatin | Non-immunogenic Non-toxic Amphoteric Non-carcinogenic Good cell adhesion, proliferation, and differentiation due to many binding sites | Thermosensitive | 2-chloro-1-methylpyridinium iodide (CMPI) | Activation of carboxylic acid sodium salt under heterogeneous reaction with high water uptake ability, reasonable biodegradability, and excellent cytocompatibility | [83,85,157] |
| Alginate | Non-toxic Non-immunogenic Good adhesion Thickening and stabilizing Gel-forming and film-forming Fiber spinning Hydrophilic Cost-effective Acidic environment neutralizer Excellent hemostatic properties | Weak mechanical strength Scarcity of efficient sites for cell adhesion, thus, poor cell attachment and proliferation. Alginate gels shrink at low pH Difficulties in sterilization, handling, and storage Difficult to control the release of alginate encapsulated material due to its porosity, permeability, and degradation | Calcium ions Sodium ions | Alginate hydrogel changed weight by 10% in pure water and 90% in an isotonic solution Selective binding to G sequences of alginate and form heat-stable three-dimensional gel networks High quantity water absorption due to ion exchange | [81,83,84,89,160,161] |
| Collagen | High antigenicity | Ethical and cultural issues Inconsistency Low mechanical strength Fast degradation rate Potential toxicity due to residual catalysts or initiators | Polypropyleneimine-octa-amine dendrimers | Supports adhesion and proliferation of human corneal epithelial cells without encouraging cellular toxicity | [92,146,157,162] |
| Cellulose | Pure A high degree of porosity Good tensile strength Low immunogenicity High relative permeability to gases and liquids High retention and ion exchange capacity | Insoluble in most solvents | Citric acid | Formation of carboxylic bridges between cellulose fibril chains, thus preventing cellulose condensation during drying Improved rehydration ability, porosity, wettability, and water swelling rate | [95,96,163] |
| Hyaluronic acid | Non-immunogenic | Usefulness degraded by hyaluronidase | N-(3-Dimethylaminopropyl)-N’-ethyl carbodiimide hydrochloride (EDC) 2-chloro-1-methylpyrinium iodide (CMPI) | Faster degradation rate and smoother surfaces, lower cytotoxicity for corneal endothelial cells, and minimal inflammatory cell infiltration or foreign body reaction after implantation Facilitates intra- and inter-molecular ester bond formation between the carboxyl and hydroxyl groups of hyaluronic and exhibits better resistance against hydrolytic degradation | [131] |
| Fibrin | Abundant and simple Resistance to degradation Fast isolation from the patient’s blood. Promotes expression of proinflammatory cytokines, cell migration, cell adhesion, and phagocytosis in monocytes, macrophages, and neutrophils. | Risk of infection transmission | Transglutaminase 2 (TG2) | Enhances proinflammatory activity to surface adhered fibrinogen | [164,165] |
| Polymer | Advantages | Disadvantages | Cross-Linking Agent or Factor | Effect | Ref |
|---|---|---|---|---|---|
| PEG | Amphiphilic High swelling index Good gelation properties Low immunogenicity | Low cell adhesion Poor cell affinity Reduced cellular response | Glutaraldehyde | Improved water flux and porosity Decreased swelling and solute diffusion through membranes Enhance water permeability due to hydroxyl hydrophilic functional group in GLA | [81,112,113,166] |
| PVA | Good thermal stability Good mechanical strength Excellent film membrane properties Viscoelastic hydrophilic non-toxic pH stable | Does not support cell proliferation and adhesion Limited elasticity and hydrophilicity | Citric acid | Uniformly distributed membrane roughness, homogeneous films, enhanced adhesion, and strength properties with good stability. | [112,116,117,167] |
| PVP | Low cytotoxicity Hydrophilic Excellent adsorption and adhesion Good thermal stability and miscibility Excellent wetting properties Rapid swelling Excellent film; | Weak mechanical properties Thermal instability | N,N′-methylene-bisacrylamide | Improved drug, vaccine, and peptides encapsulation | [74,168,169] |
| CMC | Hydrophilic Abundant Cost-effective Environmentally friendly | Low mechanical strength Low gelation properties | Glutaraldehyde | Improved mechanical properties due to covalent bonds formed using acidic catalysis. Improved tensile strength and elastic modulus. | [67,122,124,170] |
| pNIPAAM | Hydrophilic Good mechanical properties Temperature-responsive | Thermal instability Potential phase separation Monomers and crosslinkers use are mostly non-biodegradability and not biocompatibility, which may lead to toxic, carcinogenic, and teratogenic effects. | N,N′-methylene-bisacrylamide | The surface morphology of silicon wafers became thick, rough and thermo-responsive | [123,168,171] |
| Pluronic® | Amphiphilic High biocompatibility Stabilizer Retention agent | Thermosensitive Weak mechanical strength at low concentrations Lack of mucoadhesion Lower gelation temperature at high concentrations | N,N′-methylene-bisacrylamide | Improved hydrophilic properties for the solubility of poorly soluble drug olanzapine Improved drug release in both acidic and basic pH Improved safety and biocompatibility | [127,128,130,172] |
| ROA | Polymer | Crosslinker | API | Disease | In-Vivo/In-Vitro Model | In-Vivo/In-Vitro Findings | Ref |
|---|---|---|---|---|---|---|---|
| IV | PEG and PEI | Carbonyldiimidazole | Oligonucleotides | Neurodegenerative disorders | Mice | Better brain targeting with a 15-fold increase in accumulation of the drug in the brain and a 2-fold decrease in liver and spleen accumulation | [211] |
| IT | Polyglycerols | Disulfide | MicroRNA therapeutics | Glioblastoma Multiforme | Mice | Significantly inhibited tumor growth. Downregulation of miR-34a target genes, which plays key roles in the regulation of apoptosis and cell cycle arrest | [218] |
| IV | Phosphorylcholine | Azobenzene-contained crosslinker | Doxorubicin | Glioblastoma | Mice | Favorable biocompatibility and long-circulating property in blood Significantly stronger glioblastoma inhibition effect. | [219] |
| IV | Poly(N-isopropyl acrylamide-co-acrylic acid) | carbodiimide hydrochloride and N-hydroxysulfosuccinimide | Lactoferrin | Glioma | Rats | Highly sensitive and specific MR/fluorescence imaging | [220] |
| IT | Poly (propylene sulfide) 120 | Triglycerol monostearate | Curcumin | TBI | Mice | Enhanced brain drug accumulation resulting in improved regeneration and recovery of neurons | [222] |
| IS | Alginate | Calcium D-gluconate monohydrate | Paclitaxel (PTX) and Minocycline hydrochloride (MH) | Spinal cord injury (SCI) | Wistar rats | Increased neuronal regeneration after 28 days. Reduced inflammation after 7 days | [223] |
| IC | PNIPAAm | poly (amidoamine) | Activin B | PD | Male C57BL/6J mice | Prolonged release of activin B of around 5 weeks | [224] |
| IC | Sodium alginate and hyaluronic acid | Calcium carbonate (CaCO3) | Human umbilical cord mesenchymal stem cells (hUC-MSCs) | Traumatic brain injury and stem cell tissue engineering | Sprague Dawley Rats | Enhanced regeneration of endogenous nerve cells. Protected the injected hUC-MSCs | [225] |
| SC | Hyaluronic acid | - | Donepezil | AD | Rats | Increased drug T1/2, reduced Cmax value, and sustained drug release over 7 days | [226] |
| IC | PEG and Polyethyleneimine (PEI) | 1,1′-carbonyldiimidazole | Zidovudine (AZT) | HIV-1 | Mice | Low neurotoxicity and improved antiviral suppression | [227] |
| IC | Poly(ethylene glycol)-b-poly(methacrylic acid) deblock copolymer | 1,2-ethylenedia-mine, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride | Cisplatin | Intracranial gliomas | Wistar rats | Enhanced inhibition of tumor growth and increased the life span of the animals | [229] |
| IV | 2-Dimethylamino ethyl methacrylate | N,N′-methylene bisacrylamide | Phenytoin sodium | Epilepsy | Male Sprague-Dawley rats | Higher distribution in the central nervous system | [231] |
| IP | Pluronics® 407 (P 407), Pluronics® 188 (P 188) | - | Genipin | Depression | Male ICR mice | High drug release rate and improved antidepressant-like activities | [232] |
| IV | PVA | Carbodiimide | Doxorubicin | Integrin overexpressed human glioblastoma | Nude mice | Improved tumor targeting, tumor growth inhibition, and reduced side effects | [233] |
| IN | Chitosan | Polyanionic pentasodium triphosphate | Methotrexate | Brain tumor | Sprague Dawley Rats | Up to a 10-fold increase in brain concentrations of methotrexate compared to free drug. | [234] |
| ROA | Polymer Used | Cross-Linking Agent/Factor Used | API/Agent Delivered | Human Disease | In-Vivo/In-Vitro Model | In-Vivo/In-Vitro Findings | Ref |
|---|---|---|---|---|---|---|---|
| IN | Gellan gum | Heat | Sumatriptan succinate | Headaches | Sprague-Dawley rats | Improved brain targeting and bioavailability | [235] |
| IN | PF-127 | Heat | Tacrine | AD | Rats | Increased nasal residence time, improved bioavailability, and enhanced brain uptake | [236] |
| IN | PF-127 and PF-68 | Heat | Clozapine | Schizophrenia | Dialysis bag technique | Enhanced in-vitro drug release | [237] |
| IC | Pectin and poly(ethylene glycol)-block-polylactic acid (PEG-b-PLA) | Ca2+ | Olaparib | Brain tumor | Mice | High drug loading, improved in-vitro stability, and drug release over prolonged periods | [239] |
| IN | Poly(N-vinyl pyrrolidone)-co-acrylic acid | 1-ethyl-3-[3- dimethylaminopropyl] carbodiimide hydrochloride | Insulin | AD | Male C57BL/6J (B6) mice | Non-immunogenic response of the nasal mucosa. Enhanced distribution of insulin to different brain areas. | [240] |
| TC | Sodium Alginate | Aqueous solvent | Pregabalin | Epilepsy | Dialysis membrane | Faster drug release, biodegradable, biocompatible, non-toxic, non-irritant, and no reaction on the skin were observed. | [241] |
| IN | Carbopol 934 and Pluronics® 407 | Potassium persulfate | Resveratrol | Brain tumors | Wistar albino rats | Good drug release properties. Safe and tolerable to the nasal mucosa | [242] |
| IN | Chitosan | Glutaraldehyde | Liposomal donepezil HCl | AD | New Zealand white rabbits | Significant increase in blood concentration and brain content of the API, compared to the oral tablets | [243] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashabela, L.T.; Maboa, M.M.; Miya, N.F.; Ajayi, T.O.; Chasara, R.S.; Milne, M.; Mokhele, S.; Demana, P.H.; Witika, B.A.; Siwe-Noundou, X.; et al. A Comprehensive Review of Cross-Linked Gels as Vehicles for Drug Delivery to Treat Central Nervous System Disorders. Gels 2022, 8, 563. https://doi.org/10.3390/gels8090563
Mashabela LT, Maboa MM, Miya NF, Ajayi TO, Chasara RS, Milne M, Mokhele S, Demana PH, Witika BA, Siwe-Noundou X, et al. A Comprehensive Review of Cross-Linked Gels as Vehicles for Drug Delivery to Treat Central Nervous System Disorders. Gels. 2022; 8(9):563. https://doi.org/10.3390/gels8090563
Chicago/Turabian StyleMashabela, Leshasha T., Mahlako M. Maboa, Ntombi F. Miya, Taiwo O. Ajayi, Rumbidzai S. Chasara, Marnus Milne, Shoeshoe Mokhele, Patrick H. Demana, Bwalya A. Witika, Xavier Siwe-Noundou, and et al. 2022. "A Comprehensive Review of Cross-Linked Gels as Vehicles for Drug Delivery to Treat Central Nervous System Disorders" Gels 8, no. 9: 563. https://doi.org/10.3390/gels8090563
APA StyleMashabela, L. T., Maboa, M. M., Miya, N. F., Ajayi, T. O., Chasara, R. S., Milne, M., Mokhele, S., Demana, P. H., Witika, B. A., Siwe-Noundou, X., & Poka, M. S. (2022). A Comprehensive Review of Cross-Linked Gels as Vehicles for Drug Delivery to Treat Central Nervous System Disorders. Gels, 8(9), 563. https://doi.org/10.3390/gels8090563









