Three-Dimensional Dosimetry by Optical-CT and Radiochromic Gel Dosimeter of a Multiple Isocenter Craniospinal Radiation Therapy Procedure
Abstract
:1. Introduction
2. Results and Discussion
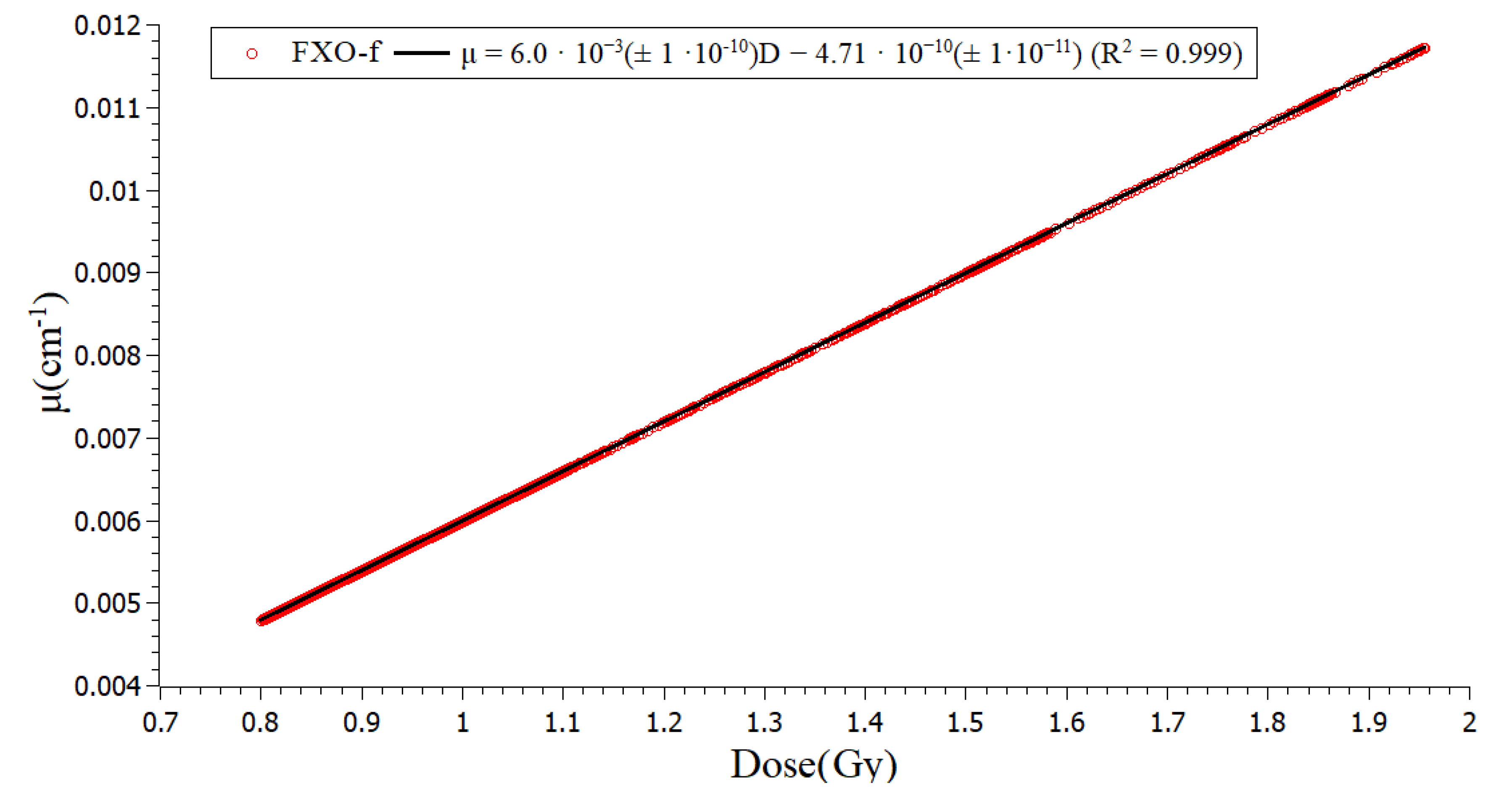
2.1. Calibration
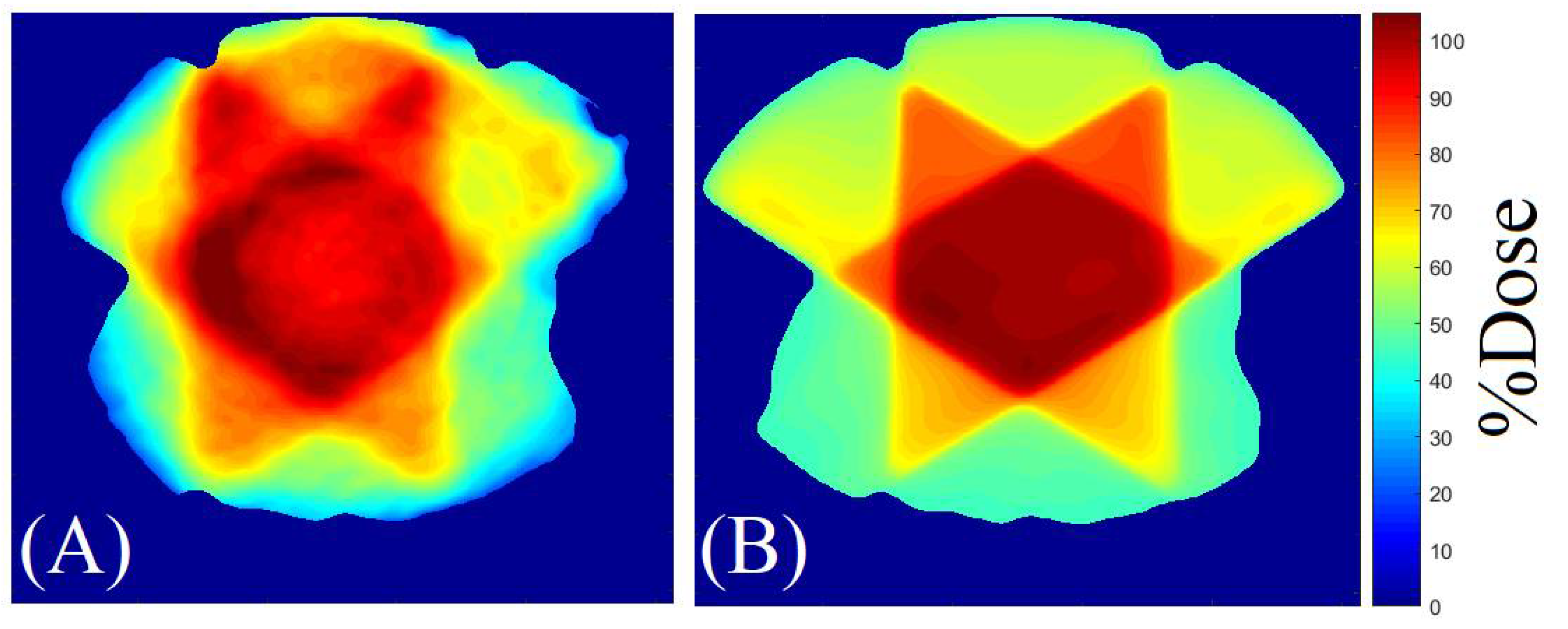
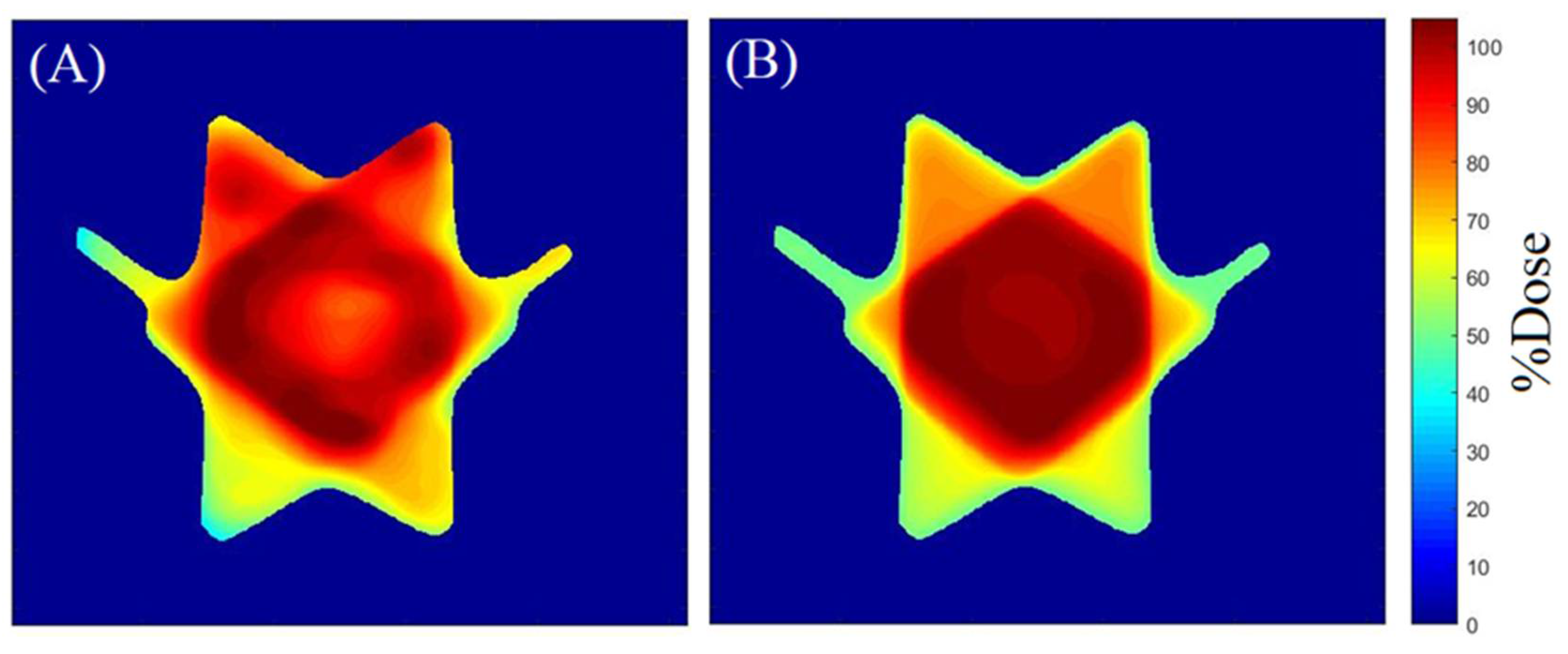
2.2. 3D QA for CSI Treatment
3. Conclusions
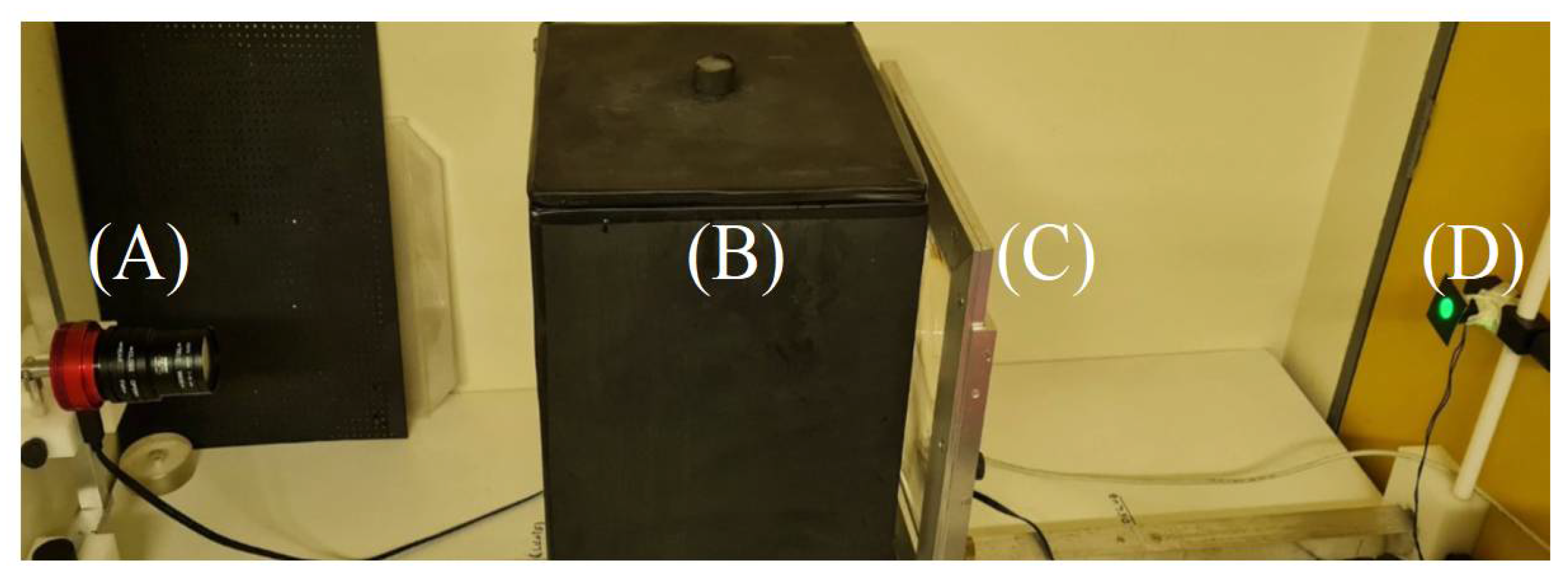
4. Materials and Methods
4.1. FXO-f Gel Dosimeter Preparation
4.2. CSI Treatment and Verification Plan Creation
4.3. Irradiations
4.4. Scanning and Image Processing
4.4.1. OCT Settings
4.4.2. Image Acquisition Protocol
4.4.3. Image Comparisons
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Massimino, M.; Biassoni, V.; Gandola, L.; Garrè, M.L.; Gatta, G.; Giangaspero, F.; Poggi, G.; Rutkowski, S. Childhood medulloblastoma, Crit. Rev. Oncol. Hematol. 2016, 105, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.; Brodeur, M.; Roberge, D.; Freeman, C. Standard and Nonstandard Craniospinal Radiotherapy Using Helical TomoTherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 926–931. [Google Scholar] [CrossRef]
- Fogliata, A.; Bergstrom, S.; Cafaro, I.; Clivio, A.; Cozzi, L.; Dipasquale, G.; Hållström, P.; Mancosu, P.; Navarria, P.; Nicolini, G.; et al. Cranio-spinal irradiation with volumetric modulated arc therapy: A multi-institutional treatment experience. Radiother. Oncol. 2011, 99, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Ramaseshan, R.; Corns, R.; Harrop, S.; Nuraney, N.; Steiner, P.; Karvat, A. A Three-Isocenter Jagged-Junction IMRT Approach for Craniospinal Irradiation Without Beam Edge Matching for Field Junctions. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, W.; Feng, Y.; Guo, Y.; Cong, Z.; Song, B. A simple approach of three-isocenter IMRT planning for craniospinal irradiation. Radiat. Oncol. 2013, 8, 217. [Google Scholar] [CrossRef]
- Biltekin, F.; Yazici, G.; Ozyigit, G. A novel inverse optimization based three-dimensional conformal radiotherapy technique in craniospinal irradiation. Phys. Eng. Sci. Med. 2021, 44, 265–275. [Google Scholar] [CrossRef]
- Foroudi, F.; Wilson, L.; Bressel, M.; Haworth, A.; Hornby, C.; Pham, D.; Cramb, J.; Gill, S.; Tai, K.H.; Kron, T. A dosimetric comparison of 3D conformal vs intensity modulated vs volumetric arc radiation therapy for muscle invasive bladder cancer. Radiat. Oncol. 2012, 7, 111. [Google Scholar] [CrossRef]
- Salimi, M.; Shirani, K.; Abi, T.; Nedaie, H.; Hassani, H.; Gharaati, H.; Samei, M.; Shahi, R.; Zarei, H. Assessment and comparison of homogeneity and conformity indexes in step-and-shoot and compensator-based intensity modulated radiation therapy (IMRT) and three-dimensional conformal radiation therapy (3D CRT) in prostate cancer. J. Med. Signals Sens. 2017, 7, 102–107. [Google Scholar] [PubMed]
- Baldock, C.; DeDeene, N.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; Mcauley, K.B.; Oldham, M.; Schreiner, L.J. Polymer gel dosimetry. Phys. Med. Biol. 2010, 55, R1. [Google Scholar] [CrossRef]
- Pavoni, J.F.; Pike, T.L.; Snow, J.; DeWerd, L.; Baffa, O. Tomotherapy dose distribution verification using MAGIC-f polymer gel dosimetry. Med. Phys. 2012, 39, 2877–2884. [Google Scholar] [CrossRef]
- Watanabe, Y.; Gopishankar, N. Three-dimensional dosimetry of Tomotherapy by MRI-based polymer gel technique. J. Appl. Clin. Med. Phys. 2010, 12, 3273–3280. [Google Scholar] [CrossRef]
- Marrale, M.; d’Errico, F. Hydrogels for Three-Dimensional Ionizing-Radiation Dosimetry. Gels 2021, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Asena, A.; Nilsson, S.; Smith, S.T.; Kairn, T.; Crowe, S.B.; Trapp, J.V. A method for obtaining three-dimensional measurements of HDR brachytherapy dose distributions using Fricke gel dosimeters and optical computed tomography. Australas. Phys. Eng. Sci. Med. 2019, 42, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Pasquale, S.; Lenardi, C.; Veronese, I.; Gueli, A.M. Effect of ionizing radiation on the colorimetric properties of PVA-GTA Xylenol Orange Fricke gel dosimeters. Dye. Pigment. 2021, 187, 109141. [Google Scholar] [CrossRef]
- Scotti, M.; Arosio, P.; Brambilla, E.; Gallo, S.; Lenardi, C.; Locarno, S.; Orsini, F.; Pignoli, E.; Pedicone, L.; Veronese, I. How Xylenol Orange and Ferrous Ammonium Sulphate Influence the Dosimetric Properties of PVA-GTA Fricke Gel Dosimeters: A Spectrophotometric Study. Gels 2022, 8, 204. [Google Scholar] [CrossRef]
- Kelly, R.G.; Jordan, K.J.; Battista, J.J. Optical CT reconstruction of 3D dose distributions using the ferrous-benzoic-xylenol (FBX) gel dosimeter. Med. Phys. 1998, 25, 1741–1750. [Google Scholar] [CrossRef]
- Silveira, M.A. Development and Characterization of an Optical Computed Tomography for Clinical Procedures. A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Department of Physics, Faculty of Philosophy, Sciences and Literature of Ribeirão Preto—SP, 2021; 118p. Available online: https://www.teses.usp.br/teses/disponiveis/59/59135/tde-07062022-092818/pt-br.php (accessed on 29 July 2021).
- Zhu, L.; Du, Y.; Xiang, X.; Zou, S.; Wang, X. Preliminary study of normoxic PAGAT polymer gel dosimeter by adding formaldehyde. J. Phys. Conf. Ser. 2019, 1305, 012047. [Google Scholar] [CrossRef]
- Jordan, K.; Turnbull, D.; Batista, J.J. Laser cone-beam computed tomography scanner geometry for large volume 3D dosimetry. J. Phys. Conf. Ser. 2013, 444, 012062. [Google Scholar] [CrossRef]
- Doran, S.J.; Krstajic, N. The history and principles of optical computed tomography for scanning 3-D radiation dosimeters. J. Phys. Conf. Ser. 2006, 56, 45–57. [Google Scholar] [CrossRef]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical cone-beam algorithm. J. Opt. Soc. Am. A 1984, 1, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Beister, M.; Kolditz, D.; Kalender, W.A. Iterative reconstruction methods in X-ray CT. Phys. Med. 2012, 28, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Matenine, D.; Goussard, Y.; Després, P. GPU-accelerated regularized iterative reconstruction for few-view cone-beam CT. Med. Phys. 2015, 42, 1505–1517. [Google Scholar] [CrossRef]
- Rudin, L.I.; Osher, S.; Fatemi, E. Nonlinear total variation based noise removal algorithms. Phys. D 1992, 60, 259–268. [Google Scholar] [CrossRef]
- Dekker, K.H.; Battista, J.J.; Jordan, K.J. Technical note: Evaluation of an iterative reconstruction algorithm for optical CT radiation dosimetry. Med. Phys. 2017, 44, 6678–6689. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, X.; Xiang, X.; Wei, Z. Evaluation of hybrid SART + OS + TV iterative reconstruction algorithm for optical-CT gel dosimeter imaging. Phys. Med. Biol. 2016, 61, 8425. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.M.; Pinter, C.; Fichtinger, G.; Olding, T.; Schreiner, L.J. Streamlined open-source gel dosimetry analysis in 3D slicer. Biomed. Phys. Eng. Express 2018, 4, 045041. [Google Scholar] [CrossRef] [PubMed]
- Babic, S.; Battista, J.; Jordan, K. Three-dimensional dose verification for intensity-modulated radiation therapy in the radiological physics centre head-and-neck phantom using optical computed tomography scans of ferrous xylenol-orange gel dosimeters. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1281–1291. [Google Scholar] [CrossRef]
- Baldok, C.; Lepage, M.; Bäck, S.A.; Murry, P.J.; Jayasekera, P.M.; Porter, D.; Kron, T. Dose resolution in radiotherapy polymer gel dosimetry: Effect of echo spacing in MRI pulse sequence. Phys. Med. Biol. 2001, 46, 449–460. [Google Scholar] [CrossRef]
- Zhou, Y.; Ai, Y.; Han, C.; Zheng, X.; Yi, J.; Xie, C.; Jin, X. Impact of setup errors on multi-isocenter volumetric modulated arc therapy for craniospinal irradiation. J. Appl. Clin. Med. Phys. 2020, 21, 115–123. [Google Scholar] [CrossRef]
- Nguyen, M.; Dang, T.; Deegan, L.; Henry, K.; Jones, S.; Pullar, A.; Cheuk, R.; Gibbs, A.; Sim, L.; Hargrave, C. Modified volumetric modulated arc therapy technique with reduced planning and treatment time for craniospinal irradiation utilising two isocentres. J. Med. Radiat. Sci. 2022, 69, 357–366. [Google Scholar] [CrossRef]
- Piotrowski, M.; Maras, P.; Wach, R.; Kadlubowski, S.; Kozicki, M. Impact of Salt on Thermal Stability and Dose Response of the Fricke-XO-Pluronic F-127 3D Radiotherapy Dosimeter. Materials 2022, 15, 5223. [Google Scholar] [CrossRef]
- Deasy, J.O.; Blanco, A.I.; Clark, V.H. CERR: A computational environment for radiotherapy research. Med. Phys. 2003, 30, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wuu, C.S.; Maryanski, M.J. Sensitivity calibration procedures in optical-CT scanning of BANG 3 polymer gel dosimeters. Med. Phys. 2010, 37, 861–868. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Absorbed dose determination in external beam radiotherapy. In Technical Report Series No. 398; IAEA: Vienna, Austria, 2000. [Google Scholar]
- Dyk, J.V.; Barnett, R.B.; Cygler, J.E.; Shragge, P.C. Commissioning and quality assurance of treatment planning computers. Int. J. Radiat. Oncol. Biol. Phys. 1993, 26, 261–273. [Google Scholar] [CrossRef]
- Low, D.; Dempsey, J. Evaluation of the gamma dose distribution comparison method. Med. Phys. 2003, 30, 2455–2464. [Google Scholar] [CrossRef] [PubMed] [Green Version]

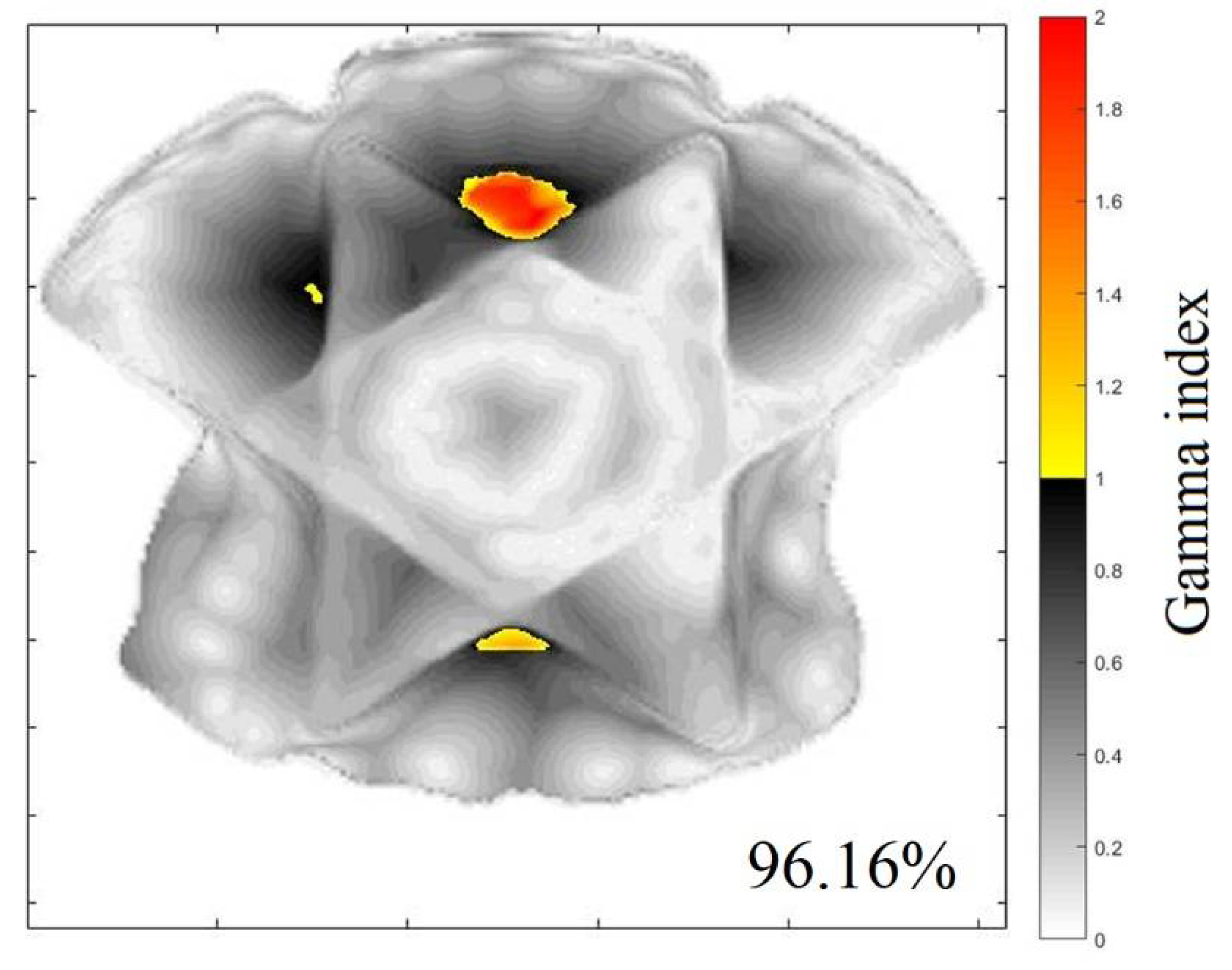
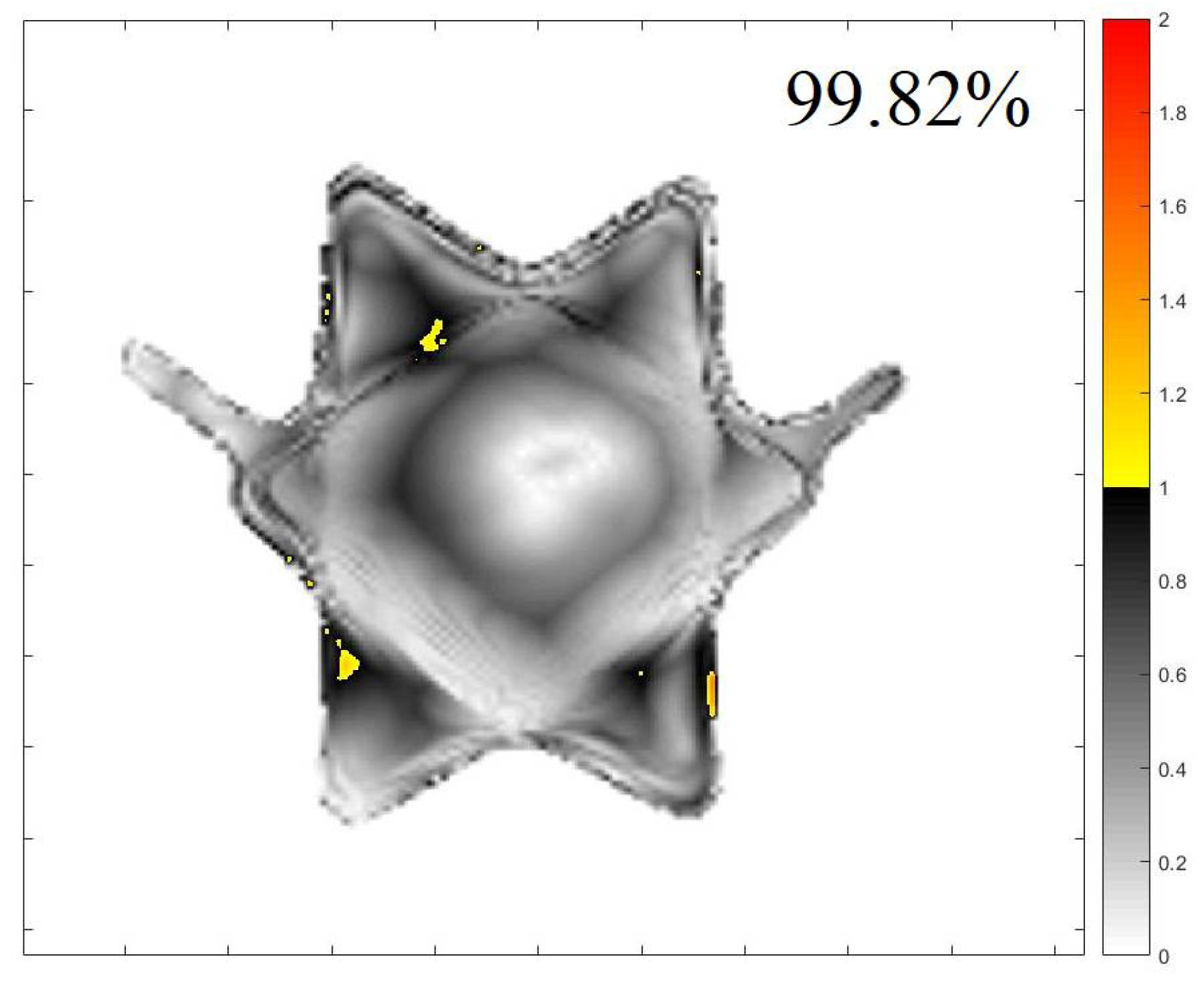



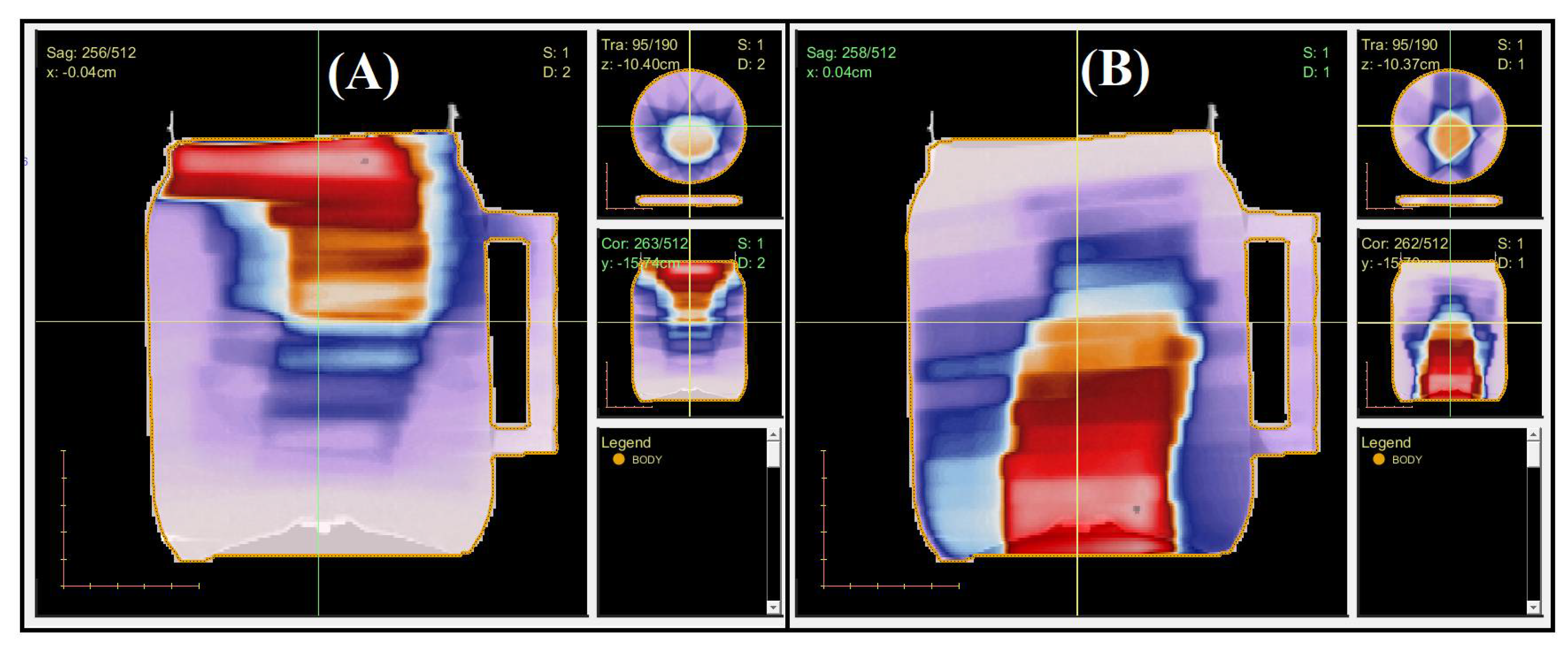
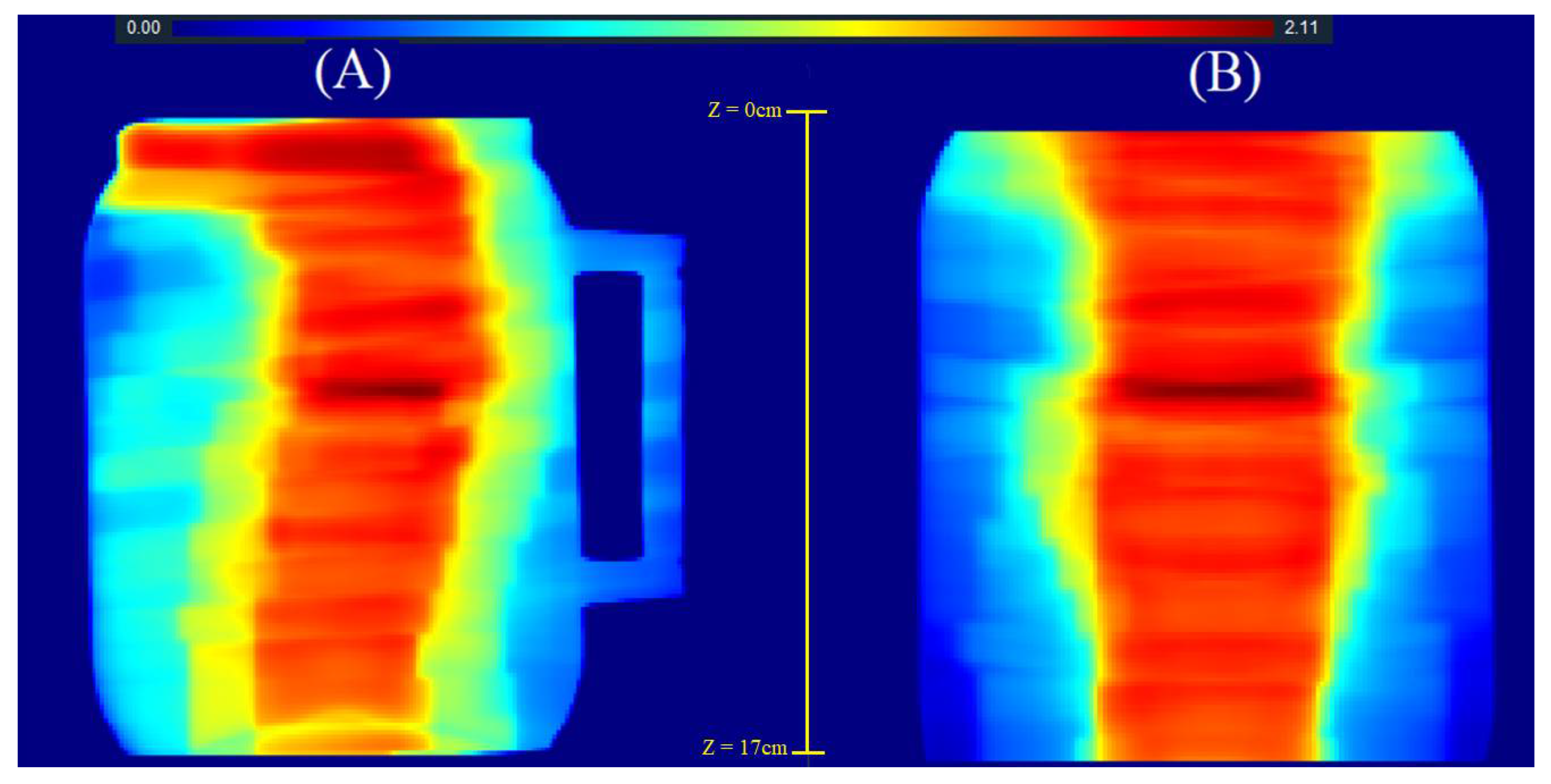
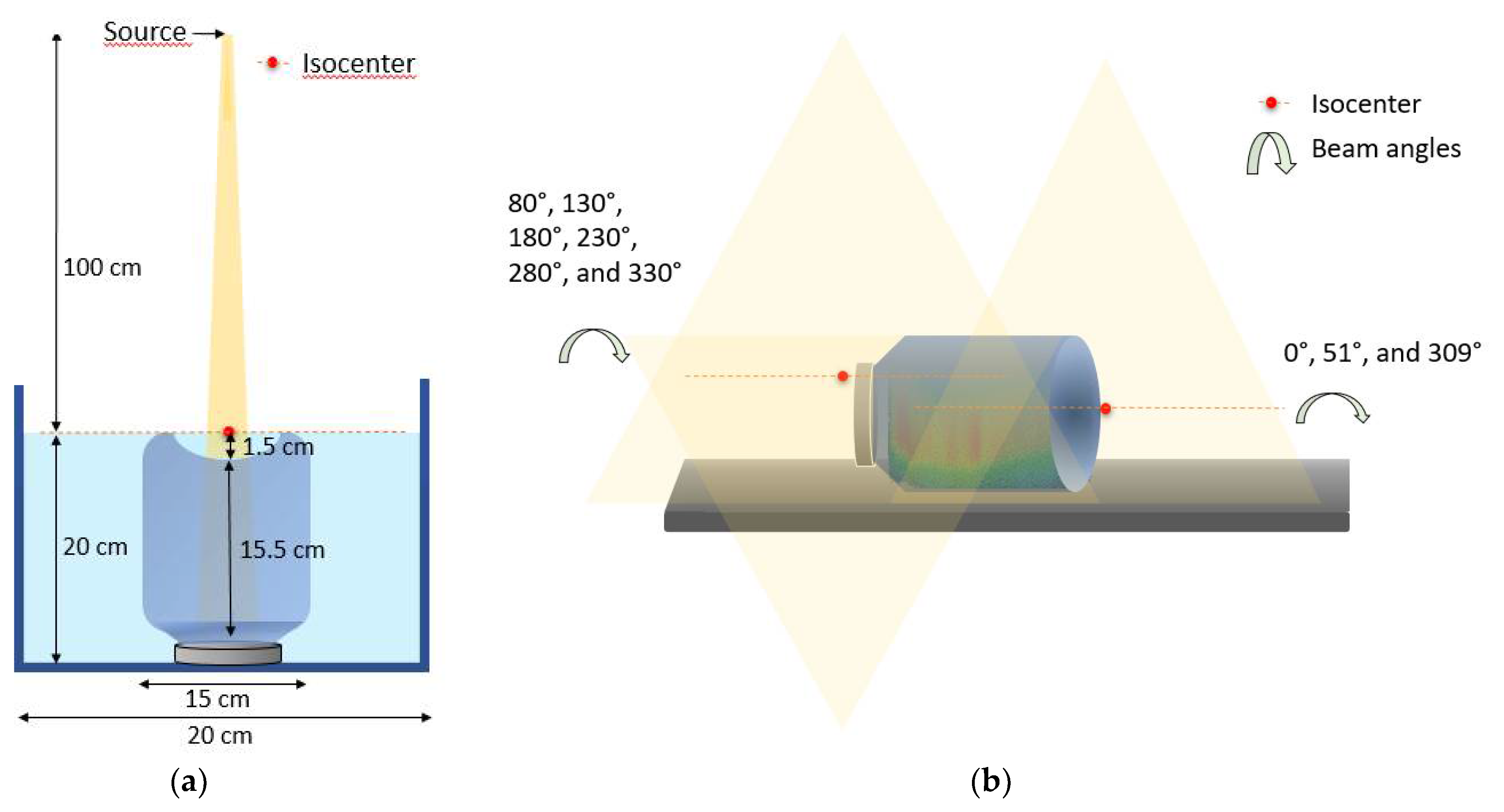
| Z (cm) | Gamma 20% (%) | Gamma 40 (%) | Z (cm) | Gamma 20% (%) | Gamma 40% (%) |
|---|---|---|---|---|---|
| 4.0 | 92.76 | 99.86 | 6.1 | 96.02 | 99.76 |
| 4.1 | 92.32 | 99.88 | 6.2 | 96.15 | 99.86 |
| 4.2 | 92.46 | 99.96 | 6.3 | 96.16 | 99.84 |
| 4.3 | 93.21 | 99.96 | 6.4 | 95.72 | 99.76 |
| 4.4 | 92.51 | 99.93 | 6.5 | 95.12 | 99.77 |
| 4.5 | 92.32 | 99.95 | 6.6 | 94.88 | 99.71 |
| 4.6 | 92.40 | 99.87 | 6.7 | 94.84 | 93.95 |
| 4.7 | 92.86 | 99.76 | 6.8 | 94.79 | 94.15 |
| 4.8 | 93.33 | 99.97 | 6.9 | 94.28 | 94.24 |
| 4.9 | 93.30 | 99.91 | 7.0 | 95.26 | 94.00 |
| 5.0 | 93.28 | 99.90 | 7.1 | 95.40 | 93.67 |
| 5.1 | 93.29 | 99.88 | 7.2 | 94.87 | 93.38 |
| 5.2 | 95.01 | 99.85 | 7.3 | 95.77 | 93.07 |
| 5.3 | 95.42 | 99.84 | 7.4 | 96.06 | 92.84 |
| 5.4 | 95.57 | 99.82 | 7.5 | 95.88 | 92.60 |
| 5.5 | 95.80 | 99.85 | 7.6 | 94.82 | 92.45 |
| 5.6 | 95.92 | 99.91 | 7.7 | 93.49 | 92.24 |
| 5.7 | 95.52 | 99.76 | 7.8 | 86.24 | 92.01 |
| 5.8 | 94.87 | 99.95 | 7.9 | 87.35 | 91.49 |
| 5.9 | 94.52 | 99.97 | 8.0 | 88.76 | 91.03 |
| 6.0 | 94.35 | 99.80 | 8.1 | 94.33 | 96.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silveira, M.A.; Pavoni, J.F.; Bruno, A.C.; Arruda, G.V.; Baffa, O. Three-Dimensional Dosimetry by Optical-CT and Radiochromic Gel Dosimeter of a Multiple Isocenter Craniospinal Radiation Therapy Procedure. Gels 2022, 8, 582. https://doi.org/10.3390/gels8090582
da Silveira MA, Pavoni JF, Bruno AC, Arruda GV, Baffa O. Three-Dimensional Dosimetry by Optical-CT and Radiochromic Gel Dosimeter of a Multiple Isocenter Craniospinal Radiation Therapy Procedure. Gels. 2022; 8(9):582. https://doi.org/10.3390/gels8090582
Chicago/Turabian Styleda Silveira, Matheus Antonio, Juliana Fernandes Pavoni, Alexandre Colello Bruno, Gustavo Viani Arruda, and Oswaldo Baffa. 2022. "Three-Dimensional Dosimetry by Optical-CT and Radiochromic Gel Dosimeter of a Multiple Isocenter Craniospinal Radiation Therapy Procedure" Gels 8, no. 9: 582. https://doi.org/10.3390/gels8090582
APA Styleda Silveira, M. A., Pavoni, J. F., Bruno, A. C., Arruda, G. V., & Baffa, O. (2022). Three-Dimensional Dosimetry by Optical-CT and Radiochromic Gel Dosimeter of a Multiple Isocenter Craniospinal Radiation Therapy Procedure. Gels, 8(9), 582. https://doi.org/10.3390/gels8090582






