Physicochemical, Morphological, Thermal, and Rheological Properties of Native Starches Isolated from Four Cultivars of Anchote (Coccinia abyssinica (Lam.) Cogn.) Tuber
Abstract
1. Introduction
2. Results and Discussion
2.1. Starch Particle Size and Morphology
2.2. Proximate Composition
2.3. Mineral Content
2.4. Amylose and Total Starch Contents
2.5. Pasting Properties
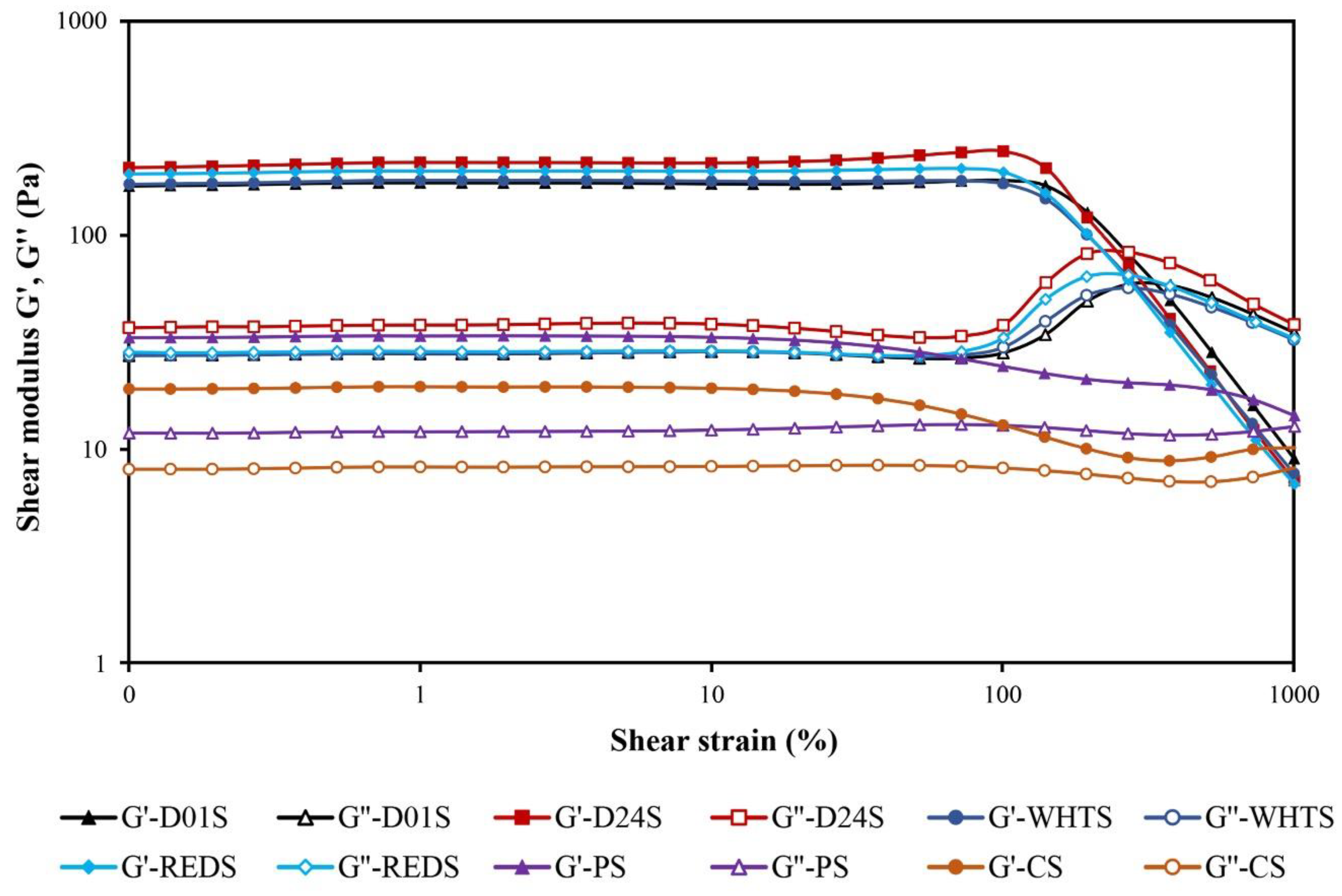
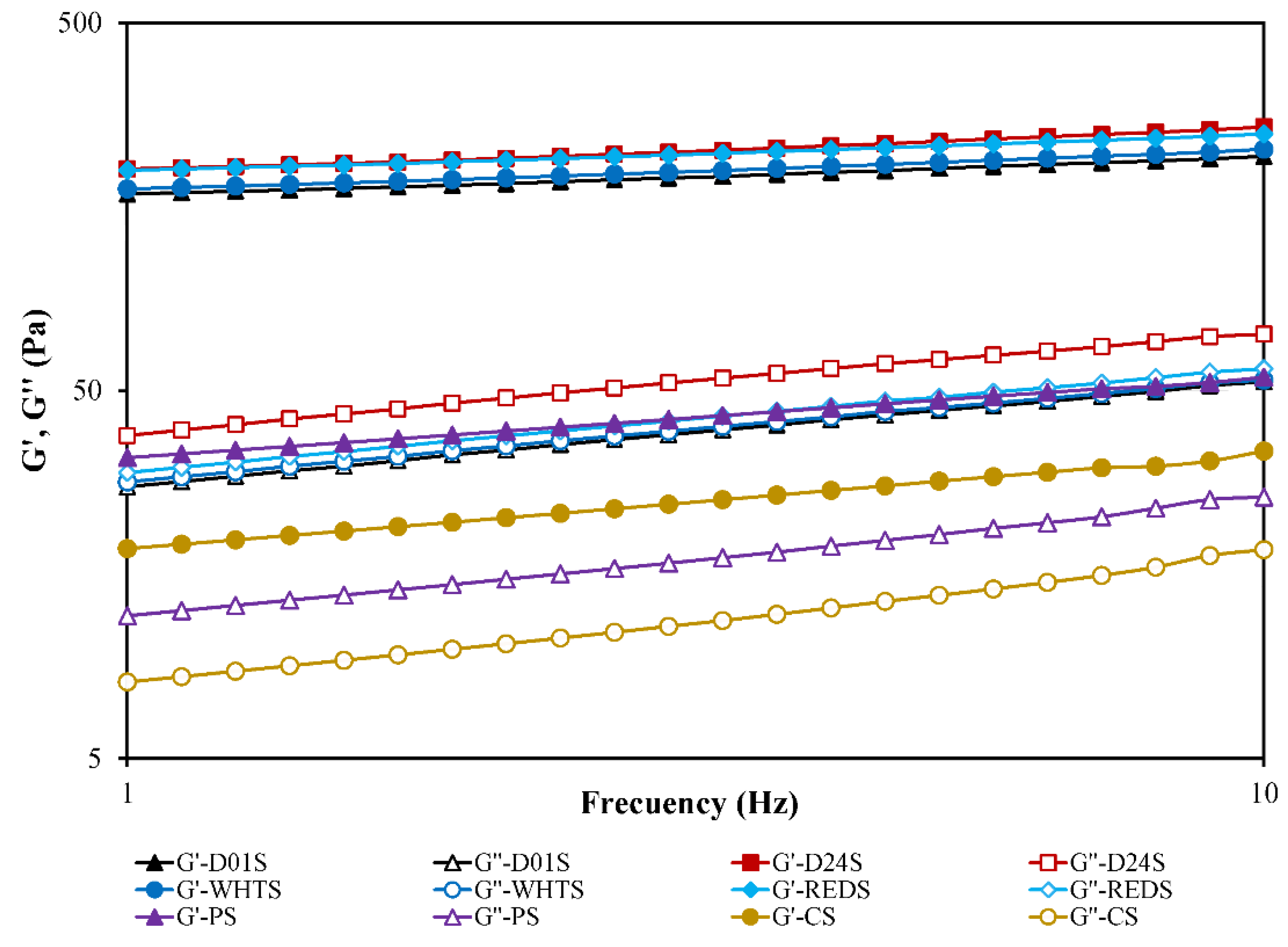
2.6. Rheological Properties
2.7. Thermal Properties
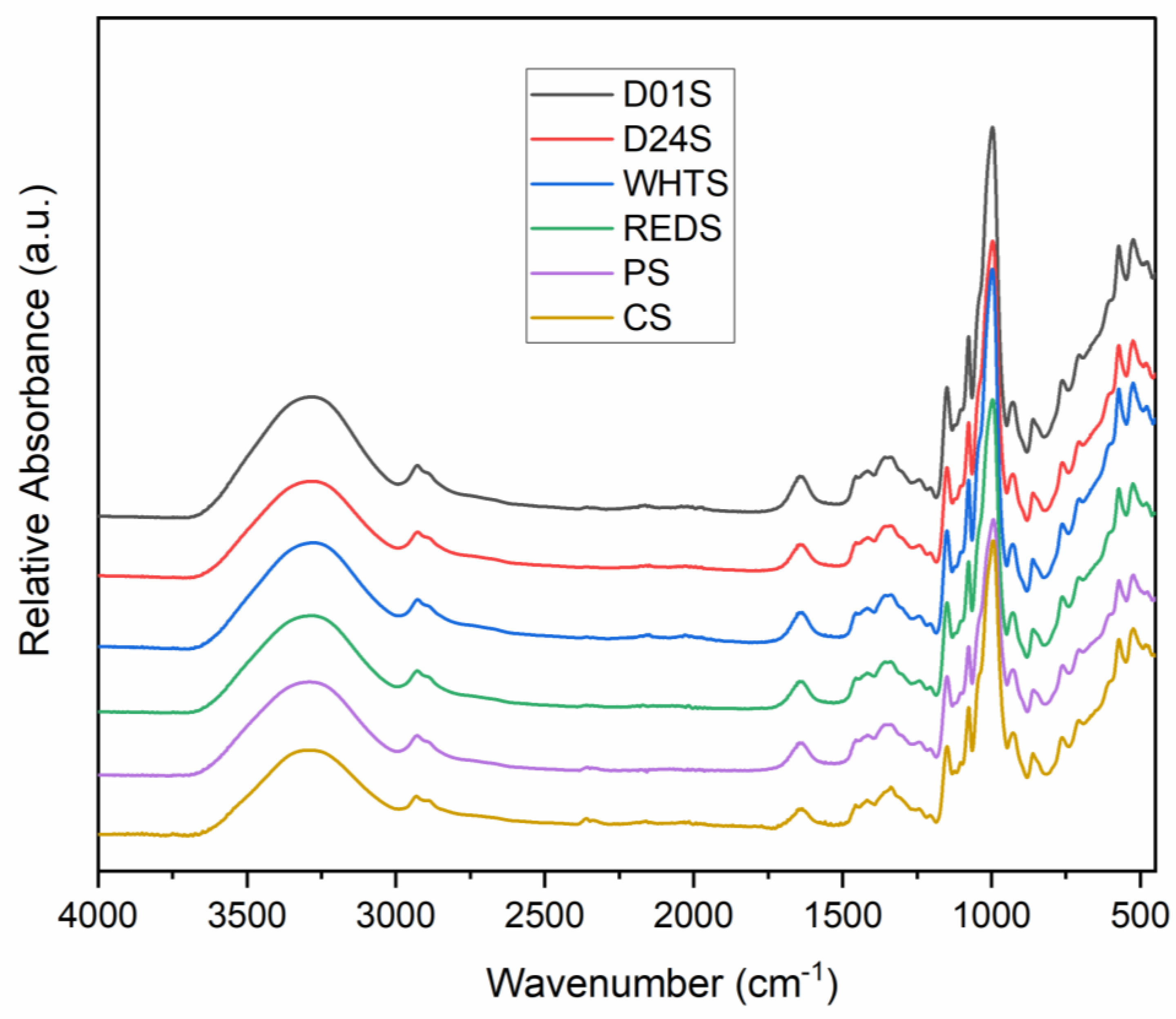
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Isolation of Starch
4.3. Starch Particle Size, Crystallinity, and Morphology
4.4. Proximate and Mineral Composition
4.5. Amylose and Total Starch Determination
4.6. Pasting Properties
4.7. Rheological Properties
4.8. Thermal Properties
4.9. Fourier Transform Infrared Spectroscopy (FTIR)
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dereje, B. Composition, Morphology and Physicochemical Properties of Starches Derived from Indigenous Ethiopian Tuber Crops: A Review. Int. J. Biol. Macromol. 2021, 187, 911–921. [Google Scholar] [CrossRef]
- Abegunde, O.K.; Mu, T.H.; Chen, J.W.; Deng, F.M. Physicochemical Characterization of Sweet Potato Starches Popularly Used in Chinese Starch Industry. Food Hydrocoll. 2013, 33, 169–177. [Google Scholar] [CrossRef]
- Ren, Y.; Wei, Q.; Lin, L.; Shi, L.; Cui, Z.; Li, Y.; Huang, C.; Wei, C. Physicochemical Properties of a New Starch from Ramie (Boehmeria nivea) Root. Int. J. Biol. Macromol. 2021, 174, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Bikila, A.M.; Tola, Y.; Esho, T.B.; Forsido, S.F. Effect of Predrying Treatment and Drying Temperature on Proximate Composition, Mineral Contents, and Thermophysical Properties of Anchote (Coccinia abyssinica (Lam.) Cogn.) Flour. Food Sci. Nutr. 2020, 8, 5532–5544. [Google Scholar] [CrossRef] [PubMed]
- Abera, G.; Woldeyes, B.; Dessalegn, H.; Miyake, G.M. International Journal of Biological Macromolecules Comparison of Physicochemical Properties of Indigenous Ethiopian Tuber Crop (Coccinia abyssinica) Starch with Commercially Available Potato and Wheat Starches. Int. J. Biol. Macromol. 2019, 140, 43–48. [Google Scholar] [CrossRef]
- Ayalew, Y.; Retta, N.; Desse, G.; Mohammed, A.; Mellesse, A. Amino Acid Profile and Protein Quality in Tuber and Leaf of Coccnia abyssinica (Lam.) (Cogn.) Accessions of Ethiopia. Food Sci. Nutr. 2017, 5, 722–729. [Google Scholar] [CrossRef]
- Tessema, A.; Admassu, H. Extraction and Characterization of Starch from Anchote (Coccinia abyssinica): Physico—Chemical, Functional, Morphological and Crystalline Properties. J. Food Meas. Charact. 2021. [Google Scholar] [CrossRef]
- Parmar, A.; Gebre, B.A.; Legesse, A.; Demelash, Y.; Fladung, K.; Hensel, O. Nutritional Comparison of White and Red Coccinia abyssinica (Lam.) Cong. Accessions: An under-Utilised Edible Tuber of the Ethiopian Highlands. Foods 2017, 6, 71. [Google Scholar] [CrossRef]
- Fekadu, H. Effect of Traditional Processing Methods on Nutritional Composition and Anti-Nutritional Factors of Anchote (Coccinia abyssinica (Lam.) Cogn) Tubers Grown in Western Ethiopia. J. Food Process. Technol. 2013, 4, 7. [Google Scholar] [CrossRef]
- Gao, H.; Cai, J.; Han, W.; Huai, H.; Chen, Y.; Wei, C. Comparison of Starches Isolated from Three Different Trapa Species. Food Hydrocoll. 2014, 37, 174–181. [Google Scholar] [CrossRef]
- Zabot, G.L.; Keven, E.; Emerick, L.B.; Herminia, M.; Felisberto, F.; Teresa, M.; Clerici, P.S.; Meireles, M.A.A. Food Hydrocolloids Physicochemical, Morphological, Thermal and Pasting Properties of a Novel Native Starch Obtained from Annatto Seeds. Food Hydrocoll. 2019, 89, 321–329. [Google Scholar] [CrossRef]
- Li, K.; Li, Q.; Jin, C.; Narayanamoorthy, S.; Zhang, T.; Sui, Z. Characterization of Morphology and Physicochemical Properties of Native Starches Isolated from 12 Lycoris Species. Food Chem. 2020, 316, 126263. [Google Scholar] [CrossRef] [PubMed]
- Chisenga, S.M.; Workneh, T.S.; Bultosa, G.; Laing, M. Characterization of Physicochemical Properties of Starches from Improved Cassava Varieties Grown in Zambia. AIMS Agric. Food 2019, 4, 939–966. [Google Scholar] [CrossRef]
- Guo, K.; Liu, T.; Xu, A.; Zhang, L.; Bian, X.; Wei, C. Food Hydrocolloids Structural and Functional Properties of Starches from Root Tubers of White, Yellow, and Purple Sweet Potatoes. Food Hydrocoll. 2019, 89, 829–836. [Google Scholar] [CrossRef]
- Lindeboom, N.; Chang, P.R.; Tyler, R.T. Analytical, Biochemical and Physicochemical Aspects of Starch Granule Size, with Emphasis on Small Granule Starches: A Review. Starch/Staerke 2004, 56, 89–99. [Google Scholar] [CrossRef]
- Chisenga, S.M.; Workneh, T.S.; Bultosa, G.; Alimi, B.A. Progress in Research and Applications of Cassava Flour and Starch: A Review. J. Food Sci. Technol. 2019, 56, 2799–2813. [Google Scholar] [CrossRef]
- Yu, B.; Li, J.; Tao, H.; Zhao, H.; Liu, P.; Cui, B. Physicochemical Properties and in Vitro Digestibility of Hydrothermal Treated Chinese Yam (Dioscorea opposita Thunb.) Starch and Flour. Int. J. Biol. Macromol. 2021, 176, 177–185. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Hu, W.; Qian, J.Y.; Ding, X.L.; Guan, C.R.; Lu, Y.Q.; Cao, Y. Multi-Scale Structures of Cassava and Potato Starch Fractions Varying in Granule Size. Carbohydr. Polym. 2018, 200, 400–407. [Google Scholar] [CrossRef]
- Zhu, F. Composition, Structure, Physicochemical Properties, and Modifications of Cassava Starch. Carbohydr. Polym. 2015, 122, 456–480. [Google Scholar] [CrossRef]
- Wang, C.; Tang, C.H.; Fu, X.; Huang, Q.; Zhang, B. Granular Size of Potato Starch Affects Structural Properties, Octenylsuccinic Anhydride Modification and Flowability. Food Chem. 2016, 212, 453–459. [Google Scholar] [CrossRef]
- Morante, N.; Ceballos, H.; Sánchez, T.; Rolland-Sabaté, A.; Calle, F.; Hershey, C.; Gibert, O.; Dufour, D. Discovery of New Spontaneous Sources of Amylose-Free Cassava Starch and Analysis of Their Structure and Techno-Functional Properties. Food Hydrocoll. 2016, 56, 383–395. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, S.; Lin, L.; Zhao, L.; Liu, A.; Wei, C. Food Hydrocolloids Properties of New Starches from Tubers of Arisaema Elephas, Yunnanense and Erubescens. Food Hydrocoll. 2016, 61, 183–190. [Google Scholar] [CrossRef]
- Schafranski, K.; Ito, V.C.; Lacerda, L.G. Impacts and Potential Applications: A Review of the Modification of Starches by Heat-Moisture Treatment (HMT). Food Hydrocoll. 2021, 117, 106690. [Google Scholar] [CrossRef]
- Noda, T.; Sulari, N.; Tsuda, S.; Mori, M.; Hashimoto, N.; Yamauchi, H. Starch Phosphorus Content in Potato (Solanum tuberosum L.) Cultivars and Its e V Ect on Other Starch Properties. Carbohydr. Polym. 2007, 68, 793–796. [Google Scholar] [CrossRef]
- Noda, T.; Tsuda, S.; Mori, M. Food Chemistry The Effect of Harvest Dates on the Starch Properties of Various Potato Cultivars. Food Chem. 2004, 86, 119–125. [Google Scholar] [CrossRef]
- Hoover, R. Composition, Molecular Structure, and Physicochemical Properties of Tuber and Root Starches: A Review. Carbohydr. Polym. 2001, 45, 253–267. [Google Scholar] [CrossRef]
- Gibson, T.S.; Solah, V.A.; McCleary, B.V. A Procedure to Measure Amylose in Cereal Starches and Flours with Concanavalin A. J. Cereal Sci. 1997, 25, 111–119. [Google Scholar] [CrossRef]
- Aina, A.J.; Falade, K.O.; Akingbala, J.O.; Titus, P. Physicochemical Properties of Caribbean Sweet Potato (Ipomoea batatas (L) Lam) Starches. Food Bioprocess Technol. 2012, 5, 576–583. [Google Scholar] [CrossRef]
- Simi, C.K.; Abraham, T.E. Physicochemical Rheological and Thermal Properties of Njavara Rice (Oryza sativa) Starch. J. Agric. Food Chem. 2008, 56, 12105–12113. [Google Scholar] [CrossRef]
- Shimelis, E.A.; Meaza, M.; Rakshit, S.K. Physico-Chemical Properties, Pasting Behavior and Functional Characteristics of Flours and Starches from Improved Bean (Phaseolus vulgaris L.) Varieties Grown in East Africa. E-J.—Int. Komm. Agrartech. 2006, VIII, 1–19. [Google Scholar]
- Ahmed, S.; Zhou, X.; Pang, Y.; Xu, Y.; Tong, C.; Bao, J. Genetic Diversity of Potato Genotypes Estimated by Starch Physicochemical Properties and Microsatellite Markers Institute of Nuclear Agricultural Science, College of Agriculture and Biotechnology, Zhejiang. Food Chem. 2018, 257, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Ronda, F.; Pérez-Quirce, S.; Villanueva, M. Rheological Properties of Gluten-Free Bread Doughs: Relationship With Bread Quality. In Advances in Food Rheology and Its Applications; Woodhead Publishing: Sawston, UK, 2017; pp. 297–334. ISBN 9780081004326. [Google Scholar]
- Villanueva, M.; Ronda, F.; Moschakis, T.; Lazaridou, A.; Biliaderis, C.G. Impact of Acidification and Protein Fortification on Thermal Properties of Rice, Potato and Tapioca Starches and Rheological Behaviour of Their Gels. Food Hydrocoll. 2018, 79, 20–29. [Google Scholar] [CrossRef]
- Gałkowska, D.; Juszczak, L. Effects of Amino Acids on Gelatinization, Pasting and Rheological Properties of Modified Potato Starches. Food Hydrocoll. 2019, 92, 143–154. [Google Scholar] [CrossRef]
- Tangsrianugul, N.; Wongsagonsup, R.; Suphantharika, M. Physicochemical and Rheological Properties of Flour and Starch from Thai Pigmented Rice Cultivars. Int. J. Biol. Macromol. 2019, 137, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, B.; Shi, J.; Xue, S.; Deng, Q.; Wei, Y.; Tian, B. Physicochemical Properties and Structure of Starches from Chinese Rice Cultivars. Food Hydrocoll. 2010, 24, 208–216. [Google Scholar] [CrossRef]
- Kumar, Y.; Singh, L.; Sharanagat, V.S.; Patel, A.; Kumar, K. Effect of Microwave Treatment (Low Power and Varying Time) on Potato Starch: Microstructure, Thermo-Functional, Pasting and Rheological Properties. Int. J. Biol. Macromol. 2020, 155, 27–35. [Google Scholar] [CrossRef]
- Falade, K.O.; Okafor, C.A. Physicochemical Properties of Five Cocoyam (Colocasia esculenta and Xanthosoma sagittifolium) Starches. Food Hydrocoll. 2013, 30, 173–181. [Google Scholar] [CrossRef]
- Abebe, W.; Collar, C.; Ronda, F. Impact of Variety Type and Particle Size Distribution on Starch Enzymatic Hydrolysis and Functional Properties of Tef Flours. Carbohydr. Polym. 2015, 115, 260–268. [Google Scholar] [CrossRef]
- Vela, A.J.; Villanueva, M.; Solaesa, Á.G.; Ronda, F. Impact of High-Intensity Ultrasound Waves on Structural, Functional, Thermal and Rheological Properties of Rice Flour and Its Biopolymers Structural Features. Food Hydrocoll. 2021, 113, 106480. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Washington, DC, USA, 2010. [Google Scholar]
- Ronda, F.; Abebe, W.; Pérez-Quirce, S.; Collar, C. Suitability of Tef Varieties in Mixed Wheat Flour Bread Matrices: A Physico-Chemical and Nutritional Approach. J. Cereal Sci. 2015, 64, 139–146. [Google Scholar] [CrossRef]
- Ronda, F.; Villanueva, M.; Collar, C. Influence of Acidification on Dough Viscoelasticity of Gluten-Free Rice Starch-Based Dough Matrices Enriched with Exogenous Protein. LWT—Food Sci. Technol. 2014, 59, 12–20. [Google Scholar] [CrossRef]
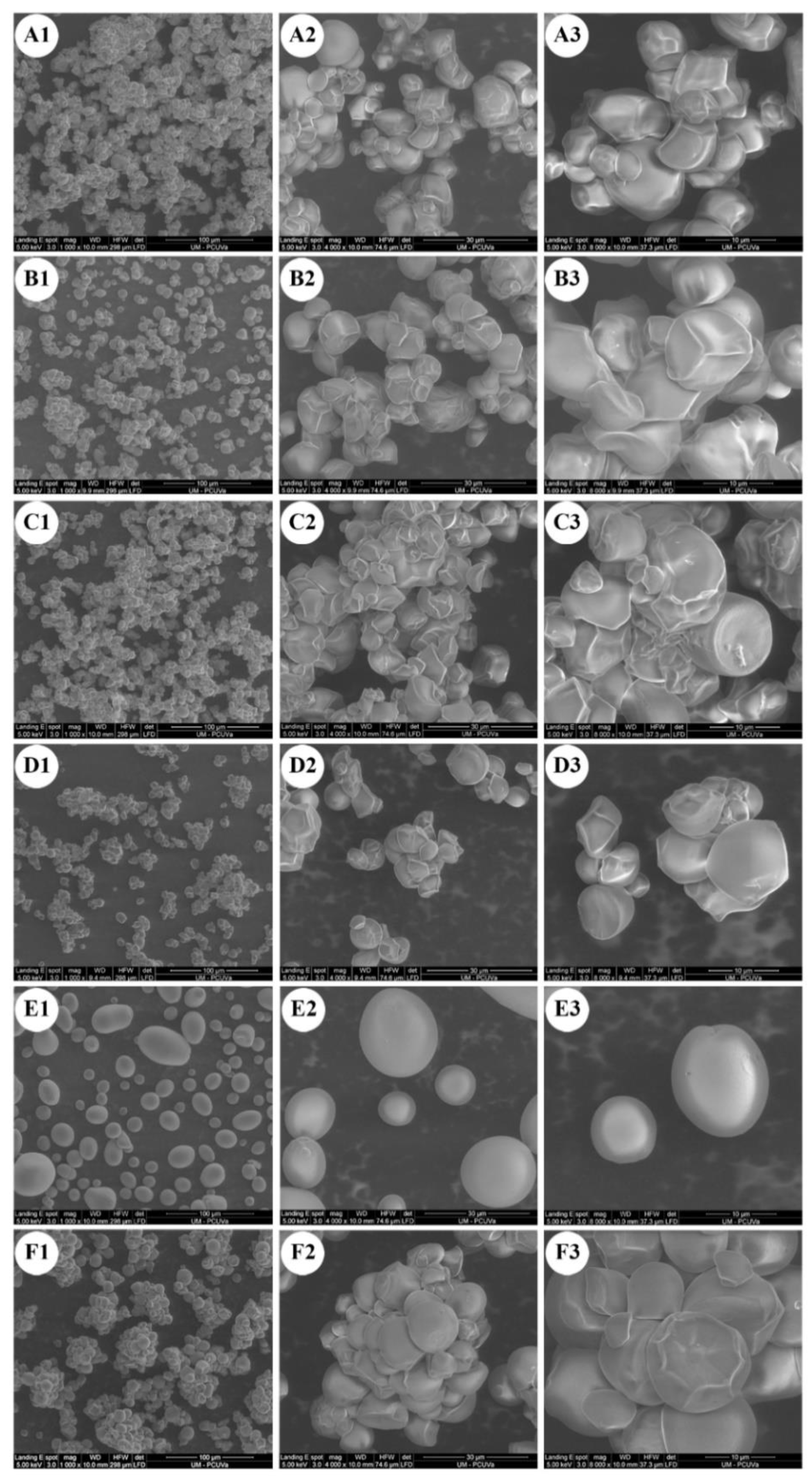
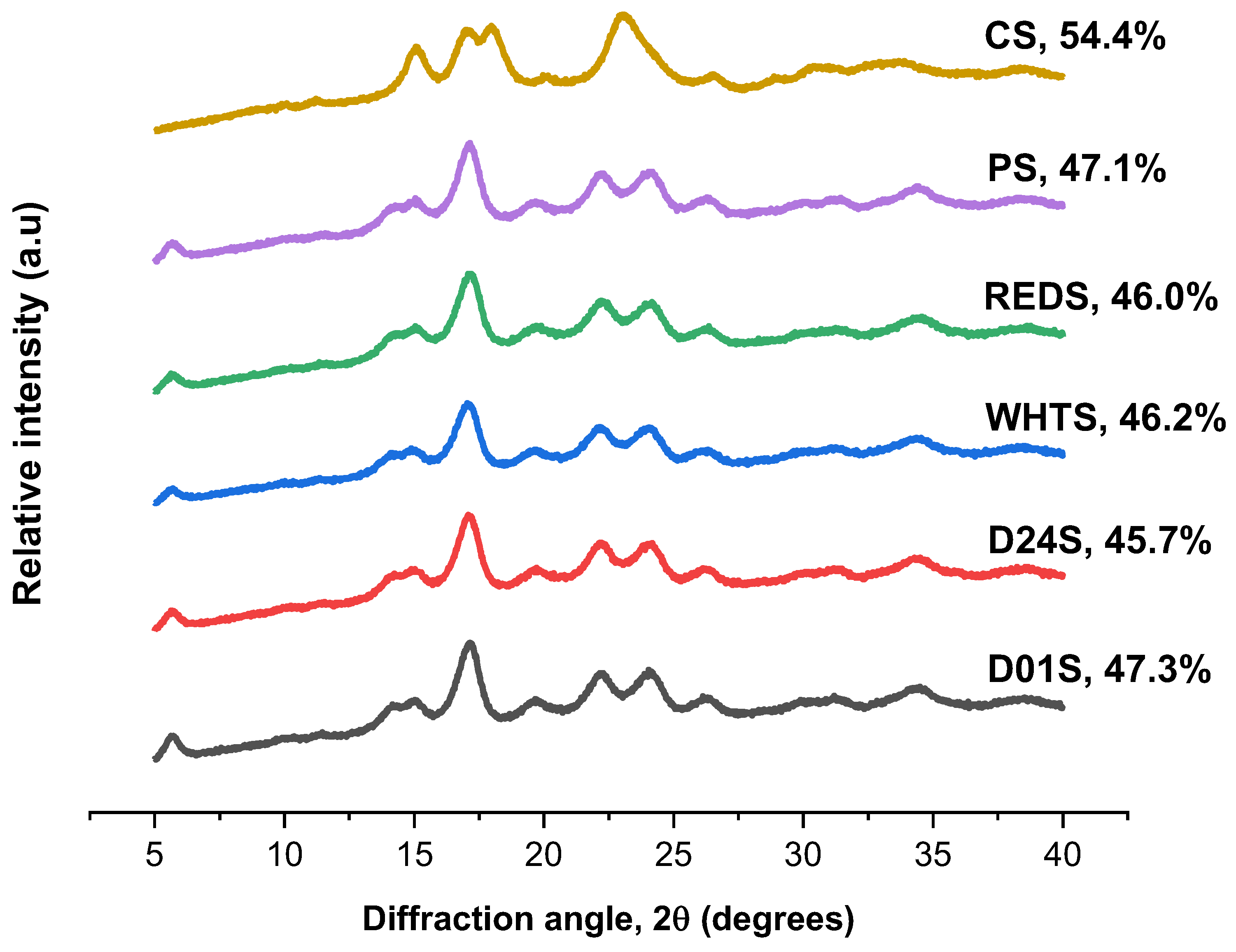

| Sample | D50 (μm) | D [3,2] (μm) | D [4,3] (μm) | (D90 − D10)/D50 |
|---|---|---|---|---|
| D01S | 11.6 ± 0.1 a | 12.0 ± 0.1 a | 63.9 ± 3.3 c | 20.6 ± 1.2 c |
| D24S | 14.4 ± 0.1 bc | 13.5 ± 0.1 bc | 66.4 ± 2.7 c | 15.5 ± 0.4 b |
| WHTS | 14.5 ± 0.7 c | 14.7 ± 0.6 c | 106.9 ± 7.8 e | 22.5 ± 0.3 d |
| REDS | 13.3 ± 0.4 b | 13.6 ± 0.5 bc | 81.7 ± 4.2 d | 20.8 ± 0.2 c |
| PS | 36.2 ± 0.6 d | 31.9 ± 0.7 d | 39.5 ± 0.6 b | 1.3 ± 0.1 a |
| CS | 13.5 ± 0.1 bc | 12.5 ± 0.1 ab | 14.3 ± 0.1 a | 1.0 ± 0.1 a |
| Sample | Moisture (%) | Ash (%) | Fat (%) | Protein (%) | Total Starch (%) | Amylose (%) | Phosphorus (mg/100 g) | Calcium (mg/100 g) |
|---|---|---|---|---|---|---|---|---|
| D01S | 20.24 ± 0.01 f | 0.38 ± 0.01 c | 0.09 ± 0.01 b | 0.65 ± 0.01 c | 80.8 ± 1.0 cd | 15.8 ± 0.9 a | 82. 8 ± 0.6 c | 45.8 ± 0.4 e |
| D24S | 17.79 ± 0.02 c | 0.39 ± 0.01 d | 0.14 ± 0.01 d | 0.64 ± 0.02 bc | 74.9 ± 0.2 b | 22.3 ± 0.6 c | 83.2 ± 0.1 c | 55.7 ± 0.5 f |
| WHTS | 19.41 ± 0.03 e | 0.47 ± 0.01 e | 0.10 ± 0.01 bc | 0.51 ± 0.03 b | 77.6 ± 1.8 bc | 17.2 ± 0.1 ab | 93.3 ± 2.4 d | 30.4 ± 0.5 c |
| REDS | 18.52 ± 0.01 d | 0.49 ± 0.01 f | 0.11 ± 0.01 c | 0.54 ± 0.07 bc | 66.8 ± 1.9 a | 22.3 ± 0.2 c | 92.2 ± 1.3 d | 36.8 ± 0.4 d |
| PS | 14.52 ± 0.01 b | 0.32 ± 0.01 b | 0.09 ± 0.01 b | 0.32 ± 0.01 a | 84.1 ± 1.6 d | 18.8 ± 0.5 b | 60.3 ± 0.3 b | 7.4 ± 0.1 a |
| CS | 12.78 ± 0.01 a | 0.18 ± 0.01 a | 0.02 ± 0.01 a | 0.36 ± 0.02 a | 96.2 ± 1.1 e | 19.0 ± 0.4 b | 5.8 ± 0.1 a | 26.3 ± 0.1 b |
| Sample | PV (mPa·s) | TV (mPa·s) | BV (mPa·s) | FV (mPa·s) | SV (mPa·s) | Ptime (min) | PT (°C) |
|---|---|---|---|---|---|---|---|
| D01S | 2726 ± 20 d | 2239 ± 22 c | 487 ± 28 bc | 3466 ± 15 d | 1228 ± 23 b | 4.89 ± 0.10 c | 70.33 ± 0.08 b |
| D24S | 2293 ± 45 b | 2046 ± 47 b | 248 ± 54 a | 3686 ± 14 e | 1640 ± 43 d | 5.29 ± 0.04 e | 72.65 ± 0.05 c |
| WHTS | 2649 ± 53 d | 2280 ± 38 c | 369 ± 27 ab | 3495 ± 21 d | 1215 ± 35 b | 5.33 ± 0.01 e | 69.38 ± 0.06 a |
| REDS | 2448 ± 28 c | 2058 ± 44 b | 389 ± 35 b | 3409 ± 20 c | 1351 ± 32 c | 5.15 ± 0.04 d | 69.5 ± 0.09 a |
| PS | 5728 ± 26 e | 1942 ± 69 b | 3786 ± 87 d | 2322 ± 21 b | 380 ± 49 a | 3.27 ± 0.01 a | 68.83 ± 0.45 a |
| CS | 1367 ± 38 a | 796 ± 14 a | 571 ± 24 c | 1222 ± 15 a | 426 ± 10 a | 4.47 ± 0.01 b | 75.65 ± 0.44 d |
| Sample | G1′ (Pa) | a | G1′′ (Pa) | b | (tan δ)1 | c | Cross Point (Pa) | τmax (Pa) |
|---|---|---|---|---|---|---|---|---|
| D01S | 169 ± 3 c | 0.11 ± 0.01 a | 28.1 ± 0.6 c | 0.29 ± 0.01 a | 0.17 ± 0.01 b | 0.18 ± 0.01 b | 285 ± 1 b | 181 ± 3 d |
| D24S | 202 ± 8 d | 0.12 ± 0.01 a | 39.1 ± 1.6 d | 0.28 ± 0.01 a | 0.19 ± 0.01 c | 0.16 ± 0.01 b | 281 ± 6 b | 171 ± 7 c |
| WHTS | 175 ± 2 c | 0.11 ± 0.01 a | 28.7 ± 0.2 c | 0.28 ± 0.01 a | 0.16 ± 0.01 b | 0.17 ± 0.01 b | 243 ± 7 a | 131 ± 2 a |
| REDS | 198 ± 4 d | 0.10 ± 0.01 a | 30.1 ± 0.9 c | 0.28 ± 0.01 a | 0.15 ± 0.01 a | 0.18 ± 0.01 b | 242 ± 1 a | 151 ± 2 b |
| PS | 34 ± 1 b | 0.22 ± 0.01 b | 12.3 ± 0.6 b | 0.32 ± 0.01 b | 0.36 ± 0.01 d | 0.11 ± 0.01 a | nd | nd |
| CS | 19 ± 1 a | 0.24 ± 0.02 c | 8.0 ± 0.1 a | 0.35 ± 0.01 c | 0.42 ± 0.01 e | 0.11 ± 0.02 a | nd | nd |
| Sample | TO (°C) | Tp (°C) | TC (°C) | ΔT (°C) | ΔH (J/g) |
|---|---|---|---|---|---|
| D01S | 61.13 ± 0.15 b | 64.11 ± 0.12 b | 67.28 ± 0.23 a | 6.16 ± 0.08 a | 17.16 ± 0.46 b |
| D24S | 61.74 ± 0.09 c | 65.07 ± 0.07 c | 69.33 ± 0.16 c | 7.59 ± 0.07 b | 18.01 ± 0.47 bc |
| WHTS | 60.97 ± 0.09 b | 63.86 ± 0.22 ab | 67.21 ± 0.23 a | 6.25 ± 0.13 a | 18.38 ± 0.47 bc |
| REDS | 61.34 ± 0.08 bc | 64.31 ± 0.15 b | 67.97 ± 0.07 b | 6.64 ± 0.01 a | 16.87 ± 0.99 ab |
| PS | 59.42 ± 0.04 a | 63.45 ± 0.11 a | 69.16 ± 0.09 c | 9.74 ± 0.05 c | 18.75 ± 0.78 c |
| CS | 64.7 ± 0.28 d | 70.76 ± 0.07 d | 81.17 ± 0.04 d | 16.47 ± 0.25 d | 15.44 ± 0.24 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolde, Y.T.; Emire, S.A.; Abebe, W.; Ronda, F. Physicochemical, Morphological, Thermal, and Rheological Properties of Native Starches Isolated from Four Cultivars of Anchote (Coccinia abyssinica (Lam.) Cogn.) Tuber. Gels 2022, 8, 591. https://doi.org/10.3390/gels8090591
Wolde YT, Emire SA, Abebe W, Ronda F. Physicochemical, Morphological, Thermal, and Rheological Properties of Native Starches Isolated from Four Cultivars of Anchote (Coccinia abyssinica (Lam.) Cogn.) Tuber. Gels. 2022; 8(9):591. https://doi.org/10.3390/gels8090591
Chicago/Turabian StyleWolde, Yohannes Tolesa, Shimelis Admassu Emire, Workineh Abebe, and Felicidad Ronda. 2022. "Physicochemical, Morphological, Thermal, and Rheological Properties of Native Starches Isolated from Four Cultivars of Anchote (Coccinia abyssinica (Lam.) Cogn.) Tuber" Gels 8, no. 9: 591. https://doi.org/10.3390/gels8090591
APA StyleWolde, Y. T., Emire, S. A., Abebe, W., & Ronda, F. (2022). Physicochemical, Morphological, Thermal, and Rheological Properties of Native Starches Isolated from Four Cultivars of Anchote (Coccinia abyssinica (Lam.) Cogn.) Tuber. Gels, 8(9), 591. https://doi.org/10.3390/gels8090591










