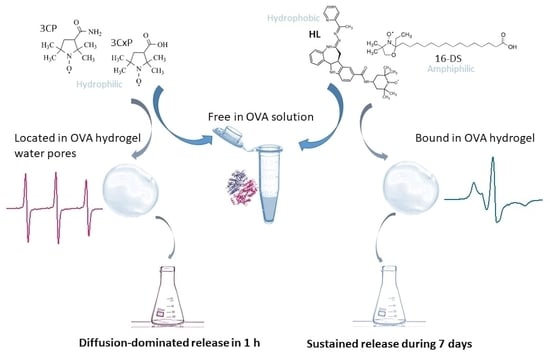
Spectroscopic Characterization of the Binding and Release of Hydrophilic, Hydrophobic and Amphiphilic Molecules from Ovalbumin Supramolecular Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Binding of Hydrophilic Spin Probes 3CP and 3CxP to OVA
2.2. The Release of Hydrophilic Spin Probes 3CP and 3CxP from OVA Hydrogels
2.3. The Binding of Amphiphilic and Hydrophobic Molecules to OVA
2.3.1. Spin-Labeled Fatty Acid 16-DS
2.3.2. Spin-Labeled Cytotoxic Ligand HL
2.4. The Release of Amphiphilic and Hydrophobic Molecules from OVA Hydrogels
2.4.1. Spin-Labeled Fatty Acid 16-DS
2.4.2. Spin-Labeled Cytotoxic Ligand HL
2.5. Raman Spectroscopy of OVA Solution and Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. EPR Spectroscopy
4.3. Sample Preparation for the EPR Binding and Release Studies
4.4. Raman Spectroscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stein, P.E.; Leslie, A.G.W.; Finch, J.T.; Carrell, R.W. Crystal Structure of Uncleaved Ovalbumin at 1·95 Å Resolution. J. Mol. Biol. 1991, 221, 941–959. [Google Scholar] [CrossRef] [PubMed]
- Huntington, J.A.; Stein, P.E. Structure and Properties of Ovalbumin. J. Chromatogr. B Biomed. Sci. Appl. 2001, 756, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Goux, W.J.; Venkatasubramanian, P.N. Metal Ion Binding Properties of Hen Ovalbumin and S-Ovalbumin: Characterization of the Metal Ion Binding Site by 31P NMR and Water Proton Relaxation Rate Enhancements. Biochemistry 1986, 25, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Xie, Y.; Wang, J.; Li, S.; Jin, Y.; Ma, M. Large-Scale Purification of Ovalbumin Using Polyethylene Glycol Precipitation and Isoelectric Precipitation. Poult. Sci. 2019, 98, 1545–1550. [Google Scholar] [CrossRef]
- Stein, P.E.; Leslie, A.G.W.; Finch, J.T.; Turnell, W.G.; McLaughlin, P.J.; Carrell, R.W. Crystal Structure of Ovalbumin as a Model for the Reactive Centre of Serpins. Nature 1990, 347, 99–102. [Google Scholar] [CrossRef]
- Hu, H.Y.; Du, H.N. α-to-β Structural Transformation of Ovalbumin: Heat and pH Effects. J. Protein Chem. 2000, 19, 177–183. [Google Scholar] [CrossRef]
- Nyemb, K.; Guérin-Dubiard, C.; Dupont, D.; Jardin, J.; Rutherfurd, S.M.; Nau, F. The Extent of Ovalbumin In Vitro Digestion and the Nature of Generated Peptides Are Modulated by the Morphology of Protein Aggregates. Food Chem. 2014, 157, 429–438. [Google Scholar] [CrossRef]
- El-Salam, M.H.A.; El-Shibiny, S. Natural Biopolymers as Nanocarriers for Bioactive Ingredients Used in Food Industries. In Nanotechnology in the Agri-Food Industry, Encapsulations; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 793–829. ISBN 9780128043073. [Google Scholar]
- Diwan, D.; Sharma, M.; Tabatabaei, M.; Gupta, V.K. Ovalbumin Production Without Poultry. Nat. Food 2021, 2, 924–925. [Google Scholar] [CrossRef]
- Abioye, R.O.; Acquah, C.; Hsu, P.C.Q.; Hüttmann, N.; Sun, X.; Udenigwe, C.C. Self-Assembly and Hydrogelation Properties of Peptides Derived from Peptic Cleavage of Aggregation-Prone Regions of Ovalbumin. Gels 2022, 8, 641. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Ying, D.; Fu, Y.; Xiong, Y.; Le, X. Ovalbumin as a Carrier to Significantly Enhance the Aqueous Solubility and Photostability of Curcumin: Interaction and Binding Mechanism Study. Int. J. Biol. Macromol. 2018, 116, 893–900. [Google Scholar] [CrossRef]
- Zeng, Q.; Zeng, W.; Jin, Y.; Sheng, L. Construction and Evaluation of Ovalbumin-Pullulan Nanogels as a Potential Delivery Carrier for Curcumin. Food Chem. 2022, 367, 130716. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Li, Y.; Kapp, J.A. Ovalbumin Injected with Complete Freund’s Adjuvant Stimulates Cytolytic Responses. Eur. J. Immunol. 1995, 25, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Kamalov, M.; Kählig, H.; Rentenberger, C.; Müllner, A.R.M.; Peterlik, H.; Becker, C.F.W. Ovalbumin Epitope SIINFEKL Self-Assembles into a Supramolecular Hydrogel. Sci. Rep. 2019, 9, 2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gou, S.; Chen, Q.; Liu, Y.; Zeng, L.; Song, H.; Xu, Z.; Kang, Y.; Li, C.; Xiao, B. Green Fabrication of Ovalbumin Nanoparticles as Natural Polyphenol Carriers for Ulcerative Colitis Therapy. ACS Sustain. Chem. Eng. 2018, 6, 12658–12667. [Google Scholar] [CrossRef]
- Hu, G.; Ma, M.; Batool, Z.; Sheng, L.; Cai, Z.; Liu, Y.; Jin, Y. Gel Properties of Heat-Induced Transparent Hydrogels from Ovalbumin by Acylation Modifications. Food Chem. 2022, 369, 130912. [Google Scholar] [CrossRef]
- Sharif, M.K.; Saleem, M.; Javed, K. Food Materials Science in Egg Powder Industry. In Role of Materials Science in Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 505–537. ISBN 9780128115008. [Google Scholar]
- Manivel, P.; Paulpandi, M.; Chen, X. Ovalbumin-Coated Fe3O4 Nanoparticles as a Nanocarrier for Chlorogenic Acid to Promote the Anticancer Efficacy on MDA-MB-231 Cells. New J. Chem. 2022, 46, 12609–12622. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a Historical Perspective: From Simple Networks to Smart Materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of Natural Hydrogels for Regenerative Medicine Applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [Green Version]
- Denzer, B.R.; Kulchar, R.J.; Huang, R.B.; Patterson, J. Advanced Methods for the Characterization of Supramolecular Hydrogels. Gels 2021, 7, 158. [Google Scholar] [CrossRef]
- Omar, J.; Ponsford, D.; Dreiss, C.A.; Lee, T.; Loh, X.J. Supramolecular Hydrogels: Design Strategies and Contemporary Biomedical Applications. Chem. Asian J. 2022, 17, e202200081. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, B. Short Peptide-Based Smart Thixotropic Hydrogels. Gels 2022, 8, 569. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ong, P.J.; Liu, S.; Thitsartarn, W.; Tan, M.J.B.H.; Suwardi, A.; Zhu, Q.; Loh, X.J. Recent Advances in Host-Guest Supramolecular Hydrogels for Biomedical Applications. Chem. Asian J. 2022, 17, e202200608. [Google Scholar] [CrossRef]
- Pramanik, B.; Ahmed, S. Peptide-Based Low Molecular Weight Photosensitive Supramolecular Gelators. Gels 2022, 8, 533. [Google Scholar] [CrossRef]
- Panahi, R.; Baghban-Salehi, M. Protein-Based Hydrogels. In Cellulose-Based Superabsorbent Hydrogels; Polymers and Polymeric Composites: A Reference Series; Mondal, M., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a Drug Carrier: Design of Prodrugs, Drug Conjugates and Nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Merlot, A.M.; Kalinowski, D.S.; Kovacevic, Z.; Jansson, P.J.; Lane, D.J.; Huang, M.L.H.; Sahni, S.; Richardson, D.R. Making a Case for Albumin—A Highly Promising Drug-Delivery System. Future Med. Chem. 2015, 7, 553–556. [Google Scholar] [CrossRef]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing Albumin as a Carrier for Cancer Therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Kandi, R.; Rao, C.P. Injectable, Self-Healing, and Stress Sustainable Hydrogel of BSA as a Functional Biocompatible Material for Controlled Drug Delivery in Cancer Cells. ACS Sustain. Chem. Eng. 2018, 6, 3321–3330. [Google Scholar] [CrossRef]
- Dantas, M.D.D.A.; de Araújo Tenório, H.; Lopes, T.I.B.; Pereira, H.J.V.; Marsaioli, A.J.; Figueiredo, I.M.; Santos, J.C.C. Interactions of Tetracyclines with Ovalbumin, the Main Allergen Protein from Egg White: Spectroscopic and Electrophoretic Studies. Int. J. Biol. Macromol. 2017, 102, 505–514. [Google Scholar] [CrossRef]
- Qian, S.; Chen, L.; Zhao, Z.; Fan, X.; Xu, X.; Zhou, G.; Zhu, B.; Ullah, N.; Feng, X. Epigallocatechin-3-Gallate Mediated Self-Assemble Behavior and Gelling Properties of the Ovalbumin with Heating Treatment. Food Hydrocoll. 2022, 131, 107797. [Google Scholar] [CrossRef]
- Nojima, T.; Iyoda, T. Water-Rich Fluid Material Containing Orderly Condensed Proteins. Angew. Chemie Int. Ed. 2017, 56, 1308–1312. [Google Scholar] [CrossRef] [PubMed]
- Nojima, T.; Iyoda, T. Egg White-Based Strong Hydrogel via Ordered Protein Condensation. NPG Asia Mater. 2018, 10, e460. [Google Scholar] [CrossRef]
- Sanaeifar, N.; Mäder, K.; Hinderberger, D. Molecular-Level Release of Coumarin-3-Carboxylic Acid and Warfarin-Derivatives from BSA-Based Hydrogels. Pharmaceutics 2021, 13, 1661. [Google Scholar] [CrossRef] [PubMed]
- Sanaeifar, N.; Mäder, K.; Hinderberger, D. Macro- and Nanoscale Effect of Ethanol on Bovine Serum Albumin Gelation and Naproxen Release. Int. J. Mol. Sci. 2022, 23, 7352. [Google Scholar] [CrossRef]
- Vesković, A.; Nakarada, Đ.; Vasiljević, O.; Dobrov, A.; Spengler, G.; Enyedy, É.A.; Arion, V.B.; Popović Bijelić, A. The Release of a Highly Cytotoxic Paullone Bearing a TEMPO Free Radical from the HSA Hydrogel: An EPR Spectroscopic Characterization. Pharmaceutics 2022, 14, 1174. [Google Scholar] [CrossRef]
- Sanaeifar, N.; Mäder, K.; Hinderberger, D. Nanoscopic Characterization of Stearic Acid Release from Bovine Serum Albumin Hydrogels. Macromol. Biosci. 2020, 20, 2000126. [Google Scholar] [CrossRef]
- Arabi, S.H.; Aghelnejad, B.; Volmer, J.; Hinderberger, D. Hydrogels from Serum Albumin in a Molten Globule-like State. Protein Sci. 2020, 29, 2459–2467. [Google Scholar] [CrossRef]
- Vesković, A.; Nakarada, Đ.; Popović Bijelić, A. A Novel Methodology for Hydrogel Water Content Determination by EPR: The Basis for Real-Time Monitoring of Controlled Drug Release and Hydrogel Swelling and Degradation. Polym. Test. 2021, 98, 107187. [Google Scholar] [CrossRef]
- Matei, I.; Ariciu, A.-M.; Popescu, E.I.; Mocanu, S.; Neculae, A.V.F.; Savonea, F.; Ionita, G. Evaluation of the Accessibility of Molecules in Hydrogels Using a Scale of Spin Probes. Gels 2022, 8, 428. [Google Scholar] [CrossRef]
- Kint, S.; Tomimatsu, Y. A Raman Difference Spectroscopic Investigation of Ovalbumin and S-Ovalbumin. Biopolymers 1979, 18, 1073–1079. [Google Scholar] [CrossRef]
- Paolinelli, C.; Barteri, M.; Boffi, F.; Forastieri, F.; Gaudiano, M.C.; Della Longa, S.; Castellano, A.C. Structural Differences of Ovalbumin and S-Ovalbumin Revealed by Denaturing Conditions. Zeitschrift Für Naturforsch. C 1997, 52, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, I.D.; Dwek, R.A. Biological Spectroscopy; Adison-Wesley: Wokimgham, UK, 1984; ISBN 0-8053-1849-6. [Google Scholar]
- Kocherginsky, N.; Swartz, H.M. Nitroxide Spin Labels. Reactions in Biology and Chemistry; CRC Press Inc.: Boca Raton, FL, USA, 1995; ISBN 978-0-8493-4204-2. [Google Scholar]
- Hagen, W. R. Biomolecular EPR Spectroscopy; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2009; ISBN 978-1-4200-5957-1. [Google Scholar]
- Marsh, D. Reaction Fields and Solvent Dependence of the EPR Parameters of Nitroxides: The Microenvironment of Spin Labels. J. Magn. Reson. 2008, 190, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Majumder, K.; Mine, Y. Egg Proteins. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 74–84. ISBN 9780128140451. [Google Scholar] [CrossRef]
- Peters, T. The Albumin Molecule: Its Structure and Chemical Properties. In All About Albumin; Peters, T., Ed.; Academic Press: Cambridge, MA, USA, 1995; pp. 9–11. ISBN1 9780125521109. ISBN2 25521109. [Google Scholar]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan Hydrogels for Sustained Drug Delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Rehfeld, S.J.; Eatough, D.J.; Plachy, W.Z. The Binding Isotherms for the Interaction of 5-Doxyl Stearic Acid with Bovine and Human Albumin. J. Lipid Res. 1978, 19, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Gantchev, T.G.; Shopova, M.B. Characterization of Spin-Labelled Fatty Acids and Hematoporphyrin Binding Sites Interactions in Serum Albumin. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1990, 1037, 422–434. [Google Scholar] [CrossRef]
- Pavićević, A.A.; Popović-Bijelić, A.D.; Mojović, M.D.; Šušnjar, S.V.; Bačić, G.G. Binding of Doxyl Stearic Spin Labels to Human Serum Albumin: An EPR Study. J. Phys. Chem. B 2014, 118, 10898–10905. [Google Scholar] [CrossRef]
- Curry, S.; Mandelkow, H.; Brick, P.; Franks, N. Crystal Structure of Human Serum Albumin Complexed with Fatty Acid Reveals an Asymmetric Distribution of Binding Sites. Nat. Struct. Biol. 1998, 5, 827–835. [Google Scholar] [CrossRef]
- Sponton, O.E.; Perez, A.A.; Carrara, C.R.; Santiago, L.G. Impact of Environment Conditions on Physicochemical Characteristics of Ovalbumin Heat-Induced Nanoparticles and on Their Ability to Bind PUFAs. Food Hydrocoll. 2015, 48, 165–173. [Google Scholar] [CrossRef]
- Dobrov, A.; Göschl, S.; Jakupec, M.A.; Popović-Bijelić, A.; Gräslund, A.; Rapta, P.; Arion, V.B. A Highly Cytotoxic Modified Paullone Ligand Bearing a TEMPO Free-Radical Unit and Its Copper(II) Complex as Potential hR2 RNR Inhibitors. Chem. Commun. 2013, 49, 10007–10009. [Google Scholar] [CrossRef] [Green Version]
- Arora, J.P.S.; Singh, S.P.; Singh, R.P.; Kumar, A. Polarographic Studies on the Binding of Copper and Cadmium Ions with Ovalbumin S. Stud. Biophys. 1984, 103, 217–223. [Google Scholar]
- Murayama, K.; Tomida, M. Heat-Induced Secondary Structure and Conformation Change of Bovine Serum Albumin Investigated by Fourier Transform Infrared Spectroscopy. Biochemistry 2004, 43, 11526–11532. [Google Scholar] [CrossRef]
- Navarra, G.; Peres, C.; Contardi, M.; Picone, P.; San Biagio, P.L.; Di Carlo, M.; Giacomazza, D.; Militello, V. Heat- and pH-Induced BSA Conformational Changes, Hydrogel Formation and Application as 3D Cell Scaffold. Arch. Biochem. Biophys. 2016, 606, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Croguennec, T.; Renault, A.; Beaufils, S.; Dubois, J.-J.; Pezennec, S. Interfacial Properties of Heat-Treated Ovalbumin. J. Colloid Interface Sci. 2007, 315, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Weijers, M.; Barneveld, P.A.; Cohen Stuart, M.A.; Visschers, R.W. Heat-Induced Denaturation and Aggregation of Ovalbumin at Neutral pH Described by Irreversible First-Order Kinetics. Protein Sci. 2003, 12, 2693–2703. [Google Scholar] [CrossRef] [Green Version]
- Howell, N.K.; Arteaga, G.; Nakai, S.; Li-Chan, E.C.Y. Raman Spectral Analysis in the C−H Stretching Region of Proteins and Amino Acids for Investigation of Hydrophobic Interactions. J. Agric. Food Chem. 1999, 47, 924–933. [Google Scholar] [CrossRef]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman Spectroscopy of Proteins: A Review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- Abrosimova, K.V.; Shulenina, O.V.; Paston, S.V. FTIR Study of Secondary Structure of Bovine Serum Albumin and Ovalbumin. J. Phys. Conf. Ser. 2016, 769, 012016. [Google Scholar] [CrossRef]
- Byrne, B.; Beattie, J.W.; Song, C.L.; Kazarian, S.G. ATR-FTIR spectroscopy and spectroscopic imaging of proteins. In Vibrational Spectroscopy in Protein Research; Ozaki, Y., Baranska, M., Lednev, I.K., Wood, B.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–22. ISBN 9780128186107. [Google Scholar]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Cai, S.; Singh, B.R. A Distinct Utility of the Amide III Infrared Band for Secondary Structure Estimation of Aqueous Protein Solutions Using Partial Least Squares Methods. Biochemistry 2004, 43, 2541–2549. [Google Scholar] [CrossRef]
- Hatta, H.; Kitabatake, N.; Doi, E. Turbidity and Hardness of a Heat-Induced Gel of Hen Egg Ovalbumin. Agric. Biol. Chem. 1986, 50, 2083–2089. [Google Scholar] [CrossRef]










| Spin Probe | a1 (G) ± 0.05 | a2 (G) ± 0.05 | τc (ns) ± 0.001 |
|---|---|---|---|
| 0.5 mM 3CP in H2O | 16.00 | 16.00 | 0.031 |
| 0.5 mM 3CP in 30 wt% OVA | 16.00 | 16.00 | 0.098 |
| 0.5 mM 3CP in 30 wt% BSA a | 16.02 | 16.03 | 0.113 |
| 0.5 mM 3CxP in H2O | 16.10 | 16.20 | 0.036 |
| 0.5 mM 3CxP in 30 wt% OVA | 16.24 | 16.30 | 0.085 |
| 0.5 mM 3CxP in 30 wt% BSA a | 16.25 | 16.27 | 0.137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesković, A.; Bajuk-Bogdanović, D.; Arion, V.B.; Popović Bijelić, A. Spectroscopic Characterization of the Binding and Release of Hydrophilic, Hydrophobic and Amphiphilic Molecules from Ovalbumin Supramolecular Hydrogels. Gels 2023, 9, 14. https://doi.org/10.3390/gels9010014
Vesković A, Bajuk-Bogdanović D, Arion VB, Popović Bijelić A. Spectroscopic Characterization of the Binding and Release of Hydrophilic, Hydrophobic and Amphiphilic Molecules from Ovalbumin Supramolecular Hydrogels. Gels. 2023; 9(1):14. https://doi.org/10.3390/gels9010014
Chicago/Turabian StyleVesković, Ana, Danica Bajuk-Bogdanović, Vladimir B. Arion, and Ana Popović Bijelić. 2023. "Spectroscopic Characterization of the Binding and Release of Hydrophilic, Hydrophobic and Amphiphilic Molecules from Ovalbumin Supramolecular Hydrogels" Gels 9, no. 1: 14. https://doi.org/10.3390/gels9010014
APA StyleVesković, A., Bajuk-Bogdanović, D., Arion, V. B., & Popović Bijelić, A. (2023). Spectroscopic Characterization of the Binding and Release of Hydrophilic, Hydrophobic and Amphiphilic Molecules from Ovalbumin Supramolecular Hydrogels. Gels, 9(1), 14. https://doi.org/10.3390/gels9010014