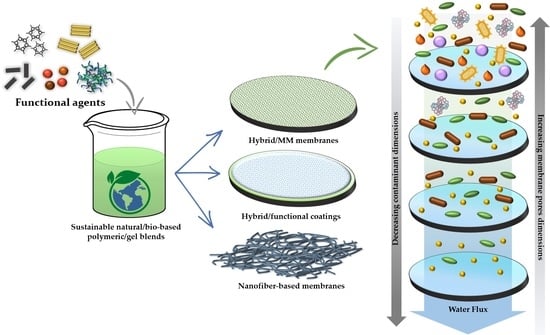
Development of Functional Hybrid Polymers and Gel Materials for Sustainable Membrane-Based Water Treatment Technology: How to Combine Greener and Cleaner Approaches
Abstract
:1. Introduction
- Microfiltration membranes (with a pore size range of 0.1–5 µm), which retain species such as algae, bacteria, suspended particles, and sediments;
- Ultrafiltration membranes (with a pore size range of 0.01–0.1 µm), which retain proteins and viruses;
- Membranes for nanofiltration (with a pore size range of 0.001–0.01 µm), which retain dissolved organic substances and divalent cations;
- Reverse osmosis membranes (with a pore size range of 0.0001–0.001 µm) either have pores or are non-porous, which work on the principle of solvent diffusion across the membrane.
2. Overview of Membrane-Based Filtration Processes, Limitations, and Innovative/Ecofriendly Approaches
- The blending of bio-based polymeric/xerogel blends with different functional molecules and nanofillers to produce mixed-matrix membranes (MMM);
- The coating of commercial membranes with bio-based blends and/or xerogels doped with reinforcing or functional agents;
- The preparation of the membranes through innovative fabrication techniques such as electrospinning starting from sustainable formulations.
3. Sustainable Hybrid/Mixed-Matrix Water Filtration Membranes
4. Hybrid/Doped Bio-Based and Functional Coatings for Filtration Membranes
5. Functional/Hybrid Electrospun Nanofiber-Based Membranes
- Double deposited as “sandwich-like” composites;
- Deposited on commercial supports;
- Deposited on nanofibrous sublayers;
- Coated with hydrogels or functional gels;
- Coated with electrospray processes;
- Not supported.
6. Final Remarks and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gude, V.G. Desalination and water reuse to address global water scarcity. Rev. Environ. Sci. Bio/Technol. 2017, 16, 591–609. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Bali, R.; Khan, H.; Mohamed, H.I.; Sharma, S.K. Improved water resource management framework for water sustainability and security. Environ. Res. 2021, 201, 111527. [Google Scholar] [CrossRef]
- Peeters, R.; Vanderschaeghe, H.; Rongé, J.; Martens, J.A. Energy performance and climate dependency of technologies for fresh water production from atmospheric water vapour. Environ. Sci. Water Res. Technol. 2020, 6, 2016–2034. [Google Scholar] [CrossRef]
- Yang, Z.; Song, J.; Cheng, D.; Xia, J.; Li, Q.; Ahamad, M.I. Comprehensive evaluation and scenario simulation for the water resources carrying capacity in Xi’an city, China. J. Environ. Manag. 2019, 230, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Qian, H. Water resources research to support a sustainable China. Int. J. Water Resour. Dev. 2018, 34, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Wei, Y.; Western, A. Evolution of the societal value of water resources for economic development versus environmental sustainability in Australia from 1843 to 2011. Glob. Environ. Chang. 2017, 42, 82–92. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Hu, M.-C.; Ni, F.-C. Supporting a circular economy: Insights from Taiwan’s plastic waste sector and lessons for developing countries. Sustain. Prod. Consum. 2021, 26, 228–238. [Google Scholar] [CrossRef]
- Grdic, Z.S.; Nizic, M.K.; Rudan, E. Circular Economy Concept in the Context of Economic Development in EU Countries. Sustainability 2020, 12, 3060. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J. The environmental benefits of water recycling and reuse. Water Supply 2003, 3, 1–10. [Google Scholar] [CrossRef]
- Jia, Z.; Cai, Y.; Chen, Y.; Zeng, W. Regionalization of water environmental carrying capacity for supporting the sustainable water resources management and development in China. Resour. Conserv. Recycl. 2018, 134, 282–293. [Google Scholar] [CrossRef]
- Howe, C.W. The effects of water resource development on economic growth: The conditions for success. In Water in a Developing World; Routledge: Oxfordshire, UK, 2019; pp. 202–218. ISBN 0429267274. [Google Scholar]
- Del Borghi, A.; Moreschi, L.; Gallo, M. Circular economy approach to reduce water–energy–food nexus. Curr. Opin. Environ. Sci. Health 2020, 13, 23–28. [Google Scholar] [CrossRef]
- Kumaraswamy, T.R.; Javeed, S.; Javaid, M.; Naika, K. Impact of Pollution on Quality of Freshwater Ecosystems. In Fresh Water Pollution Dynamics and Remediation; Qadri, H., Bhat, R.A., Mehmood, M.A., Dar, G.H., Eds.; Springer Singapore: Singapore, 2020; pp. 69–81. ISBN 978-981-13-8277-2. [Google Scholar]
- Islam, M.M.; Karim, M.R.; Zheng, X.; Li, X. Heavy Metal and Metalloid Pollution of Soil, Water and Foods in Bangladesh: A Critical Review. Int. J. Environ. Res. Public Health 2018, 15, 2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, P.K. Water Pollution: Causes, Effects and Control; New Age International: New Delhi, India, 2006; ISBN 8122418392. [Google Scholar]
- Ouda, M.; Kadadou, D.; Swaidan, B.; Al-Othman, A.; Al-Asheh, S.; Banat, F.; Hasan, S.W. Emerging contaminants in the water bodies of the Middle East and North Africa (MENA): A critical review. Sci. Total Environ. 2021, 754, 142177. [Google Scholar] [CrossRef] [PubMed]
- Li, F. Heavy Metal in Urban Soil: Health Risk Assessment and Management. In Heavy Metals; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Wickham, G.M.; Shriver, T.E. Emerging contaminants, coerced ignorance and environmental health concerns: The case of per- and polyfluoroalkyl substances (PFAS). Sociol. Health Illn. 2021, 43, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Qin, J.; Vasseghian, Y. Occurrences and removal of pharmaceutical and personal care products from aquatic systems using advanced treatment- A review. Environ. Res. 2022, 204, 112298. [Google Scholar] [CrossRef]
- Xiang, Y.; Jiang, L.; Zhou, Y.; Luo, Z.; Zhi, D.; Yang, J.; Lam, S.S. Microplastics and environmental pollutants: Key interaction and toxicology in aquatic and soil environments. J. Hazard. Mater. 2022, 422, 126843. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, L.; Zhang, X.-X. Emerging Pollutants–Part I: Occurrence, Fate and Transport. Water Environ. Res. 2018, 90, 1301–1322. [Google Scholar] [CrossRef] [Green Version]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A. A review on industrial wastewater treatment via electrocoagulation processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Miino, M.C.; Damiani, S. Treatments for color removal from wastewater: State of the art. J. Environ. Manag. 2019, 236, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Imbrogno, A.; Schäfer, A.I. Comparative study of nanofiltration membrane characterization devices of different dimension and configuration (cross flow and dead end). J. Membr. Sci. 2019, 585, 67–80. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, X.; Liang, H.; Cheng, W.; Li, G.; Zhang, Q.; Chen, J.; Chen, K.; Wang, J. Effects of Filtration Mode on the Performance of Gravity-Driven Membrane (GDM) Filtration: Cross-Flow Filtration and Dead-End Filtration. Water 2022, 14, 190. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Boyraz, E.; Maryska, J.; Kucerova, K. A Review on Membrane Technology and Chemical Surface Modification for the Oily Wastewater Treatment. Materials 2020, 13, 493. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Chapter 2—Microfiltration, ultrafiltration, nanofiltration, reverse osmosis, and forward osmosis. In Fundamental Modeling of Membrane Systems—Membrane and Process Performance; Elsevier: Amsterdam, The Netherlands, 2018; pp. 25–70. ISBN 978-0-12-813483-2. [Google Scholar]
- Gandhi, K.; Sharma, N.; Gautam, P.B.; Sharma, R.; Mann, B.; Pandey, V. Membrane Processes. In Advanced Analytical Techniques in Dairy Chemistry; Springer US: New York, NY, USA, 2022; pp. 131–145. ISBN 978-1-0716-1940-7. [Google Scholar]
- Lemming, G.; Chambon, J.C.; Binning, P.J.; Bjerg, P.L. Is there an environmental benefit from remediation of a contaminated site? Combined assessments of the risk reduction and life cycle impact of remediation. J. Environ. Manag. 2012, 112, 392–403. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, A.S.; Wei, J.; Buschjost, R. Effect of nanofiltration on photochemical integrity. Proc. SPIE 2008, 6923, 69233H. [Google Scholar] [CrossRef]
- Someya, M.; Higashino, K.; Imoto, Y.; Sakanakura, H.; Yasutaka, T. Effects of membrane filter material and pore size on turbidity and hazardous element concentrations in soil batch leaching tests. Chemosphere 2021, 265, 128981. [Google Scholar] [CrossRef] [PubMed]
- Galiano, F.; Briceño, K.; Marino, T.; Molino, A.; Christensen, K.V.; Figoli, A. Advances in biopolymer-based membrane preparation and applications. J. Membr. Sci. 2018, 564, 562–586. [Google Scholar] [CrossRef]
- Hardian, R.; Alammar, A.; Holtzl, T.; Szekely, G. Fabrication of sustainable organic solvent nanofiltration membranes using cellulose–chitosan biopolymer blends. J. Membr. Sci. 2022, 658, 120743. [Google Scholar] [CrossRef]
- Aji, M.M.; Narendren, S.; Purkait, M.K.; Katiyar, V. Biopolymer (gum arabic) incorporation in waste polyvinylchloride membrane for the enhancement of hydrophilicity and natural organic matter removal in water. J. Water Process. Eng. 2020, 38, 101569. [Google Scholar] [CrossRef]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Sivamani, S.; Sivakumar, N.; Maran, J.P.; Hosseini-Bandegharaei, A. Ecofriendly biopolymers and composites: Preparation and their applications in water-treatment. Biotechnol. Adv. 2021, 52, 107815. [Google Scholar] [CrossRef]
- Dassanayake, R.S.; Acharya, S.; Abidi, N. Recent Advances in Biopolymer-Based Dye Removal Technologies. Molecules 2021, 26, 4697. [Google Scholar] [CrossRef]
- Ielo, I.; Giacobello, F.; Sfameni, S.; Rando, G.; Galletta, M.; Trovato, V.; Rosace, G.; Plutino, M. Nanostructured Surface Finishing and Coatings: Functional Properties and Applications. Materials 2021, 14, 2733. [Google Scholar] [CrossRef]
- Libertino, S.; Plutino, M.R.; Rosace, G. Design and development of wearable sensing nanomaterials for smart textiles. AIP Conf. Proc. 2018, 1990, 020016. [Google Scholar] [CrossRef]
- Puoci, F.; Saturnino, C.; Trovato, V.; Iacopetta, D.; Piperopoulos, E.; Triolo, C.; Bonomo, M.G.; Drommi, D.; Parisi, O.I.; Milone, C.; et al. Sol–Gel Treatment of Textiles for the Entrapping of an Antioxidant/Anti-Inflammatory Molecule: Functional Coating Morphological Characterization and Drug Release Evaluation. Appl. Sci. 2020, 10, 2287. [Google Scholar] [CrossRef] [Green Version]
- Mansoori, S.; Davarnejad, R.; Matsuura, T.; Ismail, A.F. Membranes based on non-synthetic (natural) polymers for wastewater treatment. Polym. Test. 2020, 84, 106381. [Google Scholar] [CrossRef]
- Rando, G.; Sfameni, S.; Galletta, M.; Drommi, D.; Cappello, S.; Plutino, M.R. Functional Nanohybrids and Nanocomposites Development for the Removal of Environmental Pollutants and Bioremediation. Molecules 2022, 27, 4856. [Google Scholar] [CrossRef] [PubMed]
- Sfameni, S.; Rando, G.; Marchetta, A.; Scolaro, C.; Cappello, S.; Urzì, C.; Visco, A.; Plutino, M.R. Development of Eco-Friendly Hydrophobic and Fouling-Release Coatings for Blue-Growth Environmental Applications: Synthesis, Mechanical Characterization and Biological Activity. Gels 2022, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Sfameni, S.; Rando, G.; Galletta, M.; Ielo, I.; Brucale, M.; De Leo, F.; Cardiano, P.; Cappello, S.; Visco, A.; Trovato, V.; et al. Design and Development of Fluorinated and Biocide-Free Sol–Gel Based Hybrid Functional Coatings for Anti-Biofouling/Foul-Release Activity. Gels 2022, 8, 538. [Google Scholar] [CrossRef]
- Ielo, I.; Giacobello, F.; Castellano, A.; Sfameni, S.; Rando, G.; Plutino, M.R. Development of Antibacterial and Antifouling Innovative and Eco-Sustainable Sol–Gel Based Materials: From Marine Areas Protection to Healthcare Applications. Gels 2022, 8, 26. [Google Scholar] [CrossRef]
- Li, C.; Sun, W.; Lu, Z.; Ao, X.; Li, S. Ceramic nanocomposite membranes and membrane fouling: A review. Water Res. 2020, 175, 115674. [Google Scholar] [CrossRef]
- Du, X.; Shi, Y.; Jegatheesan, V.; Haq, I.U. A Review on the Mechanism, Impacts and Control Methods of Membrane Fouling in MBR System. Membranes 2020, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Enfrin, M.; Lee, J.; Le-Clech, P.; Dumée, L.F. Kinetic and mechanistic aspects of ultrafiltration membrane fouling by nano- and microplastics. J. Membr. Sci. 2020, 601, 117890. [Google Scholar] [CrossRef]
- Meng, X.; Luosang, D.; Meng, S.; Wang, R.; Fan, W.; Liang, D.; Li, X.; Zhao, Q.; Yang, L. The structural and functional properties of polysaccharide foulants in membrane fouling. Chemosphere 2021, 268, 129364. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Anantharaman, A.; Ng, A.Q.Q.; Ma, Y.; Tanis-Kanbur, M.B.; Zydney, A.L.; Chew, J.W. A review of membrane fouling by proteins in ultrafiltration and microfiltration. J. Water Process. Eng. 2022, 50, 103294. [Google Scholar] [CrossRef]
- Muhamad, N.A.S.; Mokhtar, N.M.; Lau, W.J.; Ismail, A.F.; Naim, R. Fouling studies on hydrophobic PVDF-bentonite hollow fiber membrane during membrane distillation of palm oil mill effluent. J. Water Process. Eng. 2022, 49, 102969. [Google Scholar] [CrossRef]
- Li, F.; Xing, Y.; Ding, X. Silica xerogel coating on the surface of natural and synthetic fabrics. Surf. Coatings Technol. 2008, 202, 4721–4727. [Google Scholar] [CrossRef]
- Sfameni, S.; Lawnick, T.; Rando, G.; Visco, A.; Textor, T.; Plutino, M.R. Functional Silane-Based Nanohybrid Materials for the Development of Hydrophobic and Water-Based Stain Resistant Cotton Fabrics Coatings. Nanomaterials 2022, 12, 3404. [Google Scholar] [CrossRef] [PubMed]
- Sfameni, S.; Del Tedesco, A.; Rando, G.; Truant, F.; Visco, A.; Plutino, M.R. Waterborne Eco-Sustainable Sol–Gel Coatings Based on Phytic Acid Intercalated Graphene Oxide for Corrosion Protection of Metallic Surfaces. Int. J. Mol. Sci. 2022, 23, 12021. [Google Scholar] [CrossRef] [PubMed]
- Araby, S.; Philips, B.; Meng, Q.; Ma, J.; Laoui, T.; Wang, C.H. Recent advances in carbon-based nanomaterials for flame retardant polymers and composites. Compos. Part B Eng. 2021, 212, 108675. [Google Scholar] [CrossRef]
- Zhu, M.; Li, J.; Chen, J.; Song, H.; Zhang, H. Improving thermal conductivity of epoxy resin by filling boron nitride nanomaterials: A molecular dynamics investigation. Comput. Mater. Sci. 2019, 164, 108–115. [Google Scholar] [CrossRef]
- Banerjee, S.; Bairagi, S.; Ali, S.W. A critical review on lead-free hybrid materials for next generation piezoelectric energy harvesting and conversion. Ceram. Int. 2021, 47, 16402–16421. [Google Scholar] [CrossRef]
- Andre, R.S.; Sanfelice, R.C.; Pavinatto, A.; Mattoso, L.H.C.; Correa, D.S. Hybrid nanomaterials designed for volatile organic compounds sensors: A review. Mater. Des. 2018, 156, 154–166. [Google Scholar] [CrossRef]
- Oun, A.A.; Shankar, S.; Rhim, J.-W. Multifunctional nanocellulose/metal and metal oxide nanoparticle hybrid nanomaterials. Crit. Rev. Food Sci. Nutr. 2020, 60, 435–460. [Google Scholar] [CrossRef] [PubMed]
- Stauber, J.M.; Qian, E.A.; Han, Y.; Rheingold, A.L.; Král, P.; Fujita, D.; Spokoyny, A.M. An Organometallic Strategy for Assembling Atomically Precise Hybrid Nanomaterials. J. Am. Chem. Soc. 2020, 142, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef] [PubMed]
- Si, A.; Kyzas, G.Z.; Pal, K.; de Souza, F.G., Jr. Graphene functionalized hybrid nanomaterials for industrial-scale applications: A systematic review. J. Mol. Struct. 2021, 1239, 130518. [Google Scholar] [CrossRef]
- Trovato, V.; Sfameni, S.; Rando, G.; Rosace, G.; Libertino, S.; Ferri, A.; Plutino, M.R. A Review of Stimuli-Responsive Smart Materials for Wearable Technology in Healthcare: Retrospective, Perspective, and Prospective. Molecules 2022, 27, 5709. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Alhassan, S.M.; Naushad, M. Advances in the role of natural gums-based hydrogels in water purification, desalination and atmospheric-water harvesting. Int. J. Biol. Macromol. 2022, 222, 2888–2921. [Google Scholar] [CrossRef]
- Vinod, A.; Siengchin, B.; Parameswaranpillai, J. Renewable and sustainable biobased materials: An assess-ment on biofibres, biofilms, biopolymers and biocomposites. J. Cleaner. Prod. 2020, 258, 120978. [Google Scholar] [CrossRef]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Yan, S.; Yin, X.; Chen, J. Development of alginate hydrogel modified multifunctional filtration membrane with robust anti-fouling property for efficient water purification. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123891. [Google Scholar] [CrossRef]
- Salehi, E.; Daraei, P.; Shamsabadi, A.A. A review on chitosan-based adsorptive membranes. Carbohydr. Polym. 2016, 152, 419–432. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [Green Version]
- Manohara, H.M.; Nayak, S.S.; Franklin, G.; Nataraj, S.K.; Mondal, D. Progress in marine derived renewable functional materials and biochar for sustainable water purification. Green Chem. 2021, 23, 8305–8331. [Google Scholar] [CrossRef]
- More, N.; Avhad, M.; Utekar, S.; More, A. Polylactic acid (PLA) membrane—Significance, synthesis, and applications: A review. Polym. Bull. 2022, 1–37. [Google Scholar] [CrossRef]
- Sangeetha, K.; Vinodhini, A.; Sudha, P.N.; Faleh, A.A.; Sukumaran, A. Novel chitosan based thin sheet nanofiltration membrane for rejection of heavy metal chromium. Int. J. Biol. Macromol. 2019, 132, 939–953. [Google Scholar] [CrossRef]
- Chaudhary, M.; Maiti, A. Fe–Al–Mn@chitosan based metal oxides blended cellulose acetate mixed matrix membrane for fluoride decontamination from water: Removal mechanisms and antibacterial behavior. J. Membr. Sci. 2020, 611, 118372. [Google Scholar] [CrossRef]
- Qian, X.; Li, N.; Wang, Q.; Ji, S. Chitosan/graphene oxide mixed matrix membrane with enhanced water permeability for high-salinity water desalination by pervaporation. Desalination 2018, 438, 83–96. [Google Scholar] [CrossRef]
- Wang, X.-L.; Qin, W.; Wang, L.-X.; Zhao, K.-Y.; Wang, H.-C.; Liu, H.-Y.; Wei, J.-F. Desalination of dye utilizing carboxylated TiO2/calcium alginate hydrogel nanofiltration membrane with high salt permeation. Sep. Purif. Technol. 2020, 253, 117475. [Google Scholar] [CrossRef]
- Amiri, S.; Asghari, A.; Vatanpour, V.; Rajabi, M. Fabrication and characterization of a novel polyvinyl alcohol-graphene oxide-sodium alginate nanocomposite hydrogel blended PES nanofiltration membrane for improved water purification. Sep. Purif. Technol. 2020, 250, 117216. [Google Scholar] [CrossRef]
- Alshahrani, A.; Alharbi, A.; Alnasser, S.; Almihdar, M.; Alsuhybani, M.; AlOtaibi, B. Enhanced heavy metals removal by a novel carbon nanotubes buckypaper membrane containing a mixture of two biopolymers: Chitosan and i-carrageenan. Sep. Purif. Technol. 2021, 276, 119300. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Kana, M.T.H.A. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Mallakpour, S.; Sirous, F.; Hussain, C.M. A journey to the world of fascinating ZnO nanocomposites made of chitosan, starch, cellulose, and other biopolymers: Progress in recent achievements in eco-friendly food packaging, biomedical, and water remediation technologies. Int. J. Biol. Macromol. 2021, 170, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Bessa, A.; Gonçalves, G.; Henriques, B.; Domingues, E.M.; Pereira, E.; Marques, P.A.A.P. Green Graphene–Chitosan Sorbent Materials for Mercury Water Remediation. Nanomaterials 2020, 10, 1474. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, N. Preparation of hydrophilic polyvinylidene fluoride/polyvinyl alcohol ultrafiltration membrane via polymer/non-solvent co-induced phase separation method towards enhance anti-fouling performance. J. Environ. Chem. Eng. 2021, 9, 106431. [Google Scholar] [CrossRef]
- Tummino, M.L.; Magnacca, G.; Cimino, D.; Laurenti, E.; Nisticò, R. The Innovation Comes from the Sea: Chitosan and Alginate Hybrid Gels and Films as Sustainable Materials for Wastewater Remediation. Int. J. Mol. Sci. 2020, 21, 550. [Google Scholar] [CrossRef] [Green Version]
- Otunola, B.O.; Ololade, O.O. A review on the application of clay minerals as heavy metal adsorbents for remediation purposes. Environ. Technol. Innov. 2020, 18, 100692. [Google Scholar] [CrossRef]
- Biswas, B.; Warr, L.N.; Hilder, E.F.; Goswami, N.; Rahman, M.M.; Churchman, J.G.; Vasilev, K.; Pan, G.; Naidu, R. Biocompatible functionalisation of nanoclays for improved environmental remediation. Chem. Soc. Rev. 2019, 48, 3740–3770. [Google Scholar] [CrossRef] [PubMed]
- Carniato, F.; Gatti, G.; Bisio, C. An overview of the recent synthesis and functionalization methods of saponite clay. New J. Chem. 2020, 44, 9969–9980. [Google Scholar] [CrossRef]
- Malsawmdawngzela, R.; Lalhmunsiama; Tiwari, D.; Lee, S. Synthesis of novel clay-based nanocomposite materials and its application in the remediation of arsenic contaminated water. Int. J. Environ. Sci. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Nasir, R.; Mukhtar, H.; Man, Z.; Mohshim, D.F. Material Advancements in Fabrication of Mixed-Matrix Membranes. Chem. Eng. Technol. 2013, 36, 717–727. [Google Scholar] [CrossRef]
- Qadir, D.; Mukhtar, H.; Keong, L.K. Mixed Matrix Membranes for Water Purification Applications. Sep. Purif. Rev. 2017, 46, 62–80. [Google Scholar] [CrossRef]
- Madima, N.; Mishra, S.B.; Inamuddin, I.; Mishra, A.K. Carbon-based nanomaterials for remediation of organic and inorganic pollutants from wastewater. A review. Environ. Chem. Lett. 2020, 18, 1169–1191. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Vo, D.-V.N.; Prakash, D.G.; Joseph, A.A.; Viswanathan, S.; Arun, J. Environmental applications of carbon-based materials: A review. Environ. Chem. Lett. 2021, 19, 557–582. [Google Scholar] [CrossRef]
- Athayde, A.L.; Baker, R.W.; Daniels, R.; Le, M.H.; Ly, J.H. Pervaporation for wastewater treatment. Chemtech 1997, 27, 438874. [Google Scholar]
- Khulbe, K.C.; Matsuura, T. Thin Film Composite and/or Thin Film Nanocomposite Hollow Fiber Membrane for Water Treatment, Pervaporation, and Gas/Vapor Separation. Polymers 2018, 10, 1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.Y.C.; Goh, S.S.; Liow, S.S.; Xue, K.; Loh, X.J. Molecular gel sorbent materials for environmental remediation and wastewater treatment. J. Mater. Chem. A 2019, 7, 18759–18791. [Google Scholar] [CrossRef]
- Naseem, K.; Begum, R.; Wu, W.; Usman, M.; Irfan, A.; Al-Sehemi, A.G.; Farooqi, Z.H. Adsorptive removal of heavy metal ions using polystyrene-poly(N-isopropylmethacrylamide-acrylic acid) core/shell gel particles: Adsorption isotherms and kinetic study. J. Mol. Liq. 2019, 277, 522–531. [Google Scholar] [CrossRef]
- Ajmal, M.; Siddiq, M.; Aktas, N.; Sahiner, N. Magnetic Co–Fe bimetallic nanoparticle containing modifiable microgels for the removal of heavy metal ions, organic dyes and herbicides from aqueous media. RSC Adv. 2015, 5, 43873–43884. [Google Scholar] [CrossRef]
- Arif, M.; Shahid, M.; Irfan, A.; Nisar, J.; Wang, X.; Batool, N.; Ali, M.; Farooqi, Z.H.; Begum, R. Extraction of copper ions from aqueous medium by microgel particles for in-situ fabrication of copper nanoparticles to degrade toxic dyes. Z. Für Phys. Chem. 2022, 236, 1219–1241. [Google Scholar] [CrossRef]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.; Han, E.J.; Ahn, G. Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Ding, W.; Wang, Y.; Wu, J.; Gu, Y.; He, F. Manganese oxide nanoparticles impregnated graphene oxide aggregates for cadmium and copper remediation. Chem. Eng. J. 2018, 350, 1135–1143. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, D.; Liang, Y. Nanotechnology in remediation of water contaminated by poly- and perfluoroalkyl substances: A review. Environ. Pollut. 2019, 247, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.M.; Elsayed, A.M. Carbon Nanotubes for Environmental Remediation Applications. In Handbook of Carbon Nanotubes; Abraham, J., Thomas, S., Kalarikkal, N., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–30. ISBN 978-3-319-70614-6. [Google Scholar]
- Da Silva Alves, D.C.; de Farias, B.S.; Breslin, C.; Pinto, L.A.d.A.; Cadaval, T.R.S. Chapter 18—Carbon nanotube-based materials for environmental remediation processes. In Advanced Materials for Sustainable Environmental Remediation; Giannakoudakis, D., Meili, L., Anastopoulos, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 475–513. ISBN 978-0-323-90485-8. [Google Scholar]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Dong, Y.; Wei, Z.; Xue, C. Recent advances in carrageenan-based delivery systems for bioactive ingredients: A review. Trends Food Sci. Technol. 2021, 112, 348–361. [Google Scholar] [CrossRef]
- Li, W.; Qamar, S.A.; Qamar, M.; Basharat, A.; Bilal, M.; Iqbal, H.M.N. Carrageenan-based nano-hybrid materials for the mitigation of hazardous environmental pollutants. Int. J. Biol. Macromol. 2021, 190, 700–712. [Google Scholar] [CrossRef]
- Abu-Saied, M.A.; Elnouby, M.; Taha, T.; El-shafeey, M.; Alshehri, A.G.; Alamri, S.; Alghamdi, H.; Shati, A.; Alrumman, S.; Al-Kahtani, M.; et al. Potential Decontamination of Drinking Water Pathogens through k-Carrageenan Integrated Green Bottle Fly Bio-Synthesized Silver Nanoparticles. Molecules 2020, 25, 1936. [Google Scholar] [CrossRef] [Green Version]
- Bagal-Kestwal, D.R.; Pan, M.H.; Chiang, B.-H. Properties and Applications of Gelatin, Pectin, and Carrageenan Gels. In Bio Monomers for Green Polymeric Composite Materials; John Wiley & Sons: New York, NY, USA, 2019; pp. 117–140. ISBN 9781119301714. [Google Scholar]
- Alam, J.; Alhoshan, M.; Shukla, A.K.; Aldalbahi, A.; Ali, F.A.A. k-Carrageenan—A versatile biopolymer for the preparation of a hydrophilic PVDF composite membrane. Eur. Polym. J. 2019, 120, 109219. [Google Scholar] [CrossRef]
- Ulu, A.; Alpaslan, M.; Gultek, A.; Ates, B. Eco-friendly chitosan/κ-carrageenan membranes reinforced with activated bentonite for adsorption of methylene blue. Mater. Chem. Phys. 2022, 278, 125611. [Google Scholar] [CrossRef]
- Tan, H.-F.; Ooi, B.S.; Leo, C.P. Future perspectives of nanocellulose-based membrane for water treatment. J. Water Process. Eng. 2020, 37, 101502. [Google Scholar] [CrossRef]
- Mavukkandy, M.O.; McBride, S.A.; Warsinger, D.M.; Dizge, N.; Hasan, S.W.; Arafat, H.A. Thin film deposition techniques for polymeric membranes– A review. J. Membr. Sci. 2020, 610, 118258. [Google Scholar] [CrossRef]
- Prasannan, A.; Udomsin, J.; Tsai, H.-C.; Wang, C.-F.; Lai, J.-Y. Robust underwater superoleophobic membranes with bio-inspired carrageenan/laponite multilayers for the effective removal of emulsions, metal ions, and organic dyes from wastewater. Chem. Eng. J. 2020, 391, 123585. [Google Scholar] [CrossRef]
- Bandara, P.C.; Nadres, E.T.; Rodrigues, D.F. Use of Response Surface Methodology To Develop and Optimize the Composition of a Chitosan–Polyethyleneimine–Graphene Oxide Nanocomposite Membrane Coating To More Effectively Remove Cr(VI) and Cu(II) from Water. ACS Appl. Mater. Interfaces 2019, 11, 17784–17795. [Google Scholar] [CrossRef]
- Lakra, R.; Balakrishnan, M.; Basu, S. Development of cellulose acetate-chitosan-metal organic framework forward osmosis membrane for recovery of water and nutrients from wastewater. J. Environ. Chem. Eng. 2021, 9, 105882. [Google Scholar] [CrossRef]
- Kazemi, M.; Jahanshahi, M.; Peyravi, M. Chitosan-sodium alginate multilayer membrane developed by Fe0@WO3 nanoparticles: Photocatalytic removal of hexavalent chromium. Carbohydr. Polym. 2018, 198, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Ibrar, I.; Altaee, A.; Samal, A.K.; Zhou, J. Surface modification of nanofiltration membrane with kappa-carrageenan/graphene oxide for leachate wastewater treatment. J. Membr. Sci. 2022, 659, 120776. [Google Scholar] [CrossRef]
- Zhao, S.; Tao, Z.; Chen, L.; Han, M.; Zhao, B.; Tian, X.; Wang, L.; Meng, F. An antifouling catechol/chitosan-modified polyvinylidene fluoride membrane for sustainable oil-in-water emulsions separation. Front. Environ. Sci. Eng. 2020, 15, 63. [Google Scholar] [CrossRef]
- AlAbduljabbar, F.A.; Haider, S.; Ali, F.A.A.; Alghyamah, A.A.; Almasry, W.A.; Patel, R.; Mujtaba, I.M. TiO2 nanostructured coated functionally modified and composite electrospun chitosan nanofibers membrane for efficient photocatalytic degradation of organic pollutant in wastewater. J. Mater. Res. Technol. 2021, 15, 5197–5212. [Google Scholar] [CrossRef]
- Yan, L.; Yang, X.; Zhao, Y.; Wu, Y.; Moutloali, R.M.; Mamba, B.B.; Sorokin, P.; Shao, L. Bio-inspired mineral-hydrogel hybrid coating on hydrophobic PVDF membrane boosting oil/water emulsion separation. Sep. Purif. Technol. 2022, 285, 120383. [Google Scholar] [CrossRef]
- Pan, S.; Li, J.; Noonan, O.; Fang, X.; Wan, G.; Yu, C.; Wang, L. Dual-Functional Ultrafiltration Membrane for Simultaneous Removal of Multiple Pollutants with High Performance. Environ. Sci. Technol. 2017, 51, 5098–5107. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Xue, H.; Pang, H.; Xu, Q. Metal–organic frameworks as a platform for clean energy applications. Energychem 2020, 2, 100027. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Yu, S.; Pang, H.; Huang, S.; Tang, H.; Wang, S.; Qiu, M.; Chen, Z.; Yang, H.; Song, G.; Fu, D.; et al. Recent advances in metal-organic framework membranes for water treatment: A review. Sci. Total Environ. 2021, 800, 149662. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wu, F.; Li, L.; Yang, X.; Xu, C.; Yu, P.; Ma, F.; Mao, L. Natural Leukocyte Membrane-Masked Microelectrodes with an Enhanced Antifouling Ability and Biocompatibility for In Vivo Electrochemical Sensing. Anal. Chem. 2020, 92, 11374–11379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, H.; Li, Q.; Yu, H.; Tian, X.; Zhao, M.; Zhang, H. Facile dual-functionalization of polyamide reverse osmosis membrane by a natural polypeptide to improve the antifouling and chlorine-resistant properties. J. Membr. Sci. 2020, 604, 118044. [Google Scholar] [CrossRef]
- Moradi, G.; Rahimi, M.; Zinadini, S.; Shamsipur, M.; Babajani, N. Natural deep eutectic solvent modified nanofiltration membranes with superior antifouling properties for pharmaceutical wastewater treatment. Chem. Eng. J. 2022, 448, 137704. [Google Scholar] [CrossRef]
- Bai, L.; Wu, H.; Ding, J.; Ding, A.; Zhang, X.; Ren, N.; Li, G.; Liang, H. Cellulose nanocrystal-blended polyethersulfone membranes for enhanced removal of natural organic matter and alleviation of membrane fouling. Chem. Eng. J. 2020, 382, 122919. [Google Scholar] [CrossRef]
- Lee, W.J.; Bao, Y.; Guan, C.; Hu, X.; Lim, T.-T. Ce/TiOx-functionalized catalytic ceramic membrane for hybrid catalytic ozonation-membrane filtration process: Fabrication, characterization and performance evaluation. Chem. Eng. J. 2021, 410, 128307. [Google Scholar] [CrossRef]
- Dlamini, D.S.; Matindi, C.; Vilakati, G.D.; Tesha, J.M.; Motsa, M.M.; Thwala, J.M.; Mamba, B.B.; Hoek, E.M.V.; Li, J. Fine-tuning the architecture of loose nanofiltration membrane for improved water flux, dye rejection and dye/salt selective separation. J. Membr. Sci. 2021, 621, 118930. [Google Scholar] [CrossRef]
- Xiong, Z.; Lin, H.; Zhong, Y.; Qin, Y.; Li, T.; Liu, F. Robust superhydrophilic polylactide (PLA) membranes with a TiO2 nano-particle inlaid surface for oil/water separation. J. Mater. Chem. A 2017, 5, 6538–6545. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, F.; Wang, Y.; Zhang, Q.; Ma, W.; Huang, C. Electrospun nanofiber membranes for wastewater treatment applications. Sep. Purif. Technol. 2020, 250, 117116. [Google Scholar] [CrossRef]
- Tijing, L.D.; Yao, M.; Ren, J.; Park, C.-H.; Kim, C.S.; Shon, H.K. Nanofibers for Water and Wastewater Treatment: Recent Advances and Developments. In Water and Wastewater Treatment Technologies; Bui, X.-T., Chiemchaisri, C., Fujioka, T., Varjani, S., Eds.; Springer Singapore: Singapore, 2019; pp. 431–468. ISBN 978-981-13-3259-3. [Google Scholar]
- Wang, X.; Hsiao, B.S. Electrospun nanofiber membranes. Curr. Opin. Chem. Eng. 2016, 12, 62–81. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Zhao, H.; Yue, L.; Fan, G.; Li, T.; Lu, S.; Chen, G.; Gao, S.; Asiri, A.M.; Sun, X. Recent advances in electrospun nanofibers for supercapacitors. J. Mater. Chem. A 2020, 8, 16747–16789. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2019, 19, e1800256. [Google Scholar] [CrossRef]
- Lu, T.; Cui, J.; Qu, Q.; Wang, Y.; Zhang, J.; Xiong, R.; Ma, W.; Huang, C. Multistructured Electrospun Nanofibers for Air Filtration: A Review. ACS Appl. Mater. Interfaces 2021, 13, 23293–23313. [Google Scholar] [CrossRef]
- Zanin, M.H.A.; Cerize, N.N.P.; de Oliveira, A.M. Production of Nanofibers by Electrospinning Technology: Overview and Application in Cosmetics. In Nanocosmetics and Nanomedicines: New Approaches for Skin Care; Beck, R., Guterres, S., Pohlmann, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 311–332. ISBN 978-3-642-19792-5. [Google Scholar]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Qian, Q.; Huang, H.; Chen, Y.; Wang, Z.; Chen, Q.; Yang, J.; Li, J.; Mai, Y.-W. Electrospinning-Based Strategies for Battery Materials. Adv. Energy Mater. 2021, 11, 2000845. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Bates, I.I.C.; Carrillo, I.B.S.; Germain, H.; Loranger, É.; Chabot, B. Antibacterial electrospun chitosan-PEO/TEMPO-oxidized cellulose composite for water filtration. J. Environ. Chem. Eng. 2021, 9, 106204. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, H.; Tong, X.; Wang, Z.; Crittenden, J.C.; Huang, M. Integration of a Photo-Fenton Reaction and a Membrane Filtration using CS/PAN@FeOOH/g-C3N4Electrospun Nanofibers: Synthesis, Characterization, Self-cleaning Performance and Mechanism. Appl. Catal. B Environ. 2021, 281, 119519. [Google Scholar] [CrossRef]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; Jafari rad, A.; Irani, M. Incorporation of UiO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb(II), Cd(II) and Cr(VI) ions from aqueous solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef]
- Zhijiang, C.; Cong, Z.; Ping, X.; Jie, G.; Kongyin, Z. Calcium alginate-coated electrospun polyhydroxybutyrate/carbon nanotubes composite nanofibers as nanofiltration membrane for dye removal. J. Mater. Sci. 2018, 53, 14801–14820. [Google Scholar] [CrossRef]
- Wu, S.; Li, K.; Shi, W.; Cai, J. Chitosan/polyvinylpyrrolidone/polyvinyl alcohol/carbon nanotubes dual layers nanofibrous membrane constructed by electrospinning-electrospray for water purification. Carbohydr. Polym. 2022, 294, 119756. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-L.; Zhang, J.; Wu, G.; Zhang, M.-X.; Chen, S.-C.; Wang, Y.-Z. Full-Biobased Nanofiber Membranes toward Decontamination of Wastewater Containing Multiple Pollutants. ACS Sustain. Chem. Eng. 2018, 6, 11783–11792. [Google Scholar] [CrossRef]
- Udomluck, N.; Koh, W.-G.; Lim, D.-J.; Park, H. Recent Developments in Nanofiber Fabrication and Modification for Bone Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 99. [Google Scholar] [CrossRef] [Green Version]
- Obaid, M.; Abdelkareem, M.A.; Kook, S.; Kim, H.-Y.; Hilal, N.; Ghaffour, N.; Kim, I.S. Breakthroughs in the fabrication of electrospun-nanofiber-supported thin film composite/nanocomposite membranes for the forward osmosis process: A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1727–1795. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Park, M.J.; Tijing, L.; Han, D.S.; Phuntsho, S.; Shon, H.K. Modification of Nanofiber Support Layer for Thin Film Composite forward Osmosis Membranes via Layer-by-Layer Polyelectrolyte Deposition. Membranes 2018, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.; Wang, Q.; Peng, S.; Ramakrishna, S.; Zhang, D.; Zhou, K. Electrospun Inorganic Nanofibers for Oxygen Electrocatalysis: Design, Fabrication, and Progress. Adv. Energy Mater. 2020, 10, 1902115. [Google Scholar] [CrossRef]
- Hammad, M.; Fortugno, P.; Hardt, S.; Kim, C.; Salamon, S.; Schmidt, T.C.; Wende, H.; Schulz, C.; Wiggers, H. Large-scale synthesis of iron oxide/graphene hybrid materials as highly efficient photo-Fenton catalyst for water remediation. Environ. Technol. Innov. 2021, 21, 101239. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, W.; Feng, W.; Wang, Y.; Liu, S.; Dong, Z.; Li, X. A critical review on metal complexes removal from water using methods based on Fenton-like reactions: Analysis and comparison of methods and mechanisms. J. Hazard. Mater. 2021, 414, 125517. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.M.P.; Pereira-Queiroz, N.M.; Santos, D.H.S.; Nascimento, J.R.; de Carvalho, C.M.; Tonholo, J.; Zanta, C.L.P.S. Printing ink effluent remediation: A comparison between electrochemical and Fenton treatments. J. Water Process. Eng. 2019, 31, 100803. [Google Scholar] [CrossRef]
- Yang, X.; Chen, W.; Huang, J.; Zhou, Y.; Zhu, Y.; Li, C. Rapid degradation of methylene blue in a novel heterogeneous Fe3O4 @rGO@TiO2-catalyzed photo-Fenton system. Sci. Rep. 2015, 5, 10632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raji, M.; Mirbagheri, S.A.; Ye, F.; Dutta, J. Nano zero-valent iron on activated carbon cloth support as Fenton-like catalyst for efficient color and COD removal from melanoidin wastewater. Chemosphere 2021, 263, 127945. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, S.; Huang, G.; Liu, Y.; Yang, G.; Lin, C.; Xiao, G.; Wang, Y.; Liu, M. Remediation of organic arsenic contaminants with heterogeneous Fenton process mediated by SiO2-coated nano zero-valent iron. Environ. Sci. Pollut. Res. 2020, 27, 12017–12029. [Google Scholar] [CrossRef] [PubMed]











| Polymer | Abbreviation | Chemical Structure |
|---|---|---|
| Polyethylene | UPE, HDPE |  |
| Polypropylene | PP |  |
| Polyvinylidene fluoride | PVDF |  |
| Polytetrafluoroethylene | PTFE |  |
| Polyacrylonitrile | PAN |  |
| Polyethersulfone | PES |  |
| Polycarbonate | PC |  |
| Nylon 6 | Ny6 |  |
| Nylon 6,6 | Ny6,6 |  |
| Polymer | Chemical Structure | Derivation | Ref. |
|---|---|---|---|
| Cellulose acetate 1 |  | Wood pulp | [73] |
| Alginate 2 |  | Brown algae | [74] |
| Chitosan 3 |  | Crustacean shells | [75] |
| Pectin |  | Dried citrus peels or apple pomace | [76] |
| Carrageenan 4 |  | Red seaweed | [77] |
| Polylactic acid 5 |  | Corn starch, sugarcane, and other biomasses | [78] |
| System | Preparation Method | Filtration Process | Pollutant Treated | Filtration Performances 1 | Ref. |
|---|---|---|---|---|---|
| CS/PVA/MMT 2 | Non-solvent-induced phase inversion | Dead-end | Chromium |
| [79] |
| Fe–Al–Mn@CS CA-based | Phase inversion | Cross-flow | Fluoride anions |
| [80] |
| CS/GO 3 | Casting and solvent evaporation | Pervaporation | High-salinity water |
| [81] |
| TiO2-COOH/CaAlg | Non-solvent-induced phase inversion | Cross-flow | Organic dyes |
| [82] |
| PES blended PVA-GO-NaAlg | Phase inversion by immersion precipitation | Dead-end | Organic dyes |
| [83] |
| MWCNTs/chitosan-carrageenan 4 | Vacuum filtration | Dead-end | Heavy metals (Cu2+, Cd2+, Co2+, Ni2+, Ba2+, and Pb2+) |
| [84] |
| System | Coated Membrane | Preparation Method | Filtration Process | Pollutant Treated | Filtration Performances 1 | Ref. |
|---|---|---|---|---|---|---|
| ĸ-carrageenan/laponite | h-PAN | Layer-by-layer | Dead-end | Motor oil, metal ions, BB, RB 2 |
| [121] |
| CS, polyethyleneimine, GO | Cellulose | Dip-Coating | Batch filtration | Cr(VI) and Cu(II) |
| [122] |
| Chitosan-AlFu MOF 3 | Cellulose acetate | Film coating | Forward osmosis cross-flow filtration | COD, NH4-N, NO3-N and PO4 |
| [123] |
| CS-NaAlg Fe0@WO3 NPs | PES | Layer-by-layer | Cross-flow | Cr(VI) |
| [124] |
| k-Cg/GO | UA-60 | Film coating | Dead-end | Divalent ions |
| [125] |
| Catechol/CS | PVDF | Oxidant-induced ultrafast co-deposition | Dead-end | n-hexadecane, peanut oil, and crude oil water emulsions |
| [126] |
| Polymers | Doping Agent | Support | Filtration Process | Pollutant Treated | Filtration Performances 1 | Ref. |
|---|---|---|---|---|---|---|
| CS/PEO 2 | Cu2+ | TEMPO-oxidized cellulose | Dead-end | Escherichia coli and Bacillus subtilis |
| [151] |
| CS | FeOOH/g-C3N4 particles | PAN | Dead-end | MB, ERY 3 |
| [152] |
| CS/PAN | UiO-66-NH2 | PVDF nanofibrous sublayer | Cross-flow | Pb2+, Cd2+, Cr6+ |
| [153] |
| CaAlg | CNTs | Polyhydroxybutyrate nanofibers | Custom filtration device | BB, DOS, PR, HY, and SY 4 |
| [154] |
| CS/PVP | CNTs | CS/PVP/PVA | Laboratory-scale pressure-driven membrane filtration system | Cu2+, Ni2+, Cd2+, Pb2+, MG, MB and CV 5 |
| [155] |
| PLA | β-cyclodextrin | - | Dead-end | Toluene-in-water emulsions, MB, OG 6 |
| [156] |
| System | Advantages | Disadvantages |
|---|---|---|
| Mixed-matrix membranes |
|
|
| Functional coated membranes |
|
|
| Electrospun nanofiber membranes |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rando, G.; Sfameni, S.; Plutino, M.R. Development of Functional Hybrid Polymers and Gel Materials for Sustainable Membrane-Based Water Treatment Technology: How to Combine Greener and Cleaner Approaches. Gels 2023, 9, 9. https://doi.org/10.3390/gels9010009
Rando G, Sfameni S, Plutino MR. Development of Functional Hybrid Polymers and Gel Materials for Sustainable Membrane-Based Water Treatment Technology: How to Combine Greener and Cleaner Approaches. Gels. 2023; 9(1):9. https://doi.org/10.3390/gels9010009
Chicago/Turabian StyleRando, Giulia, Silvia Sfameni, and Maria Rosaria Plutino. 2023. "Development of Functional Hybrid Polymers and Gel Materials for Sustainable Membrane-Based Water Treatment Technology: How to Combine Greener and Cleaner Approaches" Gels 9, no. 1: 9. https://doi.org/10.3390/gels9010009
APA StyleRando, G., Sfameni, S., & Plutino, M. R. (2023). Development of Functional Hybrid Polymers and Gel Materials for Sustainable Membrane-Based Water Treatment Technology: How to Combine Greener and Cleaner Approaches. Gels, 9(1), 9. https://doi.org/10.3390/gels9010009