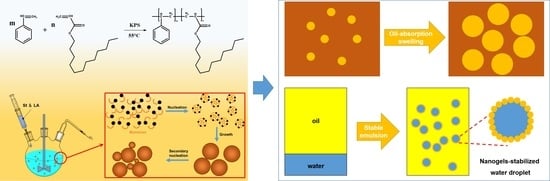
Styrene-Lauryl Acrylate Rubber Nanogels as a Plugging Agent for Oil-Based Drilling Fluids with the Function of Improving Emulsion Stability
Abstract
1. Introduction
2. Results and Discussion
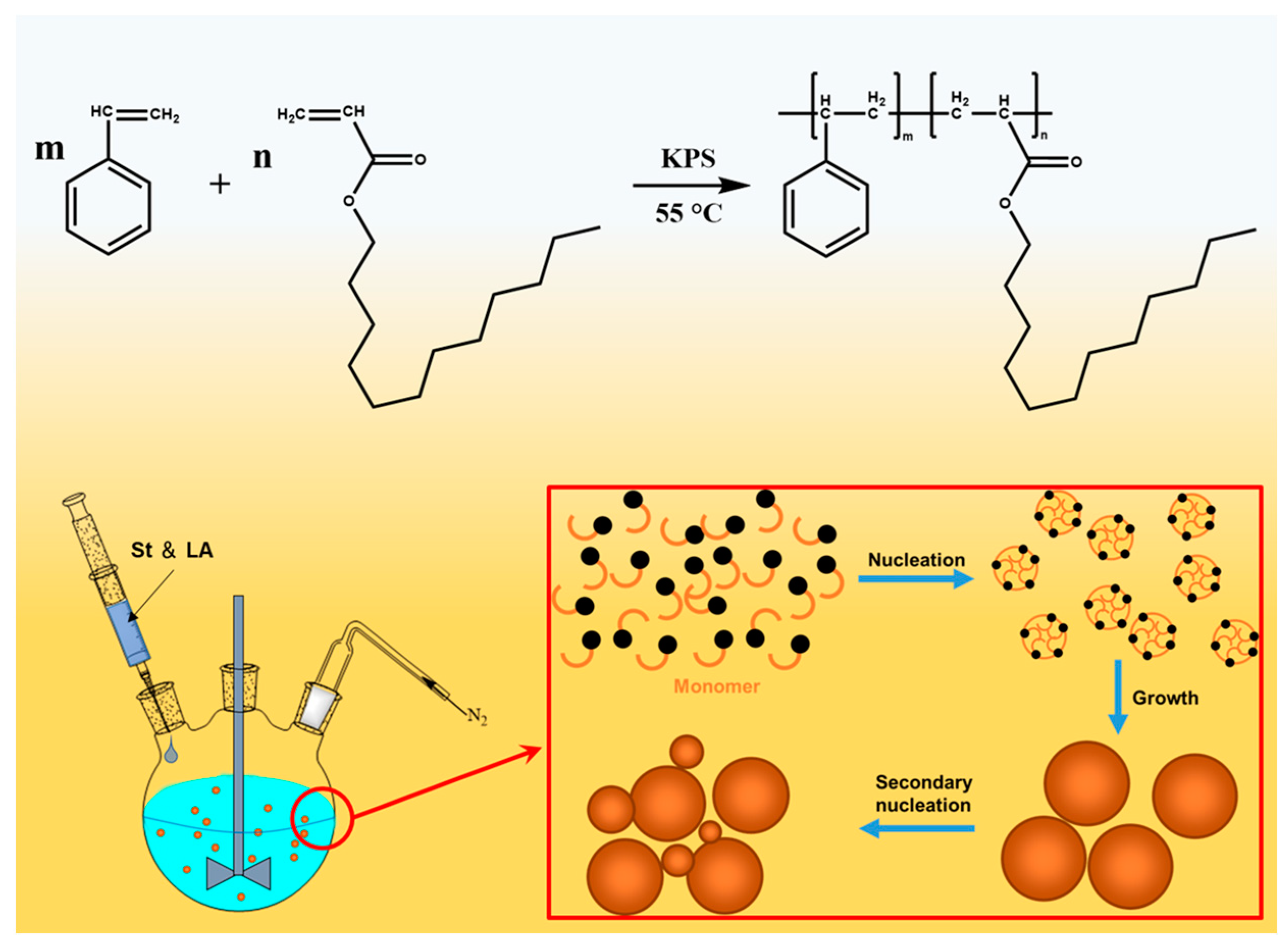
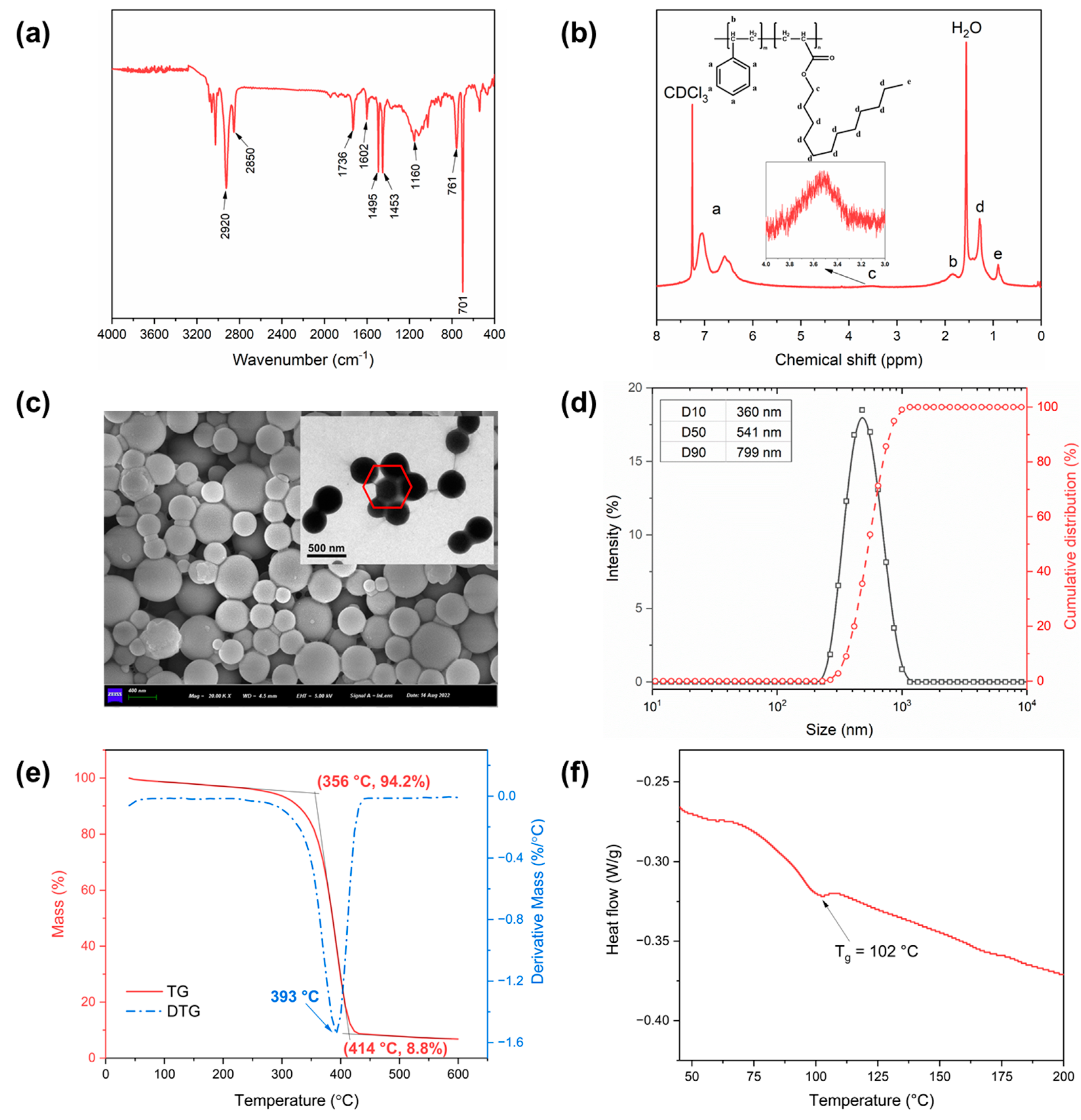
2.1. Characterization of PSL Rubber Nanogels
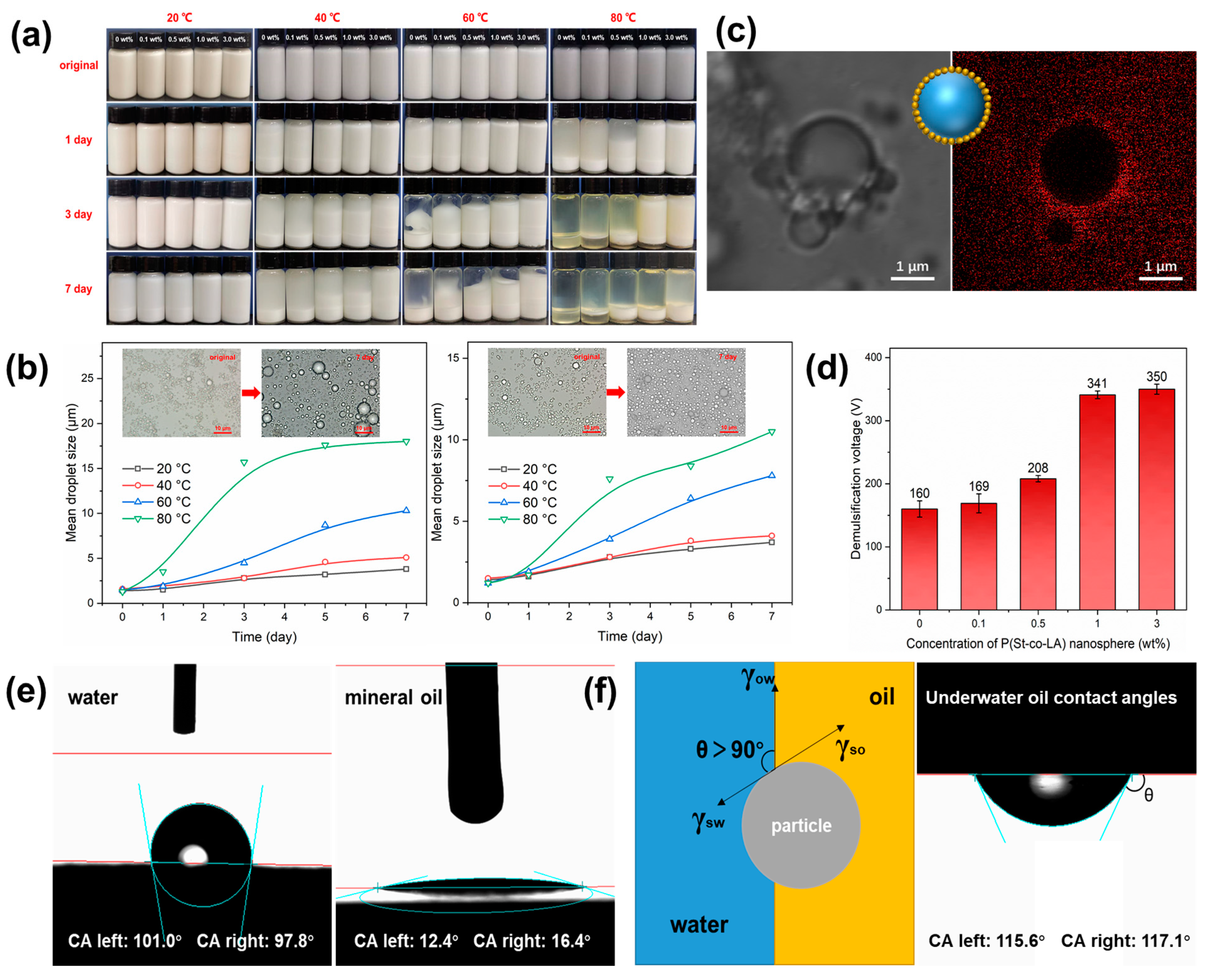
2.2. Dispersion Stability of PSL Rubber Nanogels
2.3. Swelling Behavior of PSL Rubber Nanogels
2.4. Enhancement of Emulsion Stability
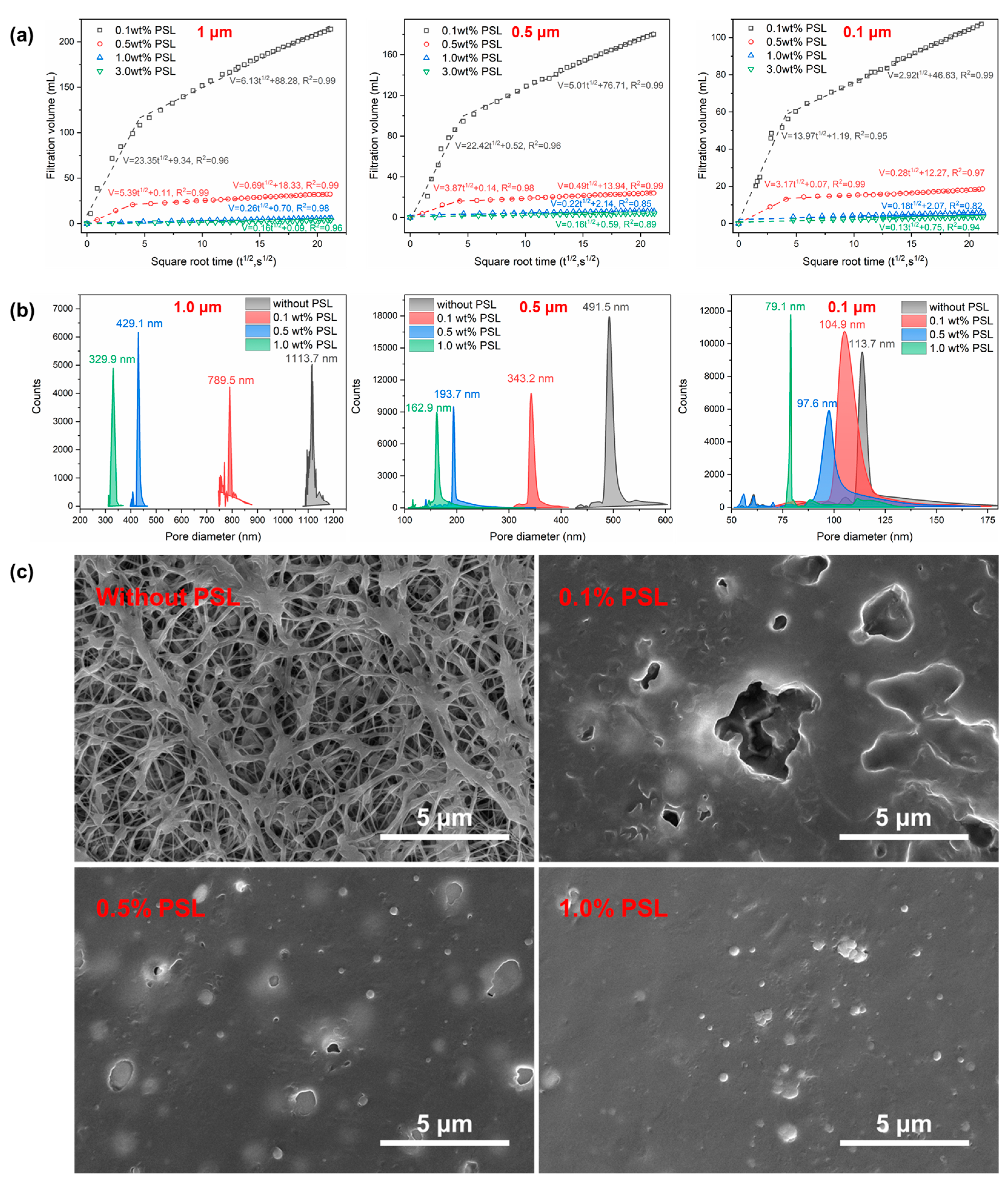
2.5. Plugging Performance of PSL
2.6. Applied in Oil-Based Drilling Fluid
2.6.1. Foaming Tests
2.6.2. Drilling Fluid Properties
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.3. Synthesis of PSL Rubber Nanogels
4.4. Characterization Methods of PSL
4.5. Preparation of Fluid
4.5.1. Reparation of Nonaqueous Suspension of PSL Rubber Nanogels
4.5.2. Reparation of W/O Emulsions
4.5.3. Preparation of Oil-Based Drilling Fluid
4.6. Dispersion Stability Tests
4.7. Swelling Behavior of PSL Nanogels in Mineral Oil
4.8. Emulsion Stability Test
4.9. Plugging Performance Tests
4.10. Compatibility with Oil Based Drilling Fluid
4.10.1. Foaming Tests
4.10.2. Drilling Fluid Properties
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Yang, Y. The current situation of shale gas in Sichuan, China. Renew. Sustain. Energy Rev. 2015, 50, 653–664. [Google Scholar]
- Wigwe, M.E.; Giussani, A.; Watson, M.C. Twelve years of unconventional oil and gas development: Production performance and economic analysis. Int. J. Energy Environ. Eng. 2021, 12, 151–174. [Google Scholar] [CrossRef]
- Sun, C.; Nie, H.; Dang, W.; Chen, Q.; Zhang, G.; Li, W.; Lu, Z. Shale Gas Exploration and Development in China: Current Status, Geological Challenges, and Future Directions. Energy Fuels 2021, 35, 6359–6379. [Google Scholar] [CrossRef]
- Bol, G.M.; Wong, S.-W.; Davidson, C.J.; Woodland, D.C. Borehole Stability in Shales. SPE Drill. Compl. 1994, 9, 87–94. [Google Scholar] [CrossRef]
- Wilson, M.J.; Wilson, L. Clay mineralogy and shale instability: An alternative conceptual analysis. Clay Miner. 2018, 49, 127–145. [Google Scholar] [CrossRef]
- Chen, G.; Chenevert, M.E.; Sharma, M.M.; Yu, M. A study of wellbore stability in shales including poroelastic, chemical, and thermal effects. J. Pet. Sci. Eng. 2003, 38, 167–176. [Google Scholar] [CrossRef]
- Díaz-Pérez, A.; Cortés-Monroy, I.; Roegiers, J.C. The role of water/clay interaction in the shale characterization. J. Pet. Sci. Eng. 2007, 58, 83–98. [Google Scholar]
- Liu, J.; Dai, Z.; Li, C.; Lv, K.; Huang, X.; Sun, J.; Wei, B. Inhibition of the Hydration Expansion of Sichuan Gas Shale by Adsorption of Compounded Surfactants. Energy Fuels 2019, 33, 6020–6026. [Google Scholar]
- Tian, Z.; Wei, W.; Zhou, S.; Wood, D.A.; Cai, J. Experimental and Fractal Characterization of the Microstructure of Shales from Sichuan Basin, China. Energy Fuels 2021, 35, 3899–3914. [Google Scholar] [CrossRef]
- He, S.; Li, H.; Qin, Q.; Long, S. Influence of Mineral Compositions on Shale Pore Development of Longmaxi Formation in the Dingshan Area, Southeastern Sichuan Basin, China. Energy Fuels 2021, 35, 10551–10561. [Google Scholar] [CrossRef]
- Wang, B.; Sun, J.; Shen, F.; Li, W.; Zhang, W. Mechanism of wellbore instability in continental shale gas horizontal sections and its water-based drilling fluid countermeasures. Nat. Gas Ind. B 2020, 7, 680–688. [Google Scholar] [CrossRef]
- Gao, D.; Xie, J.; Huang, S.; Wu, S.; Wu, P.; Huang, W.; Sheng, G. Research and Application of Evaluation Methods for Functional Characteristics of Oil-Based Drilling Fluid in Shale Gas Wells. Geofluids 2021, 2021, 8814032. [Google Scholar] [CrossRef]
- Li, X.; Yan, X.; Kang, Y. Investigation of drill-in fluids damage and its impact on wellbore stability in Longmaxi shale reservoir. J. Pet. Sci. Eng. 2017, 159, 702–709. [Google Scholar] [CrossRef]
- Geng, Y.; Sun, J.; Wang, J.; Wang, R.; Yang, J.; Wang, Q.; Ni, X. Modified Nanopolystyrene as a Plugging Agent for Oil-Based Drilling Fluids Applied in Shale Formation. Energy Fuels 2021, 35, 16543–16552. [Google Scholar] [CrossRef]
- Liu, J.; Guo, B.; Chai, J.; Wang, X. Development and Evaluation of Novel Loss Prevention Materials for Oil-Based Drilling Fluids. Int. J. Earth Sci. Eng. 2016, 9, 2609–2616. [Google Scholar]
- Tang, X.; Yang, H.; Gao, Y.; Lashari, Z.A.; Cao, C.; Kang, W. Preparation of a micron-size silica-reinforced polymer microsphere and evaluation of its properties as a plugging agent. Colloids Surf. A Physicochem. Eng. Asp. 2018, 547, 8–18. [Google Scholar] [CrossRef]
- Yan, X.; Kang, Y.; You, L. Wellbore Instability Induced by the Coupling of High-pH Fluid–Shale Reaction and Fracture Surface Sliding in Shale Gas Wells: Experimental and Field Studies. Energy Fuels 2020, 34, 5578–5588. [Google Scholar] [CrossRef]
- Deng, S.; Huang, Y.; Hu, X.; Wang, H.; Zhao, H.; He, J. Nano-Film-Forming Plugging Drilling Fluid and Bridging Cross-Linking Plugging Agent Are Used to Strengthen Wellbores in Complex Formations. ACS Omega 2022, 7, 22804–22810. [Google Scholar] [CrossRef]
- Guo, H.; Voncken, J.; Opstal, T.; Dams, R.; Zitha, P.L. Investigation of the Mitigation of Lost Circulation in Oil-Based Drilling Fluids Using Additives. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 15–17 February 2012. [Google Scholar]
- Scorsone, J.T.; Sanders, M.W.; Patel, A.D. An Improved Oil-Based Chemical Gel System for Wellbore Stabilization. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Woodlands, TX, USA, 8–9 April 2009. [Google Scholar]
- An, Y.; Jiang, G.; Qi, Y.; Huang, X.; Shi, H. High-performance shale plugging agent based on chemically modified graphene. J. Nat. Gas Sci. Eng. 2016, 32, 347–355. [Google Scholar]
- Ma, C.; Li, L.; Lu, H.; Yuan, X.B.; Wang, G. Study on the Effect of Humic Acid Acetamide on the Rheological Properties of Diesel Oil-Based Drilling Fluids. Appl. Mech. Mater. 2014, 620, 449–452. [Google Scholar] [CrossRef]
- Akhtarmanesh, S.; Shahrabi, M.J.; Atashnezhad, A. Improvement of wellbore stability in shale using nanoparticles. J. Pet. Sci. Eng. 2013, 112, 290–295. [Google Scholar] [CrossRef]
- An, Y.; Jiang, G.; Qi, Y.; Ge, Q.; Zhang, L.; Ren, Y. Synthesis of nano-plugging agent based on AM/AMPS/NVP terpolymer. J. Pet. Sci. Eng. 2015, 135, 505–514. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, R.; Zhou, J.; Chen, H.; Tan, Y. A novel hyper-cross-linked polymer for high-efficient fluid-loss control in oil-based drilling fluids. Colloids Surf. A: Physicochem. Eng. Asp. 2021, 626, 127004. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Chen, Y.; Han, Z.; Xie, G. Synthesis of the Oil-Based Nanoblocker Poly(MMA-BMA-BA-St) and the Study of the Blocking Mechanism. ACS Omega 2022, 7, 40799–40806. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Ni, X.; Yang, L.; Li, W.; Li, Y.; Deng, Z. Synthesis of superamphiphobic nanofluid as a multi-functional additive in oil-based drilling fluid, especially the stabilization performance on the water/oil interface. Colloids Surf. A Physicochem. Eng. Asp. 2020, 588, 124385. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Yan, L.; Ji, Y. Preparation of a Novel Nano-Polymer as Plugging and Filtration Loss Agent for Oil-Based Drilling Fluids. Adv. Mater. Res. 2013, 807–809, 2602–2606. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, L.; He, J.; Luo, M.; Song, F. Slightly Amphiphilic Polymer Microspheres as an HTHP Fluid Loss Control Additive for Oil-Based Drilling Fluid. Arab. J. Sci. Eng. 2022. [Google Scholar] [CrossRef]
- Cheng, R.; Lei, Z.; Bai, Y.; Zhang, J.; Hao, H.; Xie, G. Preparation of the Tetrameric Poly(VS-St-BMA-BA) Nano-Plugging Agent and Its Plugging Mechanism in Water-Based Drilling Fluids. ACS Omega 2022, 7, 28304–28312. [Google Scholar] [CrossRef]
- Lei, M.; Huang, W.; Sun, J.; Shao, Z.; Chen, Z.; Chen, W. Synthesis and characterization of high-temperature self-crosslinking polymer latexes and their application in water-based drilling fluid. Powder Technol. 2021, 389, 392–405. [Google Scholar] [CrossRef]
- Yang, J.; Lei, Z.; Dong, B.; Ai, Z.; Peng, L.; Xie, G. Synthesis and Plugging Performance of Poly (MMA-BA-ST) as a Plugging Agent in Oil-Based Drilling Fluid. Energies 2022, 15, 7626. [Google Scholar] [CrossRef]
- Li, W.; Jiang, G.; Ni, X.; Li, Y.; Wang, X.; Luo, X. Styrene butadiene resin/nano-SiO2 composite as a water-and-oil-dispersible plugging agent for oil-based drilling fluid. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125245. [Google Scholar] [CrossRef]
- Li, L.; Ma, C.; Xu, X.; Li, S.; Zhang, J.; Li, Y. Novel plugging agent for oil-based drilling fluids to overcome the borehole instability problem in shale formations. IOP Conf. Ser. Mater. Sci. Eng. 2019, 479, 012103. [Google Scholar] [CrossRef]
- Xie, G.; Luo, P.; Deng, M.; Wang, Z. Nanoplugging Performance of Hyperbranched Polyamine as Nanoplugging Agent in Oil-Based Drilling Fluid. J. Nanomater. 2015, 16, 821910. [Google Scholar] [CrossRef]
- Buron, H.; Mengual, O.; Meunier, G.; Cayré, I.; Snabre, P. Optical characterization of concentrated dispersions: Applications to laboratory analyses and on-line process monitoring and control. Polym. Int. 2004, 53, 1205–1209. [Google Scholar] [CrossRef]
- Zhong, H.; Shen, G.; Qiu, Z.; Lin, Y.; Fan, L.; Xing, X.; Li, J. Minimizing the HTHP filtration loss of oil-based drilling fluid with swellable polymer microspheres. J. Pet. Sci. Eng. 2019, 172, 411–424. [Google Scholar] [CrossRef]
- Abismaïl, B.; Canselier, J.P.; Wilhelm, A.M.; Delmas, H.; Gourdon, C. Emulsification by ultrasound: Drop size distribution and stability. Ultrason. Sonochem. 1999, 6, 75–83. [Google Scholar] [CrossRef]
- Binks, B.P.; Whitby, C.P. Nanoparticle silica-stabilised oil-in-water emulsions: Improving emulsion stability. Colloids Surf. A Physicochem. Eng. Asp. 2005, 253, 105–115. [Google Scholar] [CrossRef]
- Ba geri, B.S.; Al-Mutairi, S.H.; Mahmoud, M.A. Different Techniques for Characterizing the Filter Cake. In Proceedings of the SPE Unconventional Gas Conference and Exhibition, Muscat, Oman, 28–30 January 2013. [Google Scholar]




| Sample | Original Volume (mL) | Final Volume (mL) | Foaming Rate (%) |
|---|---|---|---|
| Without SDS | 300 | 310 | 3.33 |
| With 0.01 wt% SDS | 300 | 440 | 46.67 |
| Type | Condition | AV/mPa·s | PV/mPa·s | YP/Pa | Gel/(Pa/Pa) | FLAPI/mL | FLHTHP/mL |
|---|---|---|---|---|---|---|---|
| Original OBDF | Before aging | 32 | 28 | 4 | 4/13 | 3.2 | ---- |
| After aging | 39 | 34 | 5 | 5/14 | 3.6 | 10.2 | |
| With 3% PSL | Before aging | 39 | 34 | 5 | 5/15 | 2.4 | ---- |
| After aging | 41 | 36 | 5 | 5/15 | 2.8 | 7.8 | |
| With 3% NS | Before aging | 48 | 42 | 6 | 6/17 | 2.8 | ---- |
| After aging | 45 | 40 | 5 | 5/16 | 3.6 | 9.8 | |
| With 3% PS | Before aging | 38 | 34 | 4 | 4/11 | 2.4 | ---- |
| After aging | 41 | 37 | 4 | 3/8 | 4.2 | 9.2 |
| Adding Order | Component | Function | Amount |
|---|---|---|---|
| 1 | Mineral oil | Continuous phase | 240 mL |
| 2 | Modified fatty acid | Primary emulsifier | 9.0 g |
| 3 | Span 80 | Secondary emulsifier | 6.0 g |
| 4 | Organoclay | Adjust rheology | 3.0 g |
| 5 | Lecithin | Wetting agent | 5.4 g |
| 6 | Oxidized asphalt | Fluid loss reducer | 6.0 g |
| 7 | Brine (20 wt% CaCl2 solution) | Dispersed phase | 60 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Lv, K.; Sun, J.; Huang, X.; Shen, H. Styrene-Lauryl Acrylate Rubber Nanogels as a Plugging Agent for Oil-Based Drilling Fluids with the Function of Improving Emulsion Stability. Gels 2023, 9, 23. https://doi.org/10.3390/gels9010023
Du H, Lv K, Sun J, Huang X, Shen H. Styrene-Lauryl Acrylate Rubber Nanogels as a Plugging Agent for Oil-Based Drilling Fluids with the Function of Improving Emulsion Stability. Gels. 2023; 9(1):23. https://doi.org/10.3390/gels9010023
Chicago/Turabian StyleDu, Hongyan, Kaihe Lv, Jinsheng Sun, Xianbin Huang, and Haokun Shen. 2023. "Styrene-Lauryl Acrylate Rubber Nanogels as a Plugging Agent for Oil-Based Drilling Fluids with the Function of Improving Emulsion Stability" Gels 9, no. 1: 23. https://doi.org/10.3390/gels9010023
APA StyleDu, H., Lv, K., Sun, J., Huang, X., & Shen, H. (2023). Styrene-Lauryl Acrylate Rubber Nanogels as a Plugging Agent for Oil-Based Drilling Fluids with the Function of Improving Emulsion Stability. Gels, 9(1), 23. https://doi.org/10.3390/gels9010023