Effect of Blood Gel Derivatives on Wound Healing in Mouse Injured Tissue Models
Abstract
:1. Introduction
2. Results and Discussion
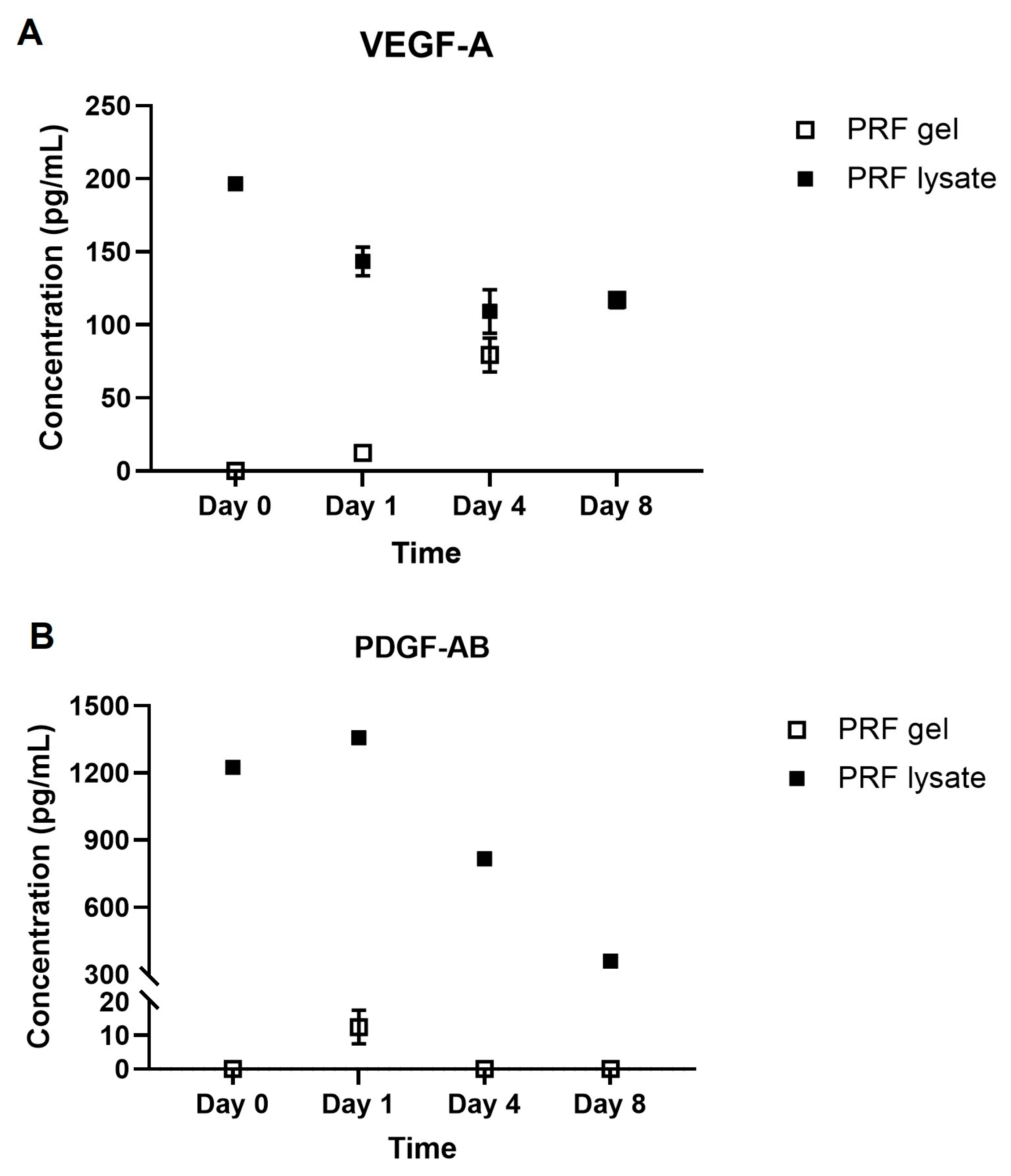
2.1. Growth Factors Quantification
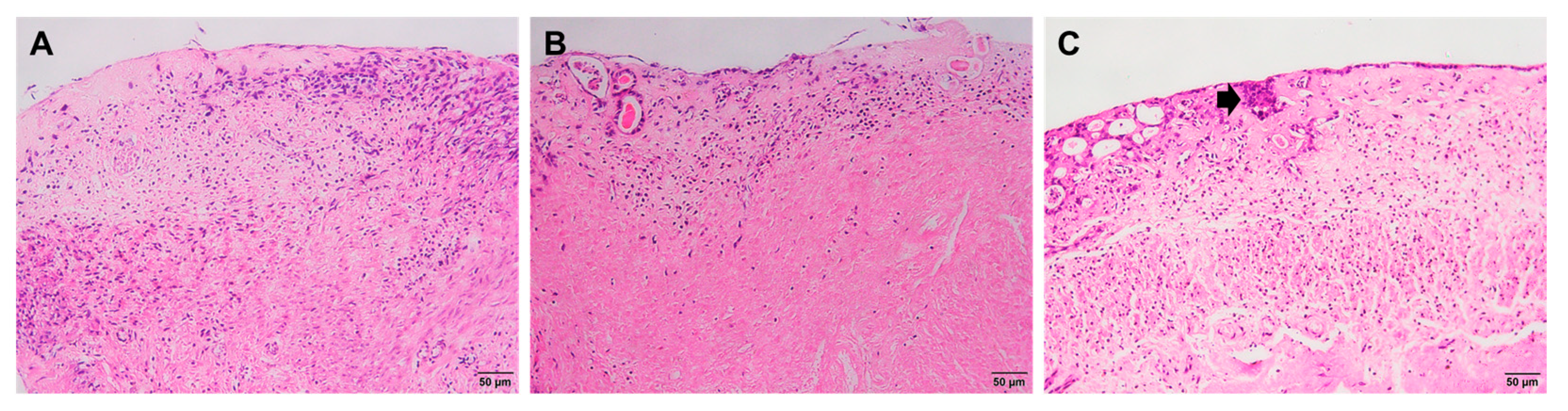
2.2. Histological Staining
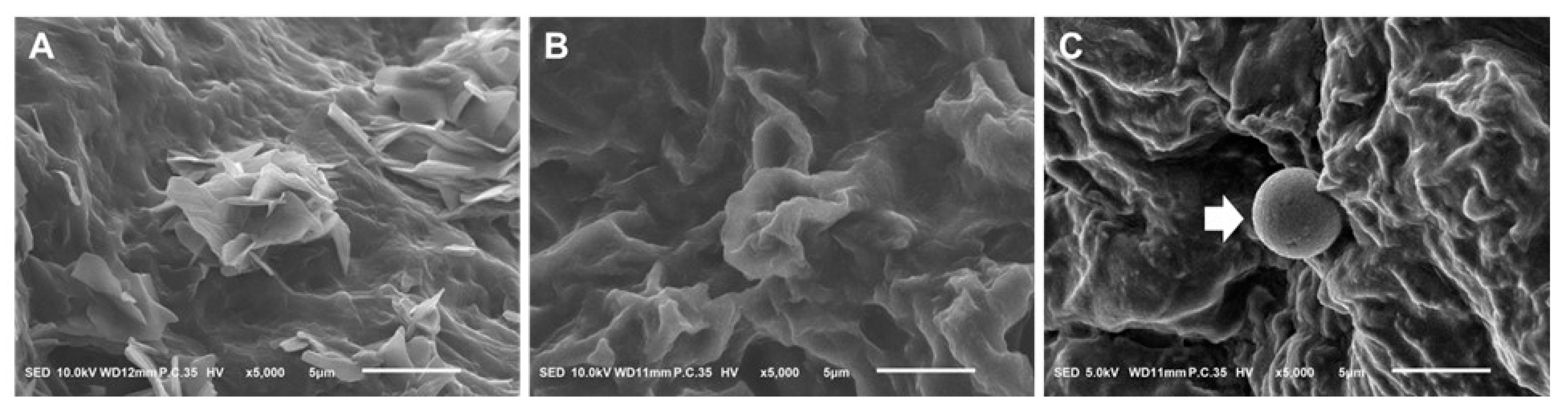
2.3. Scanning Electron Microscope
2.4. Embryo Implantation
3. Conclusions
4. Materials and Methods
4.1. Animals
4.2. PRF Gel and PRF Lysate Preparation
4.3. Growth Factor Quantification
4.4. Establishment of Mouse Injured Tissue Models
4.5. PRF Gel and PRF Lysate Treatment on Mouse Injured Tissue Models
4.6. Histological Staining
4.7. Scanning Electron Microscope
4.8. Embryo Implantation
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, Y.; Tan, D.; He, M.; Gu, M.; Wang, Z.; Zeng, G.; Duan, E. A model for implantation: Coculture of blastocysts and uterine endometrium in mice. Biol. Reprod. 2005, 72, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J. Endometrial intravascular thrombi are typically associated with shedding but may be the sentinel feature of an underlying thrombotic disorder. Histopathology 2020, 76, 919–922. [Google Scholar] [CrossRef] [PubMed]
- March, C.M. Management of Asherman’s syndrome. Reprod. BioMed. Online 2011, 23, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, H.; Zhu, H.; Zhu, X.; Tang, X.; Yan, G.; Wang, J.; Bai, D.; Wang, J.; Wang, L. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: A phase I clinical trial. Stem Cell Res. Ther. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Santamaria, X.; Cabanillas, S.; Cervelló, I.; Arbona, C.; Raga, F.; Ferro, J.; Palmero, J.; Remohí, J.; Pellicer, A.; Simón, C. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: A pilot cohort study. Hum. Reprod. 2016, 31, 1087–1096. [Google Scholar] [CrossRef]
- Marsa, R.D.; Asrianti, D.; Margono, A. The efficacy of platelet-rich fibrin lysate (PRF-L) for fibroblast cell proliferation. J. Int. Dent. Med. Res. 2017, 10, 809–813. [Google Scholar]
- Meidyawati, R.; Suprastiwi, E. The Ability of Lysate-PRF Induces Proliferation of Fibroblast Cells in Endodontic Regenerative Therapy. Open J. Stomatol. 2018, 8, 182–187. [Google Scholar] [CrossRef]
- Saluja, H.; Dehane, V.; Mahindra, U. Platelet-Rich fibrin: A second generation platelet concentrate and a new friend of oral and maxillofacial surgeons. Ann. Maxillofac. Surg. 2011, 1, 53. [Google Scholar] [CrossRef]
- Hanstede, M.M.; Van Der Meij, E.; Goedemans, L.; Emanuel, M.H. Results of centralized Asherman surgery, 2003–2013. Fertil. Steril. 2015, 104, 1561–1568.e1. [Google Scholar] [CrossRef]
- Karimi, K.; Rockwell, H. The benefits of platelet-rich fibrin. Facial Plast. Surg. Clin. 2019, 27, 331–340. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.; Schoeffler, C.; Dohan, S.; Dohan, A.; Mouhyi, J.; Dohan, D. A second-generation platelet concentrate. Part V: Histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 101, 299–303. [Google Scholar] [CrossRef]
- Lekovic, V.; Milinkovic, I.; Aleksic, Z.; Jankovic, S.; Stankovic, P.; Kenney, E.; Camargo, P. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J. Periodontal Res. 2012, 47, 409–417. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, N.D.; Jain, A. Platelet rich fibrin combined with decalcified freeze-dried bone allograft for the treatment of human intrabony periodontal defects: A randomized split mouth clinical trail. Acta Odontol. Scand. 2016, 74, 36–43. [Google Scholar] [CrossRef]
- Grecu, A.F.; Reclaru, L.; Ardelean, L.C.; Nica, O.; Ciucă, E.M.; Ciurea, M.E. Platelet-rich fibrin and its emerging therapeutic benefits for musculoskeletal injury treatment. Medicina 2019, 55, 141. [Google Scholar] [CrossRef] [PubMed]
- Soffer, E.; Ouhayoun, J.P.; Anagnostou, F. Fibrin sealants and platelet preparations in bone and periodontal healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2003, 95, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Sultan, T.; Cheah, C.W.; Ibrahim, N.B.; Asif, M.K.; Vaithilingam, R.D. Three-dimensional assessment of the extraction sockets, augmented with platelet-rich fibrin and calcium sulfate: A clinical pilot study. J. Dent. 2020, 101, 103455. [Google Scholar] [CrossRef] [PubMed]
- Beitzel, K.; McCarthy, M.B.; Cote, M.P.; Russell, R.P.; Apostolakos, J.; Ramos, D.M.; Kumbar, S.G.; Imhoff, A.B.; Arciero, R.A.; Mazzocca, A.D. Properties of biologic scaffolds and their response to mesenchymal stem cells. Arthrosc. J. Arthrosc. Relat. Surg. 2014, 30, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Trandafilovic, M.; Stojanovic, P. Platelet-rich fibrin: Basics of biological actions and protocol modifications. Open Med. 2021, 16, 446–454. [Google Scholar] [CrossRef]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef]
- Strandberg, G.; Sellberg, F.; Sommar, P.; Ronaghi, M.; Lubenow, N.; Knutson, F.; Berglund, D. Standardizing the freeze-thaw preparation of growth factors from platelet lysate. Transfusion 2017, 57, 1058–1065. [Google Scholar] [CrossRef]
- Bai, W.; Mao, L.; Wang, X.; Sun, Y.; Yang, M.; Chen, X.; Cui, L. Platelet-rich fibrin improves repair and regeneration of damaged endometrium in rats. Front. Endocrinol. 2023, 14, 1154958. [Google Scholar]
- Wang, Y.; Chen, X.; Mao, L.; Cui, L.; Bai, W. Therapeutic Effect of Platelet-Rich Fibrin Transplant on Formation of Thin Endometrium. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2021, 19, 600–608. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, M.; Mao, L.; Wang, X.; Wang, S.; Cui, G.; Hou, Z.; Yang, M.; Cui, L.; Bai, W. Efficacy and safety of autologous platelet-rich fibrin for the treatment of infertility with intrauterine adhesions. J. Obstet. Gynaecol. Res. 2021, 47, 3883–3894. [Google Scholar] [CrossRef] [PubMed]
- Weimar, C.H.; Uiterweer, E.D.P.; Teklenburg, G.; Heijnen, C.J.; Macklon, N.S. In-vitro model systems for the study of human embryo–endometrium interactions. Reprod. Biomed. Online 2013, 27, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Torry, D.S.; Holt, V.J.; Keenan, J.A.; Harris, G.; Caudle, M.R.; Torry, R.J. Vascular endothelial growth factor expression in cycling human endometrium. Fertil. Steril. 1996, 66, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Jiang, H.; Lee, K.; Tang, P.; Chow, P. Expression of vascular endothelial growth factor (VEGF) and its receptors during embryonic implantation in the golden hamster (Mesocricetus auratus). Cell Tissue Res. 1999, 296, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yi, H.; Li, T.C.; Wang, Y.; Wang, H.; Chen, X. Role of vascular endothelial growth factor (VEGF) in human embryo implantation: Clinical implications. Biomolecules 2021, 11, 253. [Google Scholar] [CrossRef]
- Matsumoto, H.; Nasu, K.; Nishida, M.; Ito, H.; Bing, S.; Miyakawa, I. Regulation of proliferation, motility, and contractility of human endometrial stromal cells by platelet-derived growth factor. J. Clin. Endocrinol. Metab. 2005, 90, 3560–3567. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; de Peppo, G.M.; Doglioli, P.; Sammartino, G. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): A gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors 2009, 27, 63–69. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-rich fibrin and soft tissue wound healing: A systematic review. Tissue Eng. Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef]
- Sahni, A.; Francis, C.W. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood J. Am. Soc. Hematol. 2000, 96, 3772–3778. [Google Scholar] [CrossRef]
- Wang, X.; Fok, M.R.; Pelekos, G.; Jin, L.; Tonetti, M.S. In Vitro and Ex Vivo Kinetic Release Profile of Growth Factors and Cytokines from Leucocyte-and Platelet-Rich Fibrin (L-PRF) Preparations. Cells 2022, 11, 2089. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.T.; Kurman, R.J. Normal endometrium and infertility evaluation. In Diagnosis of Endometrial Biopsies and Curettings; Springer: Berlin/Heidelberg, Germany, 2005; pp. 7–33. [Google Scholar]
- Quinn, C.; Casper, R. Pinopodes: A questionable role in endometrial receptivity. Hum. Reprod. Update 2009, 15, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.E.; Matson, B.C.; Wetendorf, M.; Caron, K.M. Pinopodes: Recent advancements, current perspectives, and future directions. Mol. Cell. Endocrinol. 2020, 501, 110644. [Google Scholar] [CrossRef] [PubMed]
- Rarani, F.Z.; Borhani, F.; Rashidi, B. Endometrial pinopode biomarkers: Molecules and microRNAs. J. Cell. Physiol. 2018, 233, 9145–9158. [Google Scholar] [CrossRef]
- Fukui, Y.; Hirota, Y.; Matsuo, M.; Gebril, M.; Akaeda, S.; Hiraoka, T.; Osuga, Y. Uterine receptivity, embryo attachment, and embryo invasion: Multistep processes in embryo implantation. Reprod. Med. Biol. 2019, 18, 234–240. [Google Scholar] [CrossRef]
- Wallin, R.F. A Practical Guide to ISO 10993-12: Sample Preparation and Reference Materials; MDDI: Los Angeles, CA, USA, 1998. [Google Scholar]

| Time | Groups | ||
|---|---|---|---|
| Non-Treated (Control) | PRF Gel-Treated | PRF Lysate-Treated | |
| Day 4 |
|
| |
| Day 8 | |||
| Day 12 | |||
| Day 16 | − Degradation of endometrial tissues (Figure 2(A4,B4,C4)) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.T.V.; Lam, H.M.; Nguyen, M.T.N.; Phan, N.T.H.; Huynh, T.N.K.; Le, H.N.T.; Pham, C.T.H.; Tang, V.K.H.; Hoang, T.T.T.; Hoang, T.T.D.; et al. Effect of Blood Gel Derivatives on Wound Healing in Mouse Injured Tissue Models. Gels 2023, 9, 785. https://doi.org/10.3390/gels9100785
Le TTV, Lam HM, Nguyen MTN, Phan NTH, Huynh TNK, Le HNT, Pham CTH, Tang VKH, Hoang TTT, Hoang TTD, et al. Effect of Blood Gel Derivatives on Wound Healing in Mouse Injured Tissue Models. Gels. 2023; 9(10):785. https://doi.org/10.3390/gels9100785
Chicago/Turabian StyleLe, Tuyet Thi Vi, Hoang Minh Lam, My Thi Ngoc Nguyen, Nghia Thi Hieu Phan, Trang Nguyen Khanh Huynh, Hien Nguyen Trong Le, Chau Thi Hai Pham, Van Kim Hoang Tang, Trang Thi Thuy Hoang, Tuyet Thi Diem Hoang, and et al. 2023. "Effect of Blood Gel Derivatives on Wound Healing in Mouse Injured Tissue Models" Gels 9, no. 10: 785. https://doi.org/10.3390/gels9100785
APA StyleLe, T. T. V., Lam, H. M., Nguyen, M. T. N., Phan, N. T. H., Huynh, T. N. K., Le, H. N. T., Pham, C. T. H., Tang, V. K. H., Hoang, T. T. T., Hoang, T. T. D., & Tran, H. L. B. (2023). Effect of Blood Gel Derivatives on Wound Healing in Mouse Injured Tissue Models. Gels, 9(10), 785. https://doi.org/10.3390/gels9100785








