Development and Characterization of Econazole Topical Gel
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formulation of Econazole Topical Gel
2.2. Physical Characterization of the Formulated Gel
2.3. pH Determination
2.4. Determination of Viscosity
2.5. Spreadability
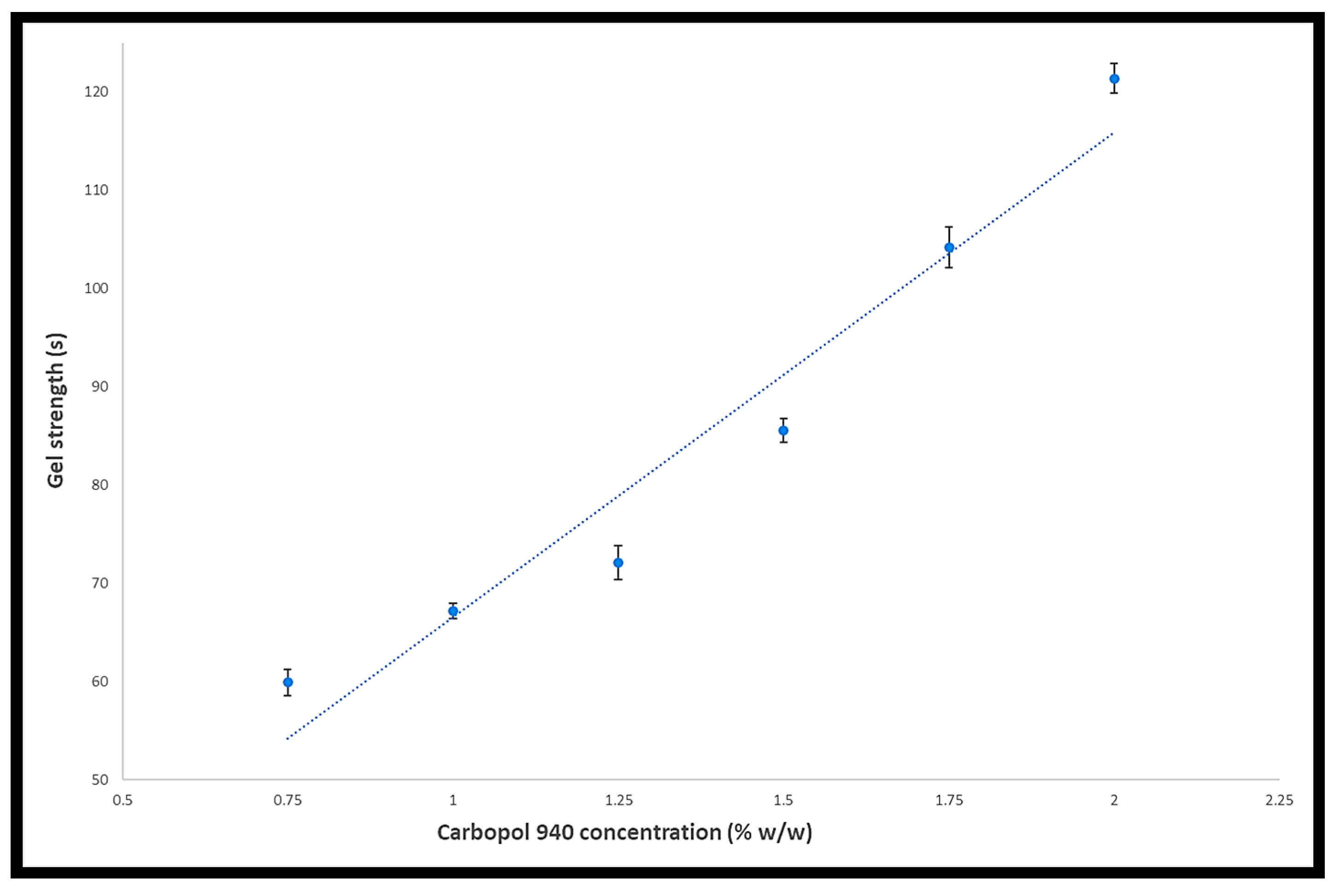
2.6. Gel Strength
2.7. Anti-Fungal Activity
2.8. Drug Content
2.9. In Vitro Release Studies
2.10. Stability Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Formulation of Econazole Topical Gel
4.2.2. Physical Characterization of the Produced Gel
4.2.3. Determination of pH
4.2.4. Viscosity Determination
4.2.5. Spreadability
4.2.6. Gel Strength
4.2.7. Anti-Fungal Activity
4.2.8. Drug Content
4.2.9. In Vitro Release Studies
4.2.10. Stability Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, A.K.; Venkataraman, M. Antifungal resistance in superficial mycoses. J. Dermatol. Treat. 2022, 33, 1888–1895. [Google Scholar] [CrossRef]
- Brown, E.M.; Clardy, J.; Xavier, R.J. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe 2023, 31, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yuan, P.; Sun, Y.; Xu, Y.; Deng, X.; Wang, X.; Liu, R.; Chen, Q.; Jiang, L. Comparison of topical antifungal agents for oral candidiasis treatment: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 282–291. [Google Scholar] [CrossRef]
- Masumoto, A.; Takagi, M.; Sugiura, K.; Matsuda, Y.; Nakamura, S.; Tatsumi, Y. A novel method for predicting the efficacy of topical drugs on onychomycosis: A comparison of efinaconazole and luliconazole. J. Med. Mycol. 2022, 32, 101259. [Google Scholar] [CrossRef] [PubMed]
- Maikan, H.K.; Jabbar, S.; Al-Haishawi, H. Isolation and Identification of Candida tropicalis as a Cause of Cutaneous Candidiasis in Kalar District, Iraq. Arch. Razi Inst. 2022, 77, 1377–1382. [Google Scholar]
- Reddy, G.K.; Padmavathi, A.R.; Nancharaiah, Y.V. Fungal infections: Pathogenesis, antifungals and alternate treatment approaches. Curr. Res. Microb. Sci. 2022, 3, 100137. [Google Scholar] [CrossRef] [PubMed]
- Chellathurai, B.J.; Anburose, R.; Alyami, M.H.; Sellappan, M.; Bayan, M.F.; Chandrasekaran, B.; Chidambaram, K.; Rahamathulla, M. Development of a Polyherbal Topical Gel for the Treatment of Acne. Gels 2023, 9, 163. [Google Scholar] [CrossRef]
- Cazan, C. Advances in Sustainable Polymeric Materials. Polymers 2022, 14, 4972. [Google Scholar] [CrossRef]
- Francavilla, A.; Corradini, M.G.; Joye, I.J. Bigels as Delivery Systems: Potential Uses and Applicability in Food. Gels 2023, 9, 648. [Google Scholar] [CrossRef]
- Bayan, M.F.; Jaradat, A.; Alyami, M.H.; Naser, A.Y. Smart Pellets for Controlled Delivery of 5-Fluorouracil. Molecules 2022, 28, 306. [Google Scholar] [CrossRef]
- Kuzina, M.A.; Kartsev, D.D.; Stratonovich, A.V.; Levkin, P.A. Organogels versus Hydrogels: Advantages, Challenges, and Applications. Adv. Funct. Mater. 2023, 33, 2301421. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Karavasili, C.; Wahane, A.; Freitas, D.; Booz, K.; Le, D.T.H.; Hua, T.; Scala, S.; Lopes, A.; Hess, K.; et al. Development of oil-based gels as versatile drug delivery systems for pediatric applications. Sci. Adv. 2022, 8, eabm8478. [Google Scholar] [CrossRef]
- Praestegaard, M.; Steele, F.; Crutchley, N. Polyaphron Dispersion Technology, A Novel Topical Formulation and Delivery System Combining Drug Penetration, Local Tolerability and Convenience of Application. Dermatol. Ther. 2022, 12, 2217–2231. [Google Scholar] [CrossRef] [PubMed]
- Witika, B.A.; Bassey, K.E.; Demana, P.H.; Siwe-Noundou, X.; Poka, M.S. Current Advances in Specialised Niosomal Drug Delivery: Manufacture, Characterization and Drug Delivery Applications. Int. J. Mol. Sci. 2022, 23, 9668. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bai, Y.; Sun, J.; Lv, K.; Han, J.; Dai, L. Experimental Study on Physicochemical Properties of a Shear Thixotropic Polymer Gel for Lost Circulation Control. Gels 2022, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Jain, S.; Abourehab, M.A.S.; Mehta, P.; Kesharwani, P. An insight on topically applied formulations for management of various skin disorders. J. Biomater. Sci. Polym. Ed. 2022, 33, 2406–2432. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.K.; Sadia, H.; Khan, M.I.; Omer, M.O.; Siddique, M.I.; Qamar, S.; Zaman, M.; Butt, M.H.; Mustafa, M.W.; Rasool, N. Development and characterization of niosomal gel of fusidic acid: In-vitro and ex-vivo approaches. Des. Monomers Polym. 2022, 25, 165–174. [Google Scholar] [CrossRef]
- Soto-Bustamante, F.; Valadez-Pérez, N.E.; Liu, Y.; Castañeda-Priego, R.; Laurati, M. Clusters in colloidal dispersions with a short-range depletion attraction: Thermodynamic identification and morphology. J. Colloid Interface Sci. 2022, 618, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, F.; Ashari, S.E.; Azmi, I.D.; Rahman, M.B. Recent advances in encapsulation of drug delivery (active substance) in cubosomes for skin diseases. J. Drug Deliv. Sci. Technol. 2022, 68, 103097. [Google Scholar] [CrossRef]
- Onugwu, A.L.; Nwagwu, C.S.; Onugwu, O.S.; Echezona, A.C.; Agbo, C.P.; Ihim, S.A.; Emeh, P.; Nnamani, P.O.; Attama, A.A.; Khutoryanskiy, V.V. Nanotechnology based drug delivery systems for the treatment of anterior segment eye diseases. J. Control. Release 2023, 354, 465–488. [Google Scholar] [CrossRef]
- Bayan, M.F.; Salem, M.S.; Bayan, R.F. Development and In Vitro Evaluation of a Large-Intestinal Drug Delivery System. Res. J. Pharm. Technol. 2022, 15, 35–39. [Google Scholar] [CrossRef]
- Sobral-Souza, D.F.; Gouveia, T.H.N.; Ortiz, M.I.G.; Condeles, A.L.; Junior, J.C.T.; Franz-Montan, M.; Aguiar, F.H.B.; Lima, D.A.N.L. Altered physical–chemical properties of home bleaching gels after an accelerated stability study and their effects on tooth enamel. Clin. Oral Investig. 2022, 26, 7229–7242. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.S.; Nawaz, A.; Asmari, M.; Uddin, J.; Ullah, H.; Ahmad, S. Formulation Development and In Vitro/In Vivo Characterization of Methotrexate-Loaded Nanoemulsion Gel Formulations for Enhanced Topical Delivery. Gels 2023, 9, 3. [Google Scholar] [CrossRef] [PubMed]





| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|
| Econazole nitrate | 1.00% | 1.00% | 1.00% | 1.00% | 1.00% | 1.00% |
| Capmul® MCM C8 | 5.00% | 5.00% | 5.00% | 5.00% | 5.00% | 5.00% |
| Carbopol® 940 | 0.75% | 1.00% | 1.25% | 1.50% | 1.75% | 2.00% |
| Propylene glycol | 20.00% | 20.00% | 20.00% | 20.00% | 20.00% | 20.00% |
| Methyl Paraben | 0.15% | 0.15% | 0.15% | 0.15% | 0.15% | 0.15% |
| Propyl paraben | 0.30% | 0.30% | 0.30% | 0.30% | 0.30% | 0.30% |
| Triethanolamine | 2.00% | 2.00% | 2.00% | 2.00% | 2.00% | 2.00% |
| Water (q. s) | 100% | 100% | 100% | 100% | 100% | 100% |
| Characteristics | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|
| Physical appearance | Transparent | Transparent | Transparent | Transparent | Transparent | Transparent |
| Color | Pale yellow | Pale yellow | Pale yellow | Pale yellow | Pale yellow | Pale yellow |
| Homogeneity | No aggregates | No aggregates | No aggregates | No aggregates | No aggregates | No aggregates |
| Formulation Code | pH | Viscosity (cps) | Spreadability (cm) | Gel Strength (s) |
|---|---|---|---|---|
| F1 | 6.1 ± 0.1 | 1341 ± 0.6 | 7.1 ± 0.2 | 59.9 ± 1.3 |
| F2 | 6.0 ± 0.1 | 1389 ± 0.9 | 6.5 ± 0.3 | 67.2 ± 0.8 |
| F3 | 6.1 ± 0.1 | 1432 ± 0.8 | 5.8 ± 0.2 | 72.1 ± 1.7 |
| F4 | 5.9 ± 0.2 | 1487 ± 0.5 | 5.3 ± 0.2 | 85.6 ± 1.2 |
| F5 | 6.2 ± 0.1 | 1515 ± 0.6 | 4.9 ± 0.1 | 104.2 ± 2.1 |
| F6 | 5.9 ± 0.1 | 1571 ± 0.8 | 4.4 ± 0.2 | 121.4 ± 1.5 |
| Sample | Aspergillus fumigatus | Candida albicans |
|---|---|---|
| Blank gel | - | - |
| F1 | 14.15 ± 1.16 | 16.47 ± 1.49 |
| F2 | 14.54 ± 1.34 | 16.22 ± 1.72 |
| F3 | 15.21 ± 1.02 | 16.63 ± 1.53 |
| F4 | 14.89 ± 1.44 | 16.45 ± 1.30 |
| F5 | 14.74 ± 1.68 | 16.88 ± 1.37 |
| F6 | 15.36 ± 1.67 | 16.21 ± 1.81 |
| Formulation | Drug Content (mg) |
|---|---|
| F1 | 0.99 ± 0.056 |
| F2 | 0.96 ± 0.030 |
| F3 | 0.98 ± 0.034 |
| F4 | 1.08 ± 0.035 |
| F5 | 1.02 ± 0.085 |
| F6 | 1.01 ± 0.081 |
| Formulation | Korsmeyer–Peppas R2 | n Value | Km |
|---|---|---|---|
| F1 | 0.853 | 0.570 | 0.240 |
| F2 | 0.873 | 0.578 | 0.214 |
| F3 | 0.909 | 0.597 | 0.186 |
| F4 | 0.956 | 0.588 | 0.157 |
| F5 | 0.985 | 0.588 | 0.133 |
| F6 | 0.986 | 0.585 | 0.115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayan, M.F.; Chandrasekaran, B.; Alyami, M.H. Development and Characterization of Econazole Topical Gel. Gels 2023, 9, 929. https://doi.org/10.3390/gels9120929
Bayan MF, Chandrasekaran B, Alyami MH. Development and Characterization of Econazole Topical Gel. Gels. 2023; 9(12):929. https://doi.org/10.3390/gels9120929
Chicago/Turabian StyleBayan, Mohammad F., Balakumar Chandrasekaran, and Mohammad H. Alyami. 2023. "Development and Characterization of Econazole Topical Gel" Gels 9, no. 12: 929. https://doi.org/10.3390/gels9120929
APA StyleBayan, M. F., Chandrasekaran, B., & Alyami, M. H. (2023). Development and Characterization of Econazole Topical Gel. Gels, 9(12), 929. https://doi.org/10.3390/gels9120929








