Effects of Counterion on the Formation and Hydration Behavior of α-Form Hydrated Crystals (α-Gels)
Abstract
:1. Introduction
2. Results and Discussion
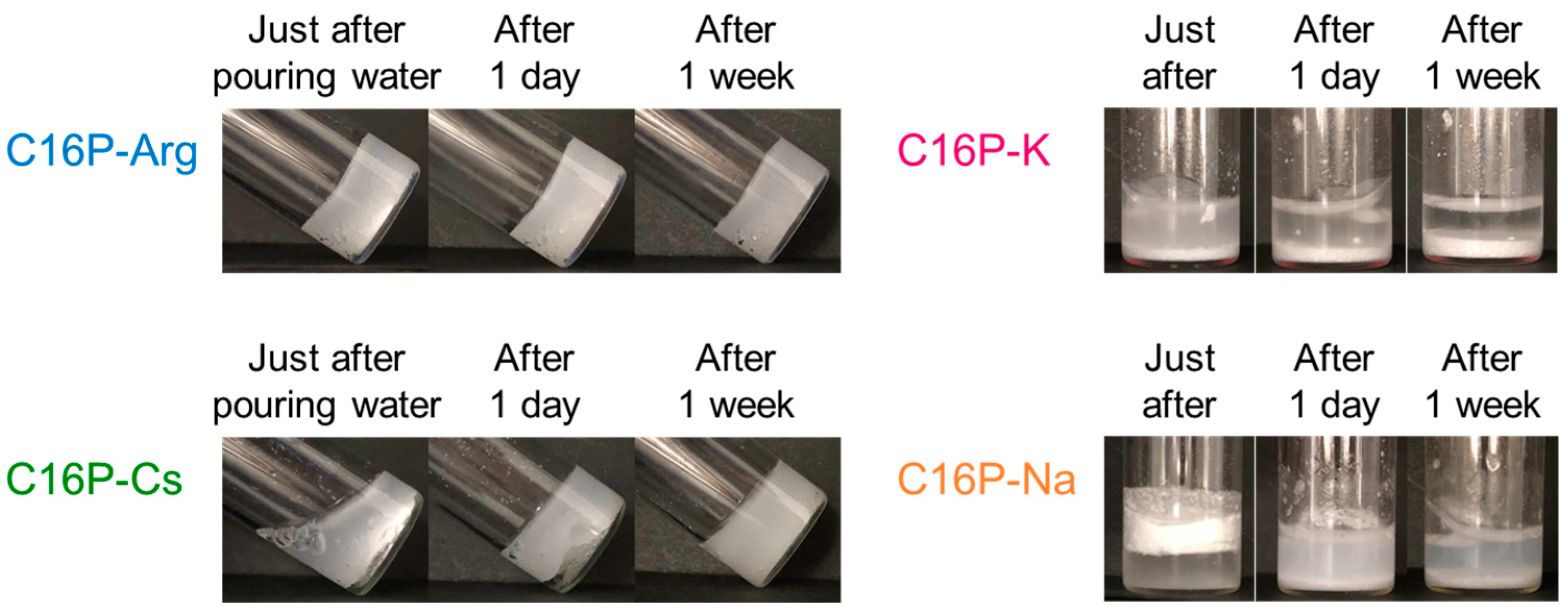
2.1. Characterization of α-Form Hydrated Crystal (α-Gel)
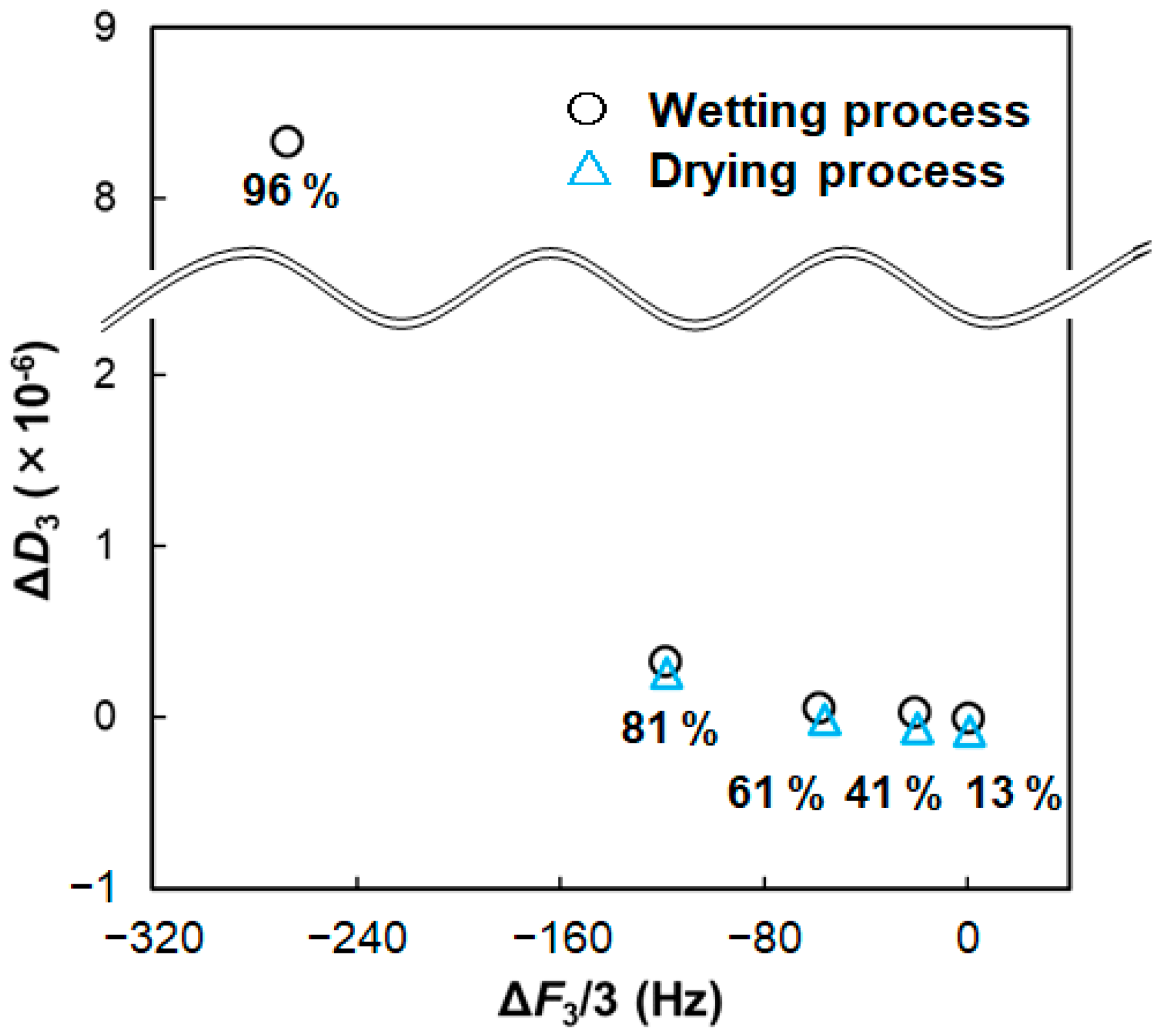
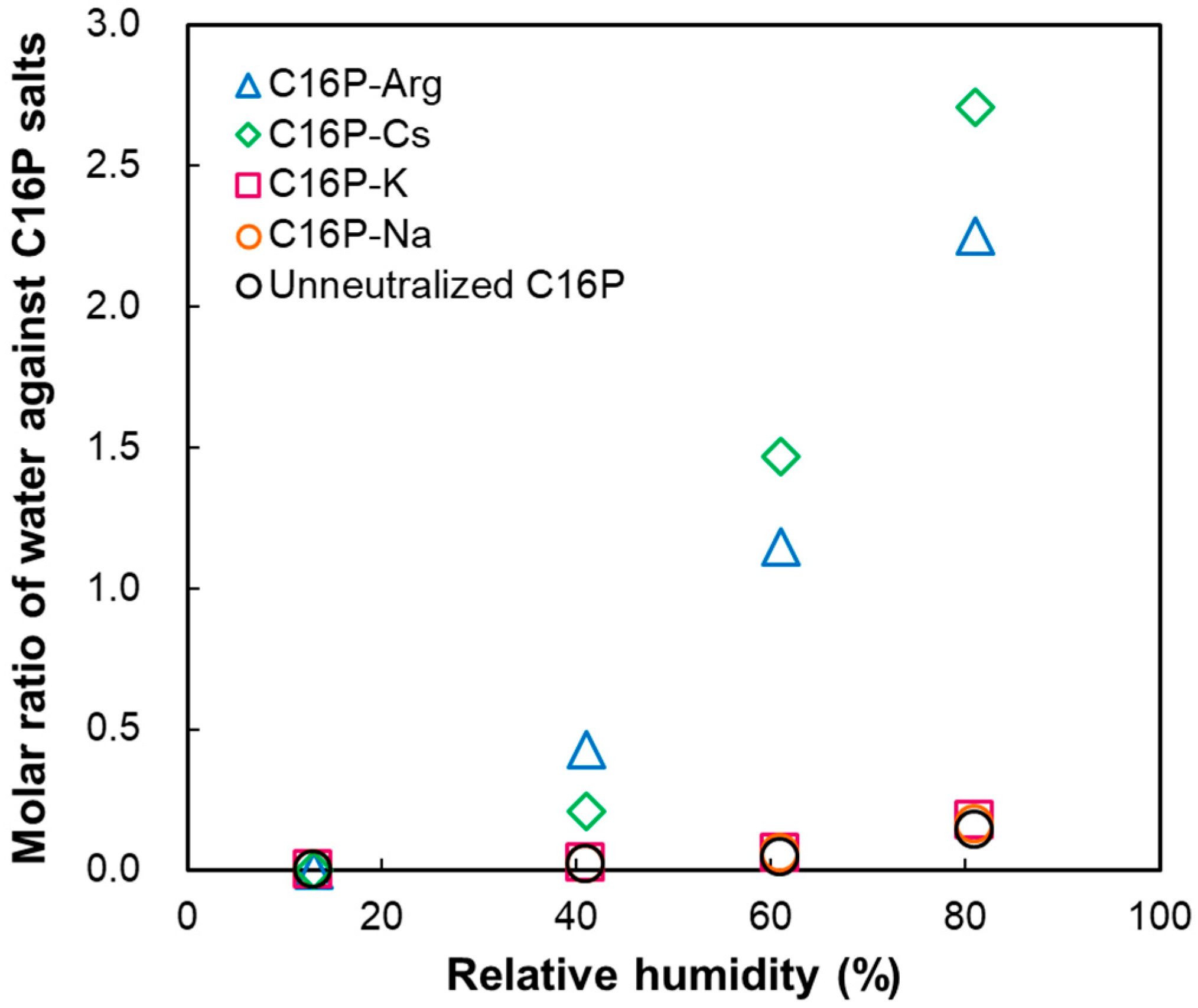
2.2. Hydration or Water Sorption from Gas Phase
3. Conclusions
4. Materials and Methods
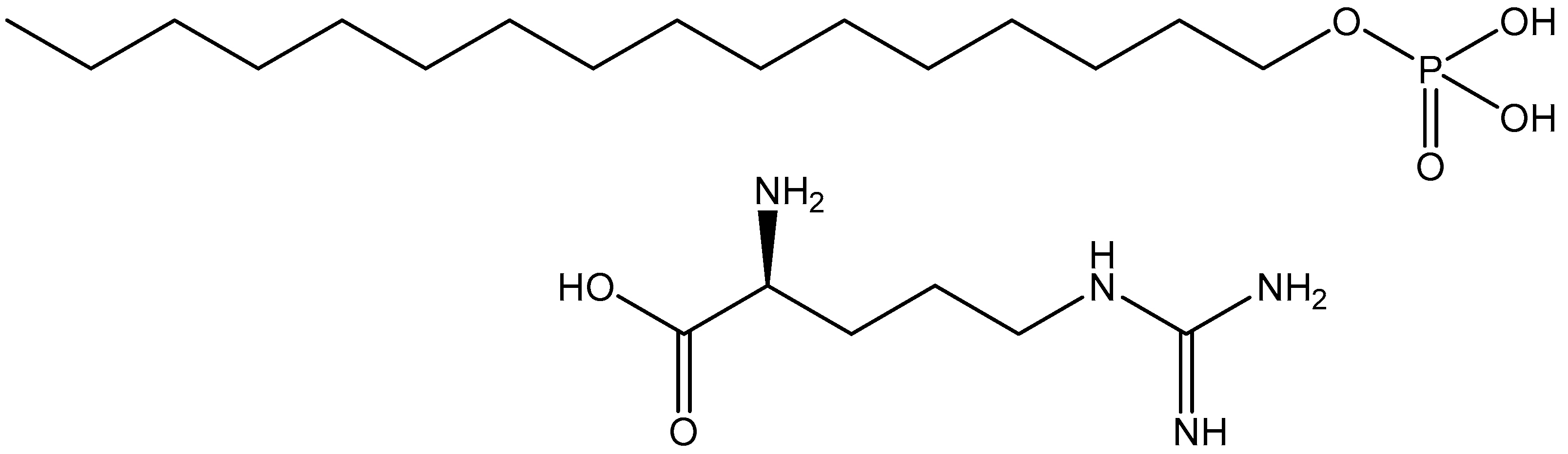
4.1. Materials
4.2. Sample Preparation
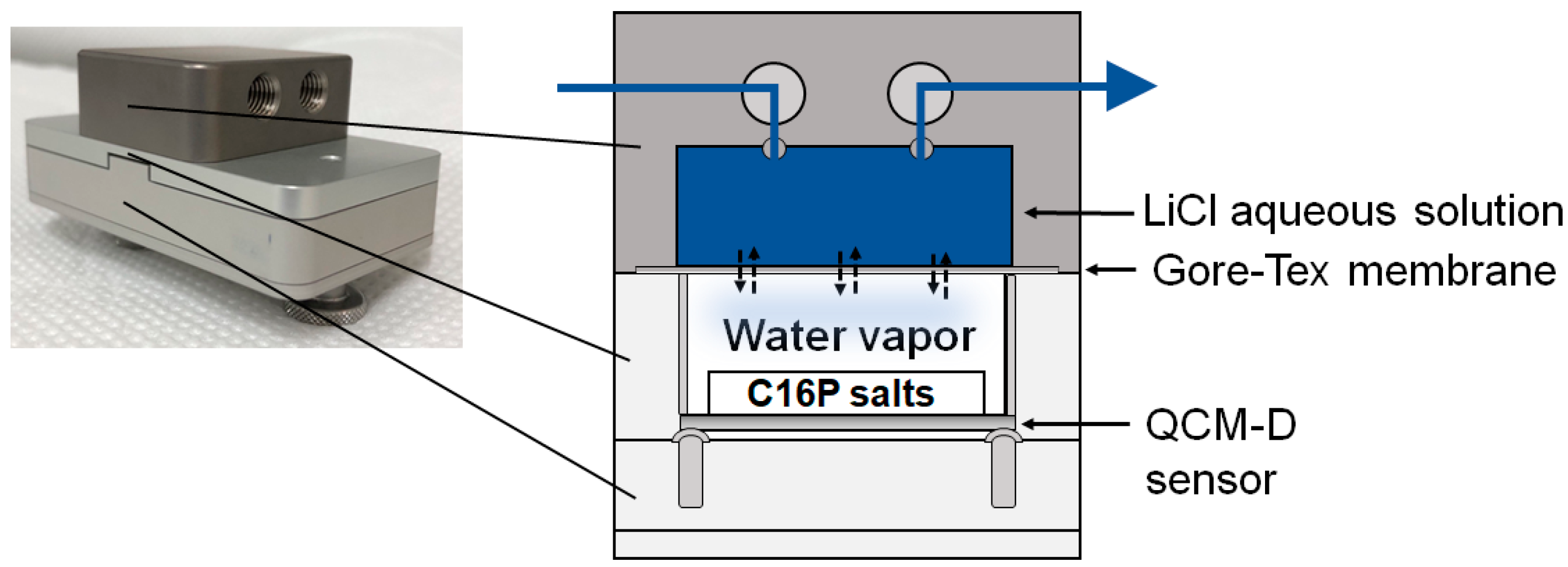
4.3. Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iwata, T. Overview of lamellar gel network. Acc. Mater. Surf. Res. 2016, 1, 99–129. [Google Scholar]
- Suzuki, T. Liquid crystal and α-gel-based emulsion and soft gel formulations. Acc. Mater. Surf. Res. 2017, 2, 21–40. [Google Scholar]
- Uyama, M.; Ikuta, K.; Teshigawara, T.; Watanabe, K.; Miyahara, R. The viscosity stability of o/w emulsion containing α-gel through an ionic-complex system. J. Oleo Sci. 2013, 62, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Uyama, M.; Araki, H.; Fukuhara, T.; Watanabe, K. Physicochemical properties of α-form hydrated crystalline phase of 3-(10-carboxydecyl)-1,1,1,3,5,5,5-heptamethyl trisiloxane/higher alcohol/polyoxyethylene (5 mol) glyceryl monostearate/water system. J. Oleo Sci. 2018, 67, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Yamaguchi, M. A phase diagram of the cetostearyl alcohol-polyethoxyoleylether-water ternary system. J. Pharm. Soc. Jpn. 1981, 101, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, Y.; Senna, M. Change in the two-step flow behavior on aging the ternary mixture comprising monoalkyl cationic surfactant, long-chain alcohol, and water I. Viscous flow preceded by incipient elastic deformation. Colloids Surf. A 1998, 132, 251–256. [Google Scholar] [CrossRef]
- Yamagata, Y.; Senna, M. Change in viscoelastic behaviors due to the phase transition of the assembly comprising cetyltrimethylammonium chloride/cetyl alcohol/water. Langmuir 1999, 15, 4388–4391. [Google Scholar] [CrossRef]
- Yamagata, Y.; Senna, M. Effects of temperature on the development of the internal structure of the cetyltrimethylammonium chloride/cetyl alcohol/water system. Langmuir 1999, 15, 7461–7463. [Google Scholar] [CrossRef]
- Yamagata, Y.; Senna, M. An electron spin resonance study on the phase transition of the molecular assembly comprising cetyltrimethylammonium chloride/cetyl alcohol/water. Langmuir 2000, 16, 6136–6140. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nakazawa, H.; Kato, S. Mechanism of gelation in hydrogenated soy-bean lecithin (PC70)/hexadecanol/water system. J. Colloid Interface Sci. 2012, 376, 146–151. [Google Scholar] [CrossRef]
- Tanaka, K.; Hirai, Y.; Suzuki, T.; Sakai, K.; Sakai, H. Phase diagram of monohexadecyl phosphate neutralized by L-arginine: α-Gel formation ability. J. Oleo Sci. 2018, 67, 851–857. [Google Scholar] [CrossRef]
- Tanaka, K.; Hirai, Y.; Suzuki, T.; Akamatsu, M.; Sakai, K.; Sakai, H. Characterizing water behavior in α-gel (α-type hydrated crystal) formed from monohexadecyl phosphate and L-arginine. J. Oleo Sci. 2019, 68, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.C.; Marangoni, A.G. Advances in the application of food emulsifier α-gel phases: Saturated monoglycerides, polyglycerol fatty acid esters, and their derivatives. J. Colloid Interface Sci. 2016, 483, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Takahashi, M.; Yamaguchi, M. Effect of cetostearyl alcohol on stabilization of oil-in-water emulsion. I. Difference in the effect by mixing cetyl alcohol with stearyl alcohol. J. Colloid Interface Sci. 1976, 57, 201–206. [Google Scholar] [CrossRef]
- Fukushima, S.; Yamaguchi, M.; Harusawa, F. Effect of cetostearyl alcohol on stabilization of oil-in-water emulsion. II. Relation between crystal form of the alcohol and stability of the emulsion. J. Colloid Interface Sci. 1977, 59, 159–165. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Acharya, D.P.; Sharma, S.C.; Aramaki, K.; Asaoka, H.; Ihara, K.; Tsunehiro, T.; Kunieda, H. Aqueous form stabilized by dispersed surfactant solid and lamellar liquid crystalline phase. J. Colloid Interface Sci. 2006, 301, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Znamenskaya, Y.; Sotres, J.; Gavryushov, S.; Engblom, J.; Arnebrant, T.; Kocherbitov, V. Water sorption and glass transition of pig gastric mucin studied by QCM-D. J. Phys. Chem. B 2013, 117, 2554–2563. [Google Scholar] [CrossRef]
- Graf, G.; Kocherbitov, V. Determination of sorption isotherm and rheological properties of lysozyme using a high-resolution humidity scanning QCM-D technique. J. Phys. Chem. B 2013, 117, 10017–10026. [Google Scholar] [CrossRef]
- Björklund, S.; Kocherbitov, V. Humidity scanning quartz crystal microbalance with dissipation monitoring setup for determination of sorption-desorption isotherms and rheological changes. Rev. Sci. Instrum. 2015, 86, 055105. [Google Scholar] [CrossRef]
- Björklund, S.; Kocherbitov, V. Water vapor sorption-desorption hysteresis in glassy surface films of mucins investigated by humidity scanning QCM-D. J. Colloid Interface Sci. 2019, 545, 289–300. [Google Scholar] [CrossRef]
- Znamenskaya, Y.; Björklund, S.; Kocherbitov, V.; Alfredsson, V. Effect of hydration and dehydration on the properties of SBA-15 layer studied by humidity scanning QCM-D. Microporous Mesoporous Mater. 2016, 230, 58–65. [Google Scholar] [CrossRef]
- Björklund, S.; Kocherbitov, V. Hydration-induced phase transitions in surfactant and lipid films. Langmuir 2016, 32, 5223–5232. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martinez, J.F.; Znamenskaya, Y.; Björklund, S.; Erkselius, S.; Rehnberg, N.; Sotres, J. Humidity-induced phase transitions of surfactants embedded in latex coatings can drastically alter their water barrier and mechanical properties. Polymers 2018, 10, 284. [Google Scholar] [CrossRef]
- Fabozzi, A.; Krauss, I.R.; Vitiello, R.; Fornasier, M.; Sicignano, L.; King, S.; Guido, S.; Jones, C.; Paduano, L.; Murgia, S.; et al. Branched alkyldimethylamine oxide surfactants: An effective strategy for the design of high concentration/low viscosity surfactant formulations. J. Colloid Interface Sci. 2019, 552, 448–463. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Aramaki, K. Effect of the behenyl trimethyl ammonium counterion on the lamellar gel property. IFSCC Mag. 2013, 16, 249–254. [Google Scholar]
- Robinson, R.A. The water activities of lithium chloride solutions up to high concentrations at 25°. Trans. Faraday Soc. 1945, 41, 756–758. [Google Scholar] [CrossRef]
- Rodahl, M.; Höök, F.; Krozer, A.; Brzezinski, P.; Kasemo, B. Quartz crystal microbalance setup for frequency and Q-factor measurements in gaseous and liquid environments. Rev. Sci. Instrum. 1995, 66, 3924–3930. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von schwingquarzen zur wägung dünner schichten und zur mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Sato, T.; Matsuoka, M.; Tanaka, K.; Ogura, T. Lamellar gel formation mechanism of monohexadecyl phosphate salt and effects of counterion species on the long-range order. In Proceedings of the Abstracts of the 72th Division Meeting of Colloid and Surface Chemistry, Online, 15–17 September 2021; Volume 1B06. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakai, K.; Nishimoto, S.; Hirai, Y.; Arakawa, K.; Akamatsu, M.; Tanaka, K.; Suzuki, T.; Sakai, H. Effects of Counterion on the Formation and Hydration Behavior of α-Form Hydrated Crystals (α-Gels). Gels 2023, 9, 928. https://doi.org/10.3390/gels9120928
Sakai K, Nishimoto S, Hirai Y, Arakawa K, Akamatsu M, Tanaka K, Suzuki T, Sakai H. Effects of Counterion on the Formation and Hydration Behavior of α-Form Hydrated Crystals (α-Gels). Gels. 2023; 9(12):928. https://doi.org/10.3390/gels9120928
Chicago/Turabian StyleSakai, Kenichi, Shuri Nishimoto, Yuki Hirai, Kyosuke Arakawa, Masaaki Akamatsu, Keisuke Tanaka, Toshiyuki Suzuki, and Hideki Sakai. 2023. "Effects of Counterion on the Formation and Hydration Behavior of α-Form Hydrated Crystals (α-Gels)" Gels 9, no. 12: 928. https://doi.org/10.3390/gels9120928
APA StyleSakai, K., Nishimoto, S., Hirai, Y., Arakawa, K., Akamatsu, M., Tanaka, K., Suzuki, T., & Sakai, H. (2023). Effects of Counterion on the Formation and Hydration Behavior of α-Form Hydrated Crystals (α-Gels). Gels, 9(12), 928. https://doi.org/10.3390/gels9120928







