A New Design of Poly(N-Isopropylacrylamide) Hydrogels Using Biodegradable Poly(Beta-Aminoester) Crosslinkers as Fertilizer Reservoirs for Agricultural Applications
Abstract
1. Introduction
2. Results and Discussion
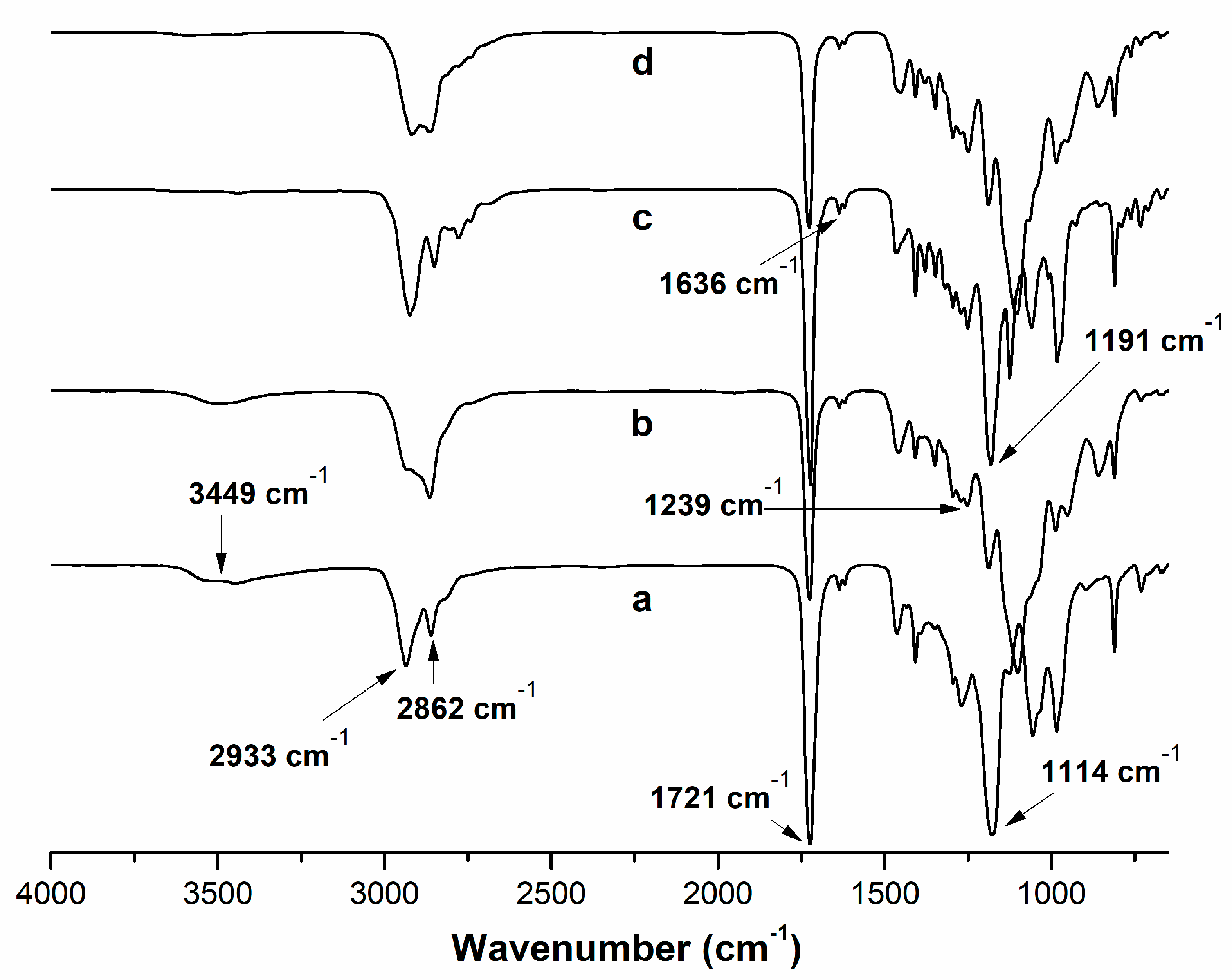
2.1. Synthesis of (PBAE) Crosslinkers
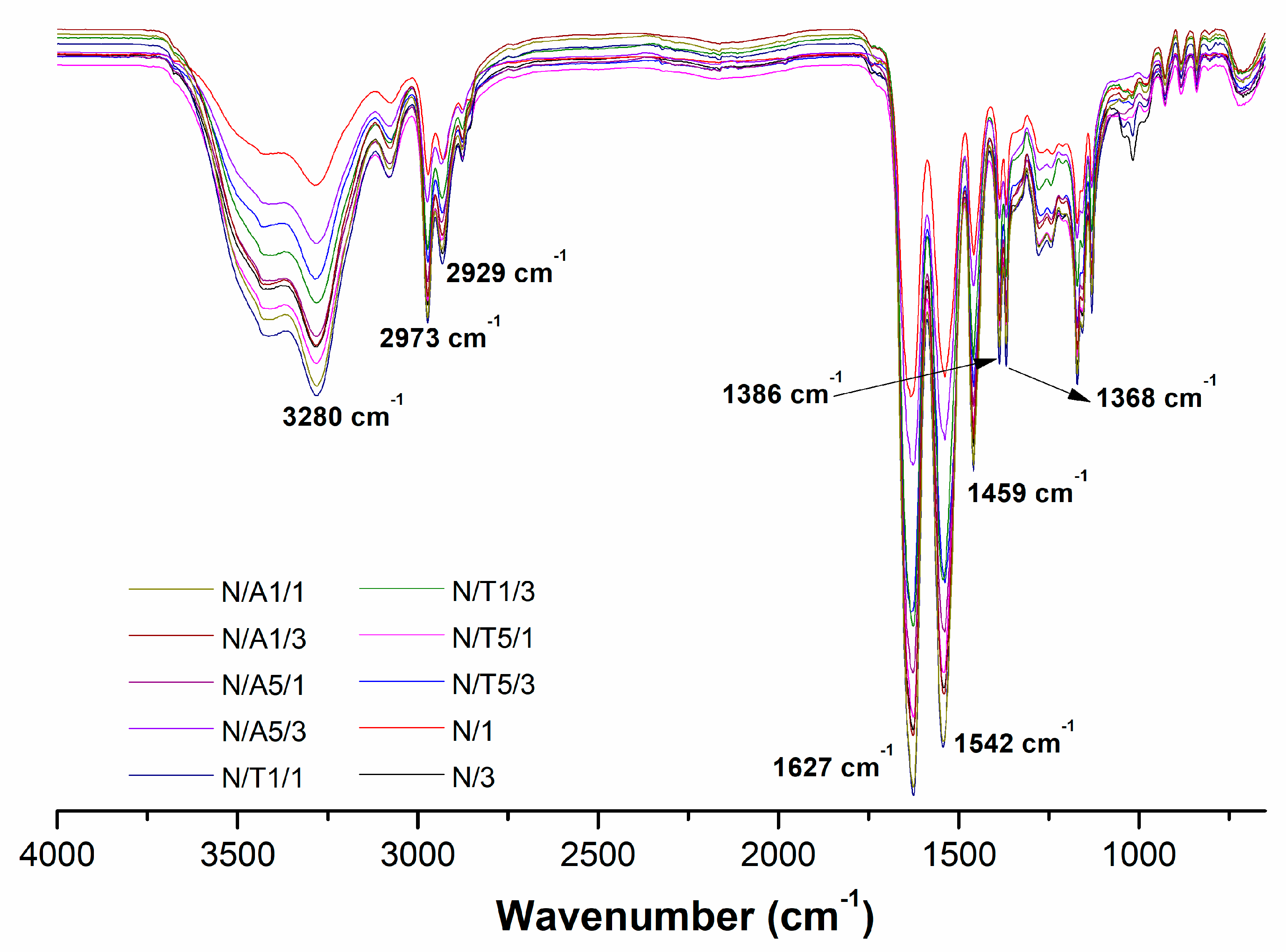
2.2. Synthesis of PBAE-Crosslinked P(NIPAAm) Hydrogels
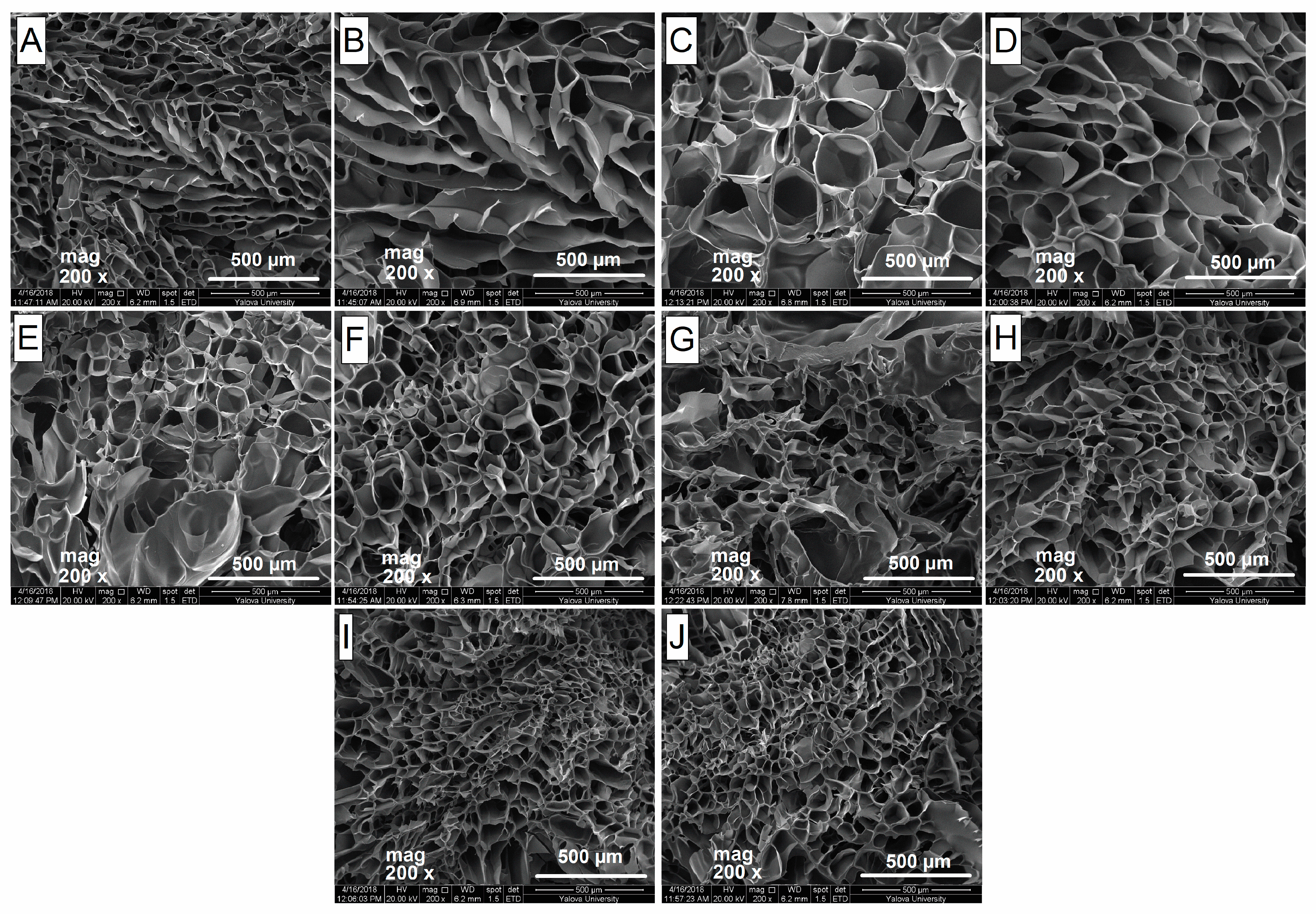
2.3. Morphology and Porosity
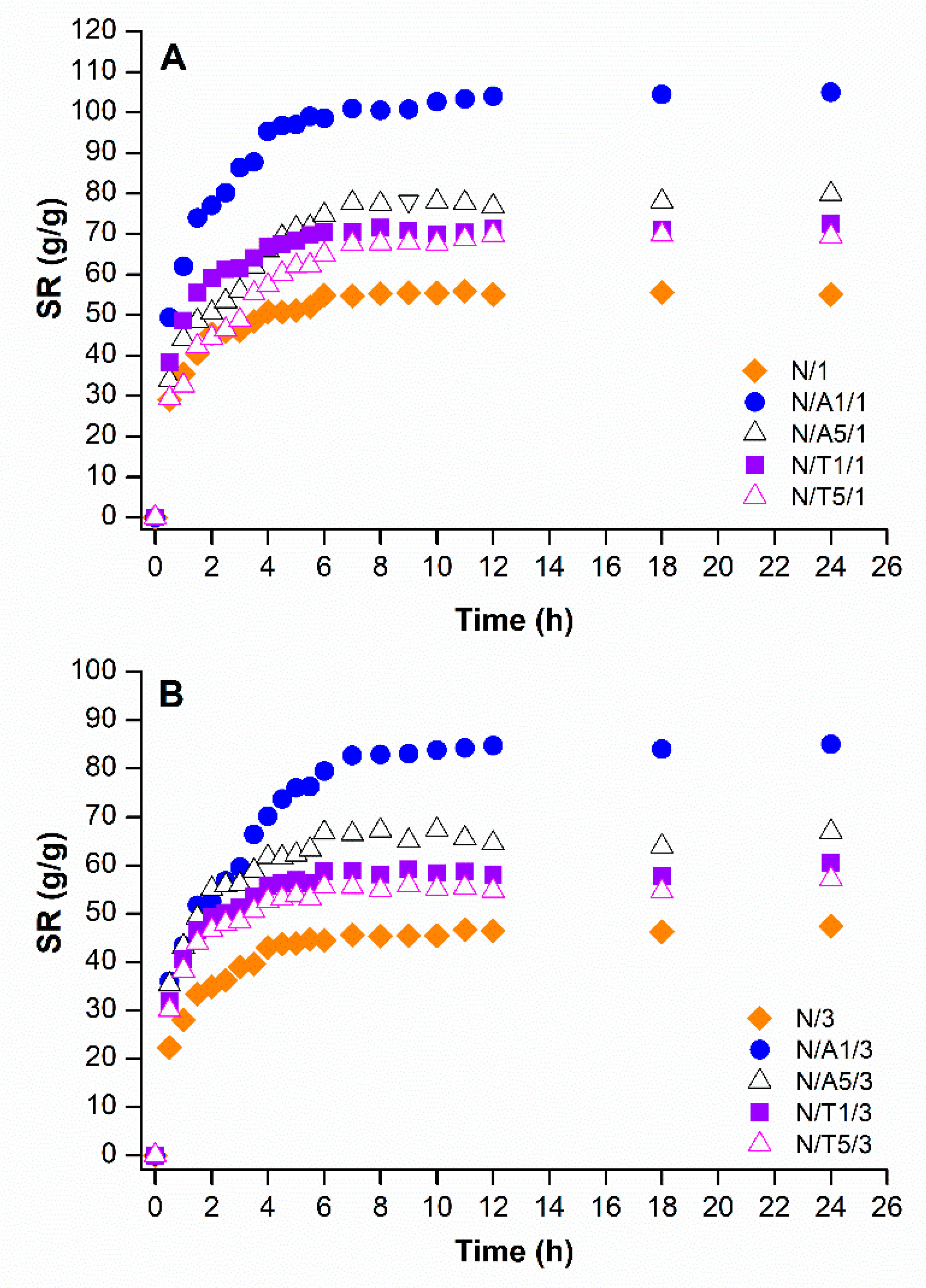
2.4. Swelling Studies
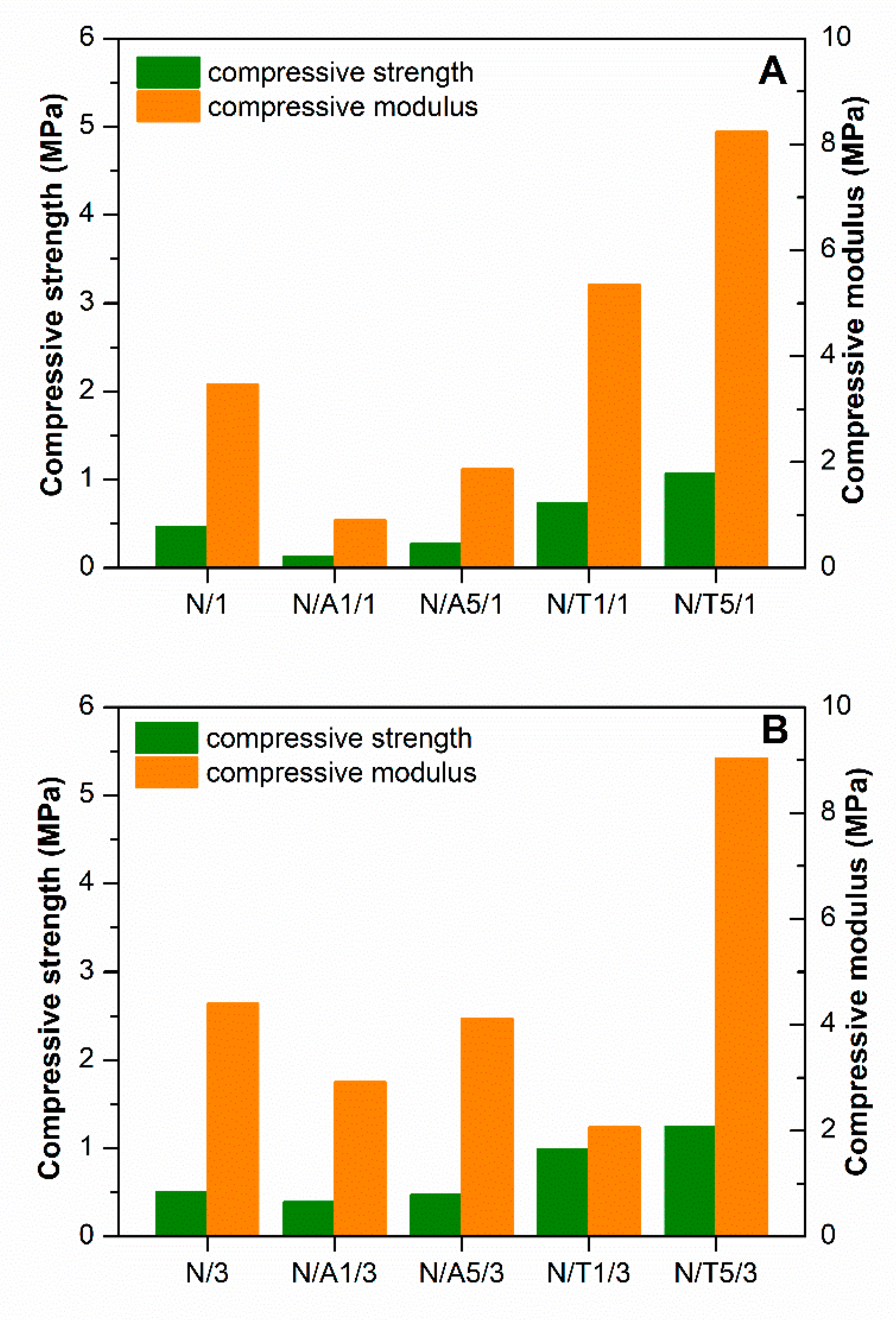
2.5. Mechanical Properties
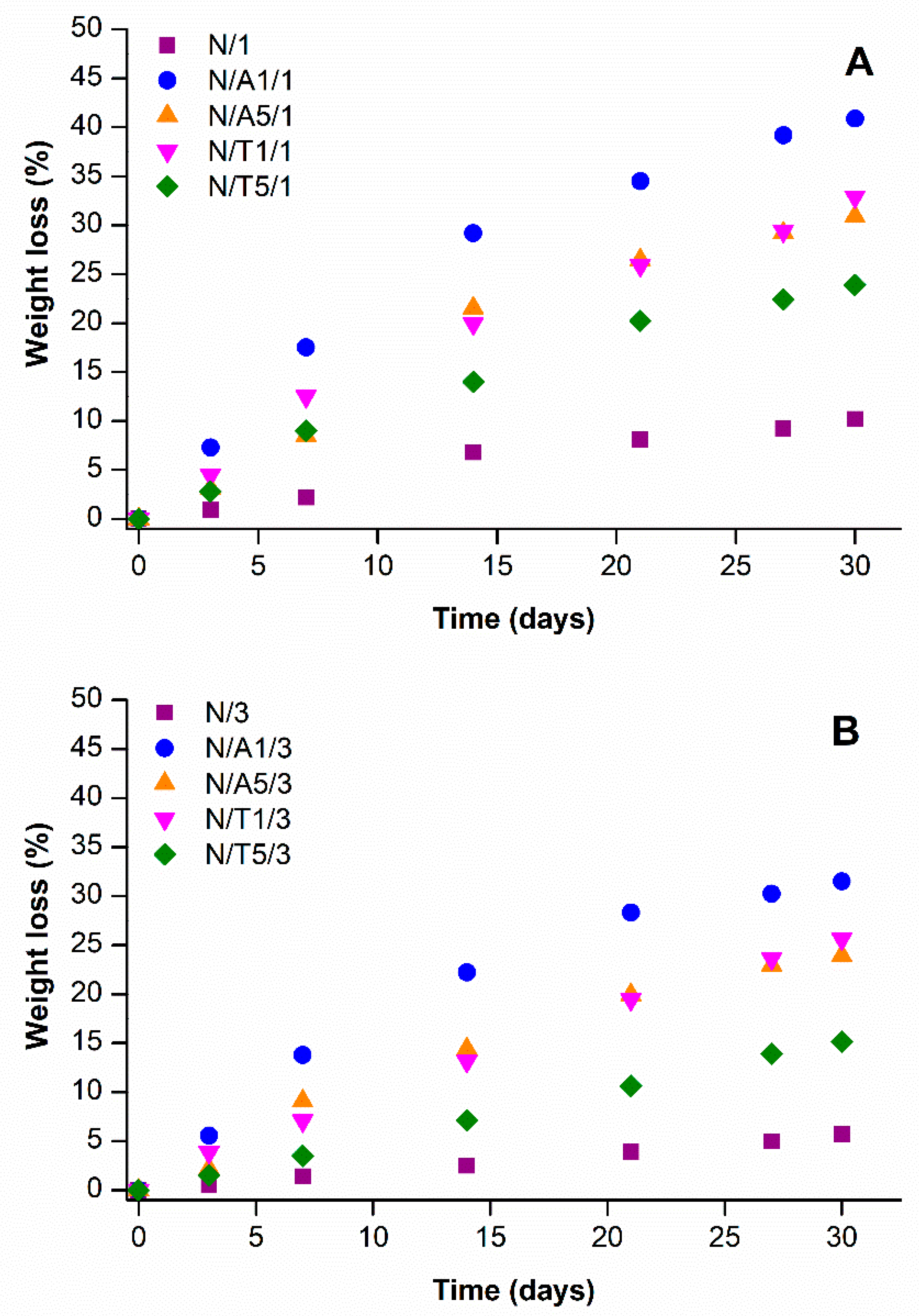
2.6. Biodegradation Studies
2.7. KNO3 Release Behavior of P(NIPAAm) Hydrogels
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Synthesis of Poly(beta-aminoester) (PBAE) Crosslinkers
4.3. Synthesis of P(NIPAAm) Hydrogels
4.4. Characterizations
4.5. Determination of Gel Fraction
4.6. Determination of the Crosslinking Density
4.7. Determination of the Porosity of Hydrogels
4.8. Swelling Studies
4.9. Mechanical Testing
4.10. Soil Biodegradation Studies
4.11. KNO3 Loading and Release Experiments
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-isopropylacrylamide)-based hydrogels for biomedical applications: A review of the state-of-the-art. Gels 2022, 8, 454. [Google Scholar] [CrossRef] [PubMed]
- Dudu, T.E.; Alpaslan, D.; Aktas, N. Superabsorbent hydrogels based on N,N-dimethylacrylamide and maleic acid for applications in agriculture as water purifier and nitrogen carrier. Polym. Bull. 2022, 79, 8551–8573. [Google Scholar] [CrossRef]
- Singh, N.; Agarwal, S.; Jain, A.; Khan, S. 3-Dimensional cross linked hydrophilic polymeric network “hydrogels”: An agriculture boom. Agric. Water Manag. 2021, 253, 106939. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, X.; Jiang, J.; Shang, S.; Song, Z. Hydrogels with high mechanical strength cross-linked by a rosin-based crosslinking agent. Rsc. Adv. 2017, 7, 42541–42548. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels—A review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremião, M.P.D. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- Di, X.; Kang, Y.; Li, F.; Yao, R.; Chen, Q.; Hang, C.; Xu, Y.; Wang, Y.; Sun, P.; Wu, G. Poly(N-isopropylacrylamide)/polydopamine/clay nanocomposite hydrogels with stretchability, conductivity, and dual light- and thermo- responsive bending and adhesive properties. Colloid. Surf. B. 2019, 177, 149–159. [Google Scholar] [CrossRef]
- Klouda, L. Thermoresponsive hydrogels in biomedical applications A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Wong, R.S.H.; Ashton, M.S.; Dodou, K. Effect of crosslinking agent concentration on the properties of unmedicated hydrogels. Pharmaceutics 2015, 7, 305–319. [Google Scholar] [CrossRef]
- Boztepe, C.; Yüceer, M.; Künkül, A.; Şölener, M.; Kabasakal, O.S. Prediction of the deswelling behaviors of pH- and temperature-responsive poly(NIPAAm-co-AAc) IPN hydrogel by artificial intelligence techniques. Res. Chem. Intermediat. 2020, 46, 409–428. [Google Scholar] [CrossRef]
- Lue, S.J.; Chen, C.H.; Shih, C.M. Tuning of lower critical solution temperature (LCST) of poly(NIsopropylacrylamide-co-Acrylic acid) hydrogels. J. Macromol. Sci. B 2011, 50, 563–579. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, S.L.; Park, S.N. Properties and in vitro drug release of pH-and temperature-sensitive double cross-linked interpenetrating polymer network hydrogels based on hyaluronic acid/poly (N-isopropylacrylamide) for transdermal delivery of luteolin. Int. J. Biol. Macromol. 2018, 118, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; Li, Z.; Xia, Q.B.; Bajalis, E.; Xi, H.X.; Lin, Y.S. Swelling/deswelling kinetics of PNIPAAm hydrogels synthesized by microwave irradiation. Chem. Eng. J. 2008, 142, 263–270. [Google Scholar] [CrossRef]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mat. Sci. Eng. C-Mater. 2017, 70, 842–855. [Google Scholar] [CrossRef]
- Gan, J.; Guan, X.; Zheng, J.; Guo, H.; Wu, K.; Liang, L.; Lu, M. Biodegradable, thermoresponsive PNIPAM-based hydrogel scaffolds for the sustained release of levofloxacin. Rsc. Adv. 2016, 6, 32967–32978. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly (N-isopropylacrylamide)-based thermoresponsive composite hydrogels for biomedical applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef] [PubMed]
- Tokarev, I.; Minko, S. Stimuli-responsive hydrogel thin films. Soft. Matter. 2009, 5, 511–524. [Google Scholar] [CrossRef]
- Alexander, A.; Ajaz, A.; Khan, J.; Saraf, S.; Saraf, S. Polyethylene glycol (PEG)-Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2014, 88, 575–585. [Google Scholar] [CrossRef]
- Ida, S.; Katsurada, A.; Tsujio, M.; Nakamura, M.; Hirokawa, Y. Crosslinker-Based regulation of swelling behavior of poly(N-isopropylacrylamide) gels in a post-polymerization crosslinking system. Gels 2020, 6, 2. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Chu, C.C. A responsive poly(N-isopropylacrylamide)/poly(ethylene glycol) diacrylate hydrogel microsphere. Colloid. Polym. Sci. 2004, 282, 1415–1420. [Google Scholar] [CrossRef]
- Wu, W.X.; Huang, Y.C.; Lee, W.F. Effect of poly(ethylene glycol)-derived crosslinkers on the properties of thermosensitive hydrogels. Iran. Polym. J. 2020, 29, 679–691. [Google Scholar] [CrossRef]
- Biswal, D.; Wattamwar, P.P.; Dziubla, T.D.; Hilt, J.Z. A single-step polymerization method for poly(b-amino ester) biodegradable hydrogels. Polymer 2011, 52, 5985–5992. [Google Scholar] [CrossRef]
- Hawkins, A.M.; Puleo, D.A.; Hilt, J.Z. Effect of macromer synthesis time on the properties of the resulting poly(β-amino ester) degradable hydrogel. J. Appl. Polym. Sci. 2011, 122, 1420–1426. [Google Scholar] [CrossRef]
- Lynn, D.M.; Langer, R. Degradable Poly(β-amino esters): Synthesis, Characterization, and Self-Assembly with Plasmid DNA. J. Am. Chem. Soc. 2000, 122, 10761–10768. [Google Scholar] [CrossRef]
- McBath, R.A.; Shipp, D. Swelling and degradation of hydrogels synthesized with degradable poly(b-amino ester) crosslinkers. Polym. Chem. 2010, 1, 860–865. [Google Scholar] [CrossRef]
- Safranski, D.L.; Crabtree, J.C.; Huq, Y.R.; Gall, K. Thermo-mechanical properties of semi-degradable Poly(b-amino ester)-co-methyl methacrylate networks under simulated physiological conditions. Polymer 2011, 52, 4920–4927. [Google Scholar] [CrossRef]
- Lakhera, N.; Laursen, C.; Safranski, D.; Frick, C.P. Biodegradable thermoset shape-memory polymer developed from poly(β-amino ester) networks. J. Polym. Sci. Pol. Phys. 2012, 50, 777–789. [Google Scholar] [CrossRef]
- Calcagnile, P.; Sibillano, T.; Giannini, C.; Sannino, A.; Demitri, C. Biodegradable poly(lactic acid)/cellulose-based superabsorbent hydrogel composite material as water and fertilizer reservoir in agricultural applications. J. Appl. Polym. Sci. 2019, 136, 47546. [Google Scholar] [CrossRef]
- Salimi, M.; Motamedi, E.; Motesharezedeh, B.; Mirseyed, H.; Alikhani, H.A. Starch-g-poly(acrylic acid-co-acrylamide) composites reinforced with natural char nanoparticles toward environmentally benign slow-release urea fertilizers. J. Environ. Chem. Eng. 2020, 8, 103765. [Google Scholar] [CrossRef]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohyd. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef]
- Ma, Z.; Jia, X.; Hu, J.; Liu, Z.; Wang, H.; Zhou, F. Mussel-Inspired thermosensitive polydopamine-graft poly(N-isopropylacrylamide) coating for controlled-release fertilizer. J. Agric. Food Chem. 2013, 61, 12232–12237. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.S.; Tamer, T.M.; El-Meligy, M.A.; Eldin, M.S.M. Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arab. J. Chem. 2015, 8, 38–47. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Characterization of nanocellulose reinforced semi-interpenetrating polymer network of poly(vinyl alcohol) & polyacrylamide composite films. Carbohyd. Polym. 2015, 134, 240–250. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, J.C.; Lee, H.Y.; Kim, J.D.; Yang, J.H. Release property of temperature-sensitive alginate beads containing poly(N-isopropylacrylamide). Colloid. Surf. B 2005, 46, 57. [Google Scholar] [CrossRef]
- Khan, A. Preparation and characterization of N-isopropylacrylamide/acrylic acid copolymer core-shell microgel particles. J. Colloid. Interf. Sci. 2007, 313, 697–704. [Google Scholar] [CrossRef]
- Hoti, G.; Caldera, F.; Cecone, C.; Pedrazzo, A.R.; Anceschi, A.; Appleton, S.L.; Monfared, Y.K.; Trotta, F. Effect of the cross-linking density on the swelling and rheological behavior of ester-bridged β-cyclodextrin nanosponges. Materials 2021, 14, 478. [Google Scholar] [CrossRef]
- Schweitzer, J.; Merad, S.; Schrodj, G.; Gall, F.B.; Vonna, L. Determination of the crosslinking density of a silicone elastomer. J. Chem. Educ. 2019, 96, 1472–1478. [Google Scholar] [CrossRef]
- Hou, X.; Yang, W.; Li, A.; Hou, J.; Zhang, C. Effects of incorporating acrylolsobutyl polyhedral oligomeric silsesquioxane on the properties of P(N-isopropylacrylamide-co-poly(ethylene glycol) diacrylate) hybrid hydrogels. Polym. Bull. 2017, 74, 1831–1847. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Murthy, P.S.K.; Raju, K.M. Preparation and swelling behavior of macroporous poly(acrylamide-co-sodium methacrylate) superabsorbent hydrogels. J. Appl. Polym. Sci. 2006, 101, 3202–3214. [Google Scholar] [CrossRef]
- Adhikary, K.B.; Pang, S.; Staiger, M.P. Long-Term moisture absorption and thickness swelling behaviour of recycled thermoplastics reinforced with pinus radiata sawdust. Chem. Eng. J. 2008, 142, 190–198. [Google Scholar] [CrossRef]
- Alfrey, T.; Gurnee, E.F.; Lloyd, W.G. Diffusion in Glassy Polymers. J. Polym. Sci. Pol. Sym. 1966, 12, 249–261. [Google Scholar] [CrossRef]
- Ganji, F.; Farahani, S.V.; Farahani, E.V. Theoretical description of hydrogel swelling: A review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Abdullah, Z.W.; Dong, Y. Biodegradable and water resistant poly(vinyl) alcohol (PVA)/starch (ST)/glycerol (GL)/halloysite nanotube (HNT) nanocomposite films for sustainable food packaging. Front. Mater. 2019, 6, 58. [Google Scholar] [CrossRef]
- Rizzarelli, P.; Innocenti, F.D.; Valenti, G.; Rapisarda, M. Biodegradation of green polymer composites: Laboratory procedures and standard test methods. Mater. Res. 2020, 68, 133–144. [Google Scholar] [CrossRef]
- Anderson, D.G.; Tweedie, C.A.; Hossain, N.; Navarro, S.M.; Brey, D.M.; Van Vliet, K.J.; Langer, R.; Burdick, J.A. A Combinatorial Library of Photocrosslinkable and Degradable Materials. Adv. Mater. 2006, 18, 2614. [Google Scholar] [CrossRef]
- Flory, P.J. Statistical mechanics of swelling of network structures. J. Chem. Phys. 1950, 18, 108–111. [Google Scholar] [CrossRef]
- Tao, C.T.; Young, T.H. Phase behavior of poly(N-isopropylacrylamide) in water–methanol cononsolvent mixtures and its relevance to membrane formation. Polymer. 2005, 46, 10077–10084. [Google Scholar] [CrossRef]
- Wu, N.; Yu, H.; Sun, M.; Li, Z.; Zhao, F.; Ao, Y.; Chen, H. Investigation on the structure and mechanical properties of highly tunable elastomeric silk fibroin hydrogels cross-linked by γ-ray radiation. ACS Appl. Bio Mater. 2020, 3, 721–734. [Google Scholar] [CrossRef]
- Peppas, N.A.; Franson, N.M. The swelling interface number as a criterion for prediction of diffusional solute release mechanism in swellable polymers. J. Polym. Sci. Polym. Phys. Ed. 1983, 21, 983–997. [Google Scholar] [CrossRef]
- Saraydin, D.; Karadağ, E.; Güven, O. Super Water-Retainer hydrogels: Crosslinked acrylamide/succinic acid copolymers. Polym. J. 1997, 29, 631–636. [Google Scholar] [CrossRef]
- George, K.A.; Wentrup-Byrne, E.; Hill, D.J.T.; Whittaker, A.K. Investigation into the diffusion of water into HEMA-co-MOEP hydrogels. Biomacromolecules 2004, 5, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Alomayri, T.S.; Assaedi, H.; Shaikh, F.U.A.; Low, I.M. Effect of water absorption on the mechanical properties of cotton fabric-reinforced geopolymer composites. J. Asian Ceram. Soc. 2014, 2, 223–230. [Google Scholar] [CrossRef]
- Debandi, M.V.; Bernal, C.; Francois, N.J. Development of biodegradable films based on chitosan/glycerol blends suitable for biomedical applications. J. Tissue. Sci. Eng. 2016, 7, 187. [Google Scholar] [CrossRef]
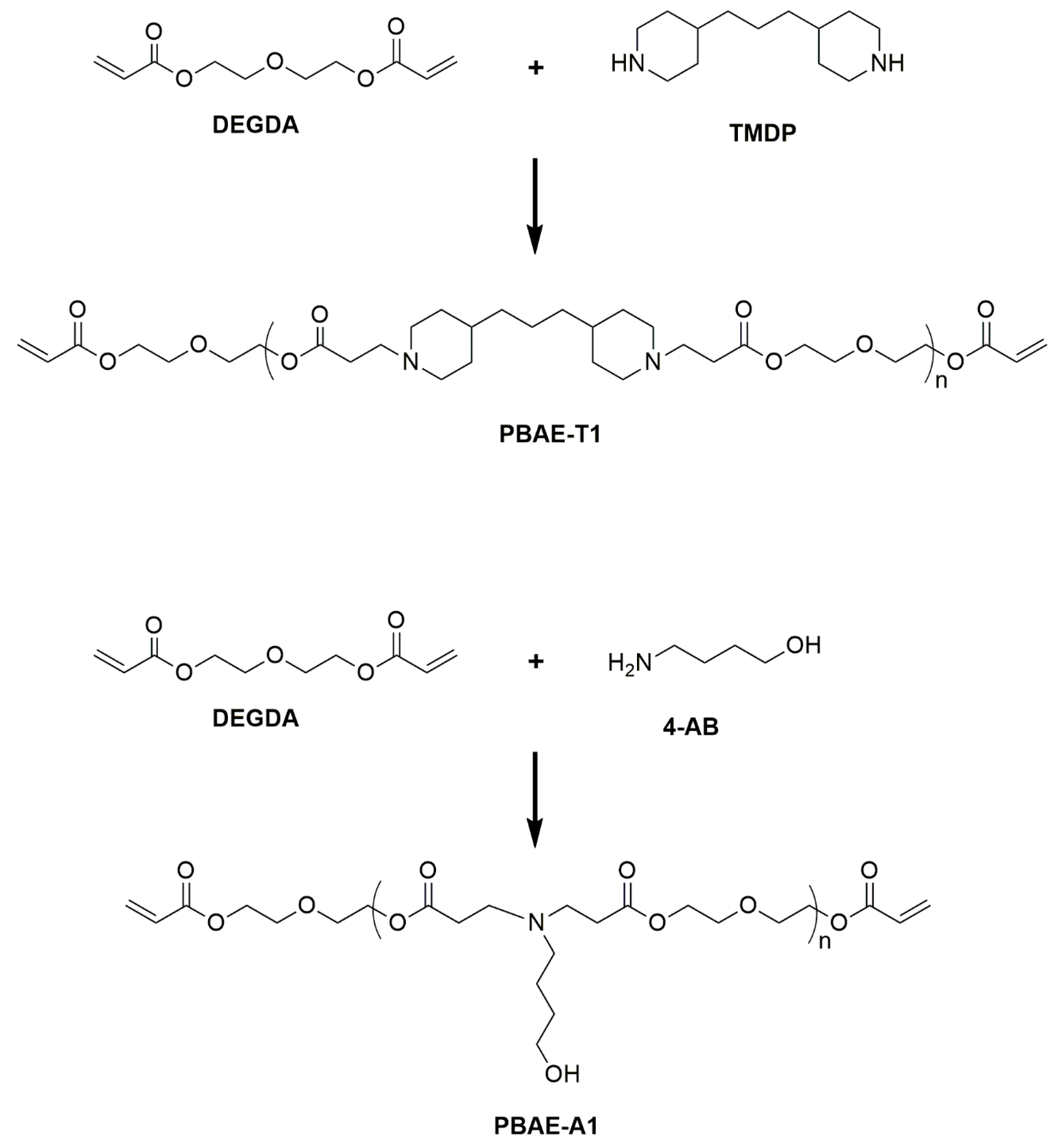


| Crosslinker | Composition (g) | Diacrylate/Amine Ratio | Mn | PDI | ||
|---|---|---|---|---|---|---|
| 4-AB | TMDP | DEGDA | ||||
| PBAE-A1 | 2.43 | - | 6.42 | 1.1:1 | 2399 | 1.74 |
| PBAE-A5 | 1.78 | - | 6.42 | 1.5:1 | 1534 | 1.32 |
| PBAE-T1 | - | 5.74 | 6.42 | 1.1:1 | 3604 | 1.45 |
| PBAE-T5 | - | 4.20 | 6.42 | 1.5:1 | 1976 | 1.82 |
| Sample | Crosslinker | Crosslinker Amount (%) | ν × 10−5 (mol cm−3) | Mc × 103 (g mol−1) |
|---|---|---|---|---|
| N/1 | MBA | 1.0 | 7.30 ± 2.24 | 10.82 ± 2.12 |
| N/3 | MBA | 3.0 | 6.29 ± 0.71 | 13.04 ± 1.78 |
| N/A1/1 | PBAE-A1 | 1.0 | 1.95 ± 0.89 | 40.12 ± 2.52 |
| N/A1/3 | PBAE-A1 | 3.0 | 2.77 ± 0.54 | 30.69 ± 1.45 |
| N/A5/1 | PBAE-A5 | 1.0 | 2.23 ± 1.53 | 35.43 ± 0.94 |
| N/A5/3 | PBAE-A5 | 3.0 | 3.21 ± 0.61 | 25.12 ± 1.46 |
| N/T1/1 | PBAE-T1 | 1.0 | 2.74 ± 2.17 | 27.74 ± 1.91 |
| N/T1/3 | PBAE-T1 | 3.0 | 3.98 ± 0.79 | 20.81 ± 0.76 |
| N/T5/1 | PBAE-T5 | 1.0 | 2.85 ± 0.64 | 27.72 ± 2.70 |
| N/T5/3 | PBAE-T5 | 3.0 | 4.97 ± 0.37 | 15. 29 ± 1.73 |
| Sample Code | n | k × 10−2 (s−1) | R2 | D (cm2 s−1) |
|---|---|---|---|---|
| N/1 | 0.79 | 3.51 | 0.996 | 3.96 |
| N/3 | 0.72 | 3.6 | 0.998 | 4.42 |
| N/A1/1 | 0.81 | 1.48 | 0.997 | 2.67 |
| N/A1/3 | 0.83 | 2.17 | 0.992 | 3.38 |
| N/A5/1 | 0.72 | 1.92 | 0.998 | 2.97 |
| N/A5/3 | 0.69 | 2.35 | 0.996 | 3.65 |
| N/T1/1 | 0.76 | 2.20 | 0.989 | 3.05 |
| N/T1/3 | 0.89 | 2.92 | 0.996 | 3.56 |
| N/T5/1 | 0.77 | 2.24 | 0.991 | 3.16 |
| N/T5/3 | 0.81 | 3.21 | 0.994 | 3.79 |
| Sample Code | KNO3 Loading (%) |
|---|---|
| N/1 | 51.79 |
| N/3 | 47.72 |
| N/A1/1 | 68.72 |
| N/A1/3 | 64.38 |
| N/A5/1 | 65.27 |
| N/A5/3 | 57.24 |
| N/T1/1 | 64.76 |
| N/T1/3 | 56.89 |
| N/T5/1 | 54.77 |
| N/T5/3 | 52.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balçık Tamer, Y. A New Design of Poly(N-Isopropylacrylamide) Hydrogels Using Biodegradable Poly(Beta-Aminoester) Crosslinkers as Fertilizer Reservoirs for Agricultural Applications. Gels 2023, 9, 127. https://doi.org/10.3390/gels9020127
Balçık Tamer Y. A New Design of Poly(N-Isopropylacrylamide) Hydrogels Using Biodegradable Poly(Beta-Aminoester) Crosslinkers as Fertilizer Reservoirs for Agricultural Applications. Gels. 2023; 9(2):127. https://doi.org/10.3390/gels9020127
Chicago/Turabian StyleBalçık Tamer, Yasemin. 2023. "A New Design of Poly(N-Isopropylacrylamide) Hydrogels Using Biodegradable Poly(Beta-Aminoester) Crosslinkers as Fertilizer Reservoirs for Agricultural Applications" Gels 9, no. 2: 127. https://doi.org/10.3390/gels9020127
APA StyleBalçık Tamer, Y. (2023). A New Design of Poly(N-Isopropylacrylamide) Hydrogels Using Biodegradable Poly(Beta-Aminoester) Crosslinkers as Fertilizer Reservoirs for Agricultural Applications. Gels, 9(2), 127. https://doi.org/10.3390/gels9020127







