Ultrasound-Assisted Extraction of Mango (Mangifera indica) Kernel Starch: Chemical, Techno-Functional, and Pasting Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yield Extraction
2.2. Chemical Composition
2.3. Total Phenolic Compounds (TPC) and Antioxidant Activity
2.4. Technological Properties
2.4.1. Water Holding Capacity (WHC) and Oil Holding Capacity (OHC)
2.4.2. Solubility and Swelling Power (SP)
2.5. Rheological Analysis
2.6. Pasting Properties
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Ultrasound-Assisted Extraction (UAE) of Mango Kernel Starch
4.2.2. Determination of Amylose Content
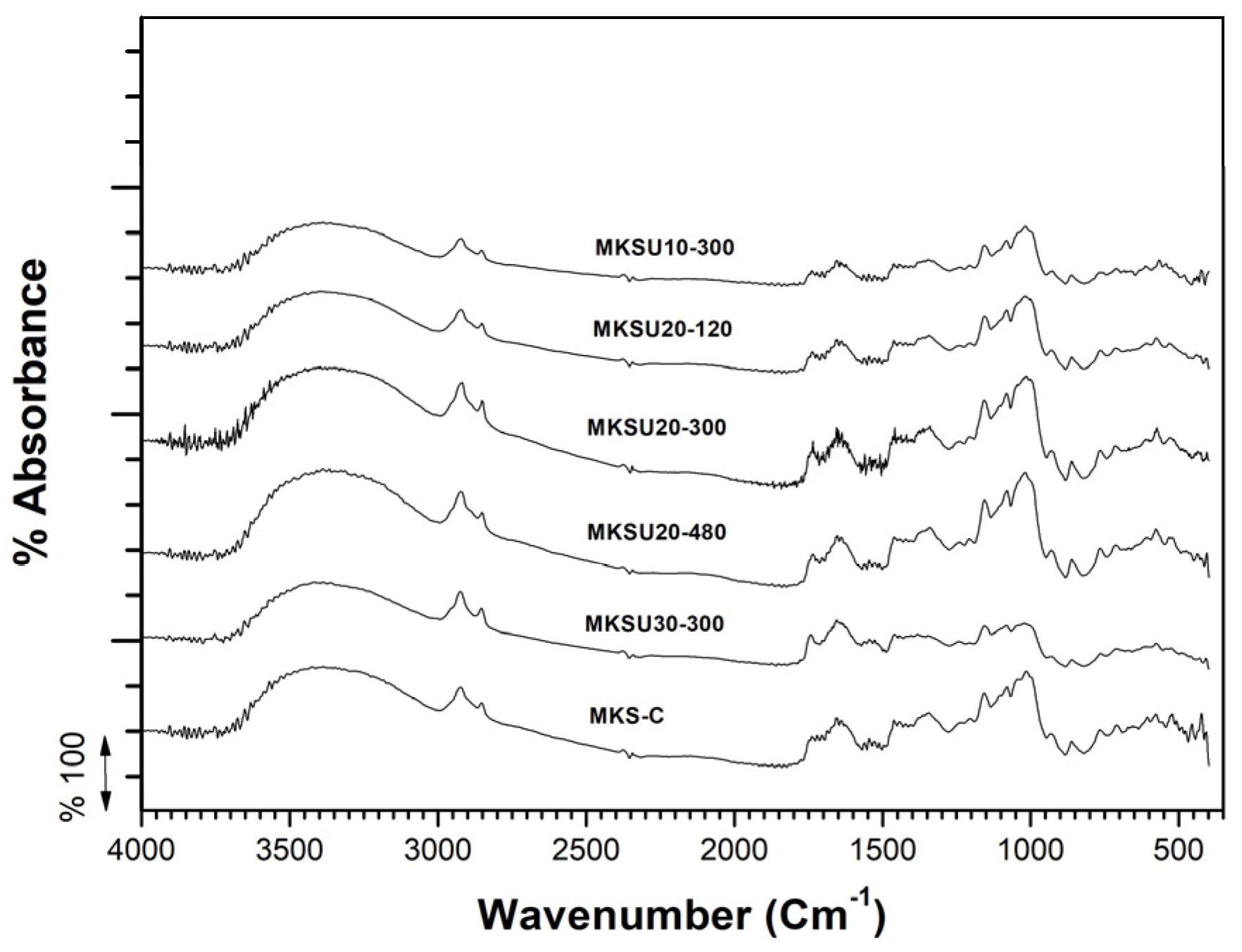
4.2.3. FTIR Analysis
4.2.4. Determination of TPC and Antioxidant Activity
4.2.5. Technological Properties
4.2.6. Rheological Analysis
4.2.7. Pasting Properties
4.2.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Statistics Division of Food and Agriculture Organization of the United Nations (FAOSTAT). [En Línea]. Available online: http://faostat.fao.org/ (accessed on 15 July 2022).
- Punia Bangar, S.; Kumar, M.; Whiteside, W.S. Mango seed starch: A sustainable and eco-friendly alternative to increasing industrial requirements. Int. J. Biol. Macromol. 2021, 183, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Panghal, A.; Attkan, A.K.; Singh, V.K.; Garg, M.K. Physicochemical characteristics, bioactive compounds and industrial applications of mango kernel and its products: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2421–2446. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, T.; Johakimu, J.K.; Chavan, R.B.; Sithole, B.; Ramjugernath, D. Valorisation of mango seed via extraction of starch: Preliminary techno-economic analysis. Clean Technol. Environ. Policy 2018, 20, 81–94. [Google Scholar] [CrossRef]
- Emeje, H.O. Chemical Properties of Starch and Its Application in the Food Industry; IntechOpen: Rijeka, Croatia, 2019; Chapter 5; ISBN 978-1-83880-116-8. [Google Scholar]
- Avérous, L.; Halley, P.J. Chapter 1—Starch Polymers: From the Field to Industrial Products. In Starch Polymers; Halley, P.J., Avérous, L.B.T.-S.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–10. ISBN 978-0-444-53730-0. [Google Scholar]
- Tagliapietra, B.L.; Felisberto, M.H.F.; Sanches, E.A.; Campelo, P.H.; Clerici, M.T.P.S. Non-conventional starch sources. Curr. Opin. Food Sci. 2021, 39, 93–102. [Google Scholar] [CrossRef]
- Li, D.; Zhu, F. Physicochemical properties of kiwifruit starch. Food Chem. 2017, 220, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Turola Barbi, R.C.; Teixeira, G.L.; Hornung, P.S.; Ávila, S.; Hoffmann-Ribani, R. Eriobotrya japonica seed as a new source of starch: Assessment of phenolic compounds, antioxidant activity, thermal, rheological and morphological properties. Food Hydrocoll. 2018, 77, 646–658. [Google Scholar] [CrossRef]
- Guo, K.; Lin, L.; Fan, X.; Zhang, L.; Wei, C. Comparison of structural and functional properties of starches from five fruit kernels. Food Chem. 2018, 257, 75–82. [Google Scholar] [CrossRef]
- Moo-Huchin, V.M.; Ac-Chim, D.M.; Chim-Chi, Y.A.; Ríos-Soberanis, C.R.; Ramos, G.; Yee-Madeira, H.T.; Ortiz-Fernández, A.; Estrada-León, R.J.; Pérez-Pacheco, E. Huaya (Melicoccus bijugatus) seed flour as a new source of Starch: Physicochemical, morphological, thermal and functional characterization. J. Food Meas. Charact. 2020, 14, 3299–3309. [Google Scholar] [CrossRef]
- Morales-Trejo, F.; Trujillo-Ramírez, D.; Aguirre-Mandujano, E.; Lobato-Calleros, C.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Ultrasound-assisted extraction of lychee (Litchi chinensis Sonn.) seed starch: Physicochemical and functional properties. Starch-Stärke 2022, 74, 2100092. [Google Scholar] [CrossRef]
- Clerici, M.T.P.S.; Schmiele, M. Starches for Food Application: Chemical, Technological and Health Properties; Academic Press: Cambridge, MA, USA, 2018; ISBN 0128134348. [Google Scholar]
- Obadi, M.; Xu, B. Review on the physicochemical properties, modifications, and applications of starches and its common modified forms used in noodle products. Food Hydrocoll. 2021, 112, 106286. [Google Scholar] [CrossRef]
- Helen Nwakego, A.-O.; Omotayo Opeyemi, J.; Olugbenga Olufemi, A.; Timilehin David, O. Physicochemical, functional, pasting properties and fourier transform infrared spectroscopy of native and modified Cardaba banana (Musa ABB) starches. Food Chem. Adv. 2022, 1, 100076. [Google Scholar] [CrossRef]
- Cárcel, J.A.; García-Pérez, J.V.; Benedito, J.; Mulet, A. Food process innovation through new technologies: Use of ultrasound. J. Food Eng. 2012, 110, 200–207. [Google Scholar] [CrossRef]
- Sit, N.; Misra, S.; Deka, S.C. Yield and functional properties of taro starch as affected by ultrasound. Food Bioproc. Technol. 2014, 7, 1950–1958. [Google Scholar] [CrossRef]
- Karaman, M.; Tuncel, N.B.; Yılmaz Tuncel, N. The effect of ultrasound-assisted extraction on yield and properties of some pulse starches. Starch-Stärke 2017, 69, 1600307. [Google Scholar] [CrossRef]
- González-Lemus, L.B.; Calderón-Domínguez, G.; de la Paz Salgado-Cruz, M.; Díaz-Ramírez, M.; Ramírez-Miranda, M.; Chanona-Pérez, J.J.; Gϋemes-Vera, N.; Farrera-Rebollo, R.R. Ultrasound-assisted extraction of starch from frozen jicama (P. erosus) roots: Effect on yield, structural characteristics and thermal properties. CyTA—J. Food 2018, 16, 738–746. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Castanha, N.; Rojas, M.L.; Augusto, P.E. Emerging technologies to enhance starch performance. Curr. Opin. Food Sci. 2021, 37, 26–36. [Google Scholar] [CrossRef]
- Ferraz, C.A.; Fontes, R.L.S.; Fontes-Sant’Ana, G.C.; Calado, V.; López, E.O.; Rocha-Leão, M.H.M. Extraction, modification, and chemical, thermal and morphological characterization of starch from the agro-industrial residue of mango (Mangifera indica L.) var. Ubá. Starch-Stärke 2019, 71, 1800023. [Google Scholar] [CrossRef]
- Bharti, I.; Singh, S.; Saxena, D.C. Exploring the influence of heat moisture treatment on physicochemical, pasting, structural and morphological properties of mango kernel starches from Indian cultivars. LWT 2019, 110, 197–206. [Google Scholar] [CrossRef]
- Tesfaye, T.; Sithole, B.B. Valorisation of mango seeds via extractioof starch: Using response surface methodology to optimise the extraction process. In Opportunities for Biomass and Organic Waste Valorisation; Routledge: Abingdon-on-Thames, UK, 2018. [Google Scholar]
- Tan, S.X.; Andriyana, A.; Lim, S.; Ong, H.C.; Pang, Y.L.; Ngoh, G.C. Rapid ultrasound-assisted starch extraction from sago pith waste (SPW) for the fabrication of sustainable bioplastic film. Polymers 2021, 13, 4398. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lan, T.; Lei, Y.; Suo, J.; Zhao, Q.; Wang, H.; Lei, J.; Sun, X.; Ma, T. Optimization of ultrasonic-assisted enzymatic extraction of kiwi starch and evaluation of its structural, physicochemical, and functional characteristics. Ultrason. Sonochem. 2021, 81, 105866. [Google Scholar] [CrossRef]
- Lemos, P.V.F.; Barbosa, L.S.; Ramos, I.G.; Coelho, R.E.; Druzian, J.I. Characterization of amylose and amylopectin fractions separated from potato, banana, corn, and cassava starches. Int. J. Biol. Macromol. 2019, 132, 32–42. [Google Scholar] [CrossRef]
- Przetaczek-Rożnowska, I. Physicochemical properties of starches isolated from pumpkin compared with potato and corn starches. Int. J. Biol. Macromol. 2017, 101, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Rosicka-Kaczmarek, J.; Makowski, B.; Nebesny, E.; Tkaczyk, M.; Komisarczyk, A.; Nita, Z. Composition and thermodynamic properties of starches from facultative wheat varieties. Food Hydrocoll. 2016, 54, 66–76. [Google Scholar] [CrossRef]
- Hernández-Medina, M.; Torruco-Uco, J.G.; Chel-Guerrero, L.; Betancur-Ancona, D. Caracterización fisicoquímica de almidones de tubérculos cultivados en Yucatán, México. Ciênc. Tecnol. Aliment. 2008, 28, 718–726. [Google Scholar] [CrossRef]
- Mieles-Gómez, L.; Lastra-Ripoll, S.E.; Torregroza-Fuentes, E.; Quintana, S.E.; García-Zapateiro, L.A. Rheological and microstructural properties of oil-in-water emulsion gels containing natural plant extracts stabilized with carboxymethyl cellulose/mango (Mangiferaindica) starch. Fluids 2021, 6, 312. [Google Scholar] [CrossRef]
- Patiño-Rodríguez, O.; Bello-Pérez, L.A.; Agama-Acevedo, E.; Pacheco-Vargas, G. Pulp and peel of unripe stenospermocarpic mango (Mangifera indica L. Cv Ataulfo) as an alternative source of starch, polyphenols and dietary fibre. Food Res. Int. 2020, 138, 109719. [Google Scholar] [CrossRef]
- De Souza, J.C.A.; Macena, J.F.F.; Andrade, I.H.P.; Camilloto, G.P.; Cruz, R.S. Functional characterization of mango seed starch (Mangifera indica L.). Res. Soc. Dev. 2021, 10, e30310310118. [Google Scholar] [CrossRef]
- Patiño-Rodríguez, O.; Agama-Acevedo, E.; Ramos-Lopez, G.; Bello-Pérez, L.A. Unripe mango kernel starch: Partial characterization. Food Hydrocoll. 2020, 101, 105512. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z.; Wan, N.; Wang, X.; Yang, M. Extraction of high-amylose starch from Radix puerariae using high-intensity low-frequency ultrasound. Ultrason. Sonochem. 2019, 59, 104710. [Google Scholar] [CrossRef]
- Kacuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Kačuráková, M.; Mathlouthi, M. FTIR and laser-Raman spectra of oligosaccharides in water: Characterization of the glycosidic bond. Carbohydr. Res. 1996, 284, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Sammon, C.; Bajwa, G.; Timmins, P.; Melia, C.D. The application of attenuated total reflectance Fourier transform infrared spectroscopy to monitor the concentration and state of water in solutions of a thermally responsive cellulose ether during gelation. Polymer 2006, 47, 577–584. [Google Scholar] [CrossRef]
- Pal, S.; Mal, D.; Singh, R.P. Cationic starch: An effective flocculating agent. Carbohydr. Polym. 2005, 59, 417–423. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Xu, L.; Jia, Y.; Xue, Z.; Zhang, M.; Phisalaphong, M.; Chen, H. Ultrasound-assisted modified pectin from unripe fruit pomace of raspberry (Rubus chingii Hu): Structural characterization and antioxidant activities. LWT 2020, 134, 110007. [Google Scholar] [CrossRef]
- Wang, J.; Lv, X.; Lan, T.; Lei, Y.; Suo, J.; Zhao, Q.; Lei, J.; Sun, X.; Ma, T. Modification in structural, physicochemical, functional, and in vitro digestive properties of kiwi starch by high-power ultrasound treatment. Ultrason. Sonochem. 2022, 86, 106004. [Google Scholar] [CrossRef]
- Alañón, M.E.; Pimentel-Moral, S.; Arráez-Román, D.; Segura-Carretero, A. HPLC-DAD-Q-ToF-MS profiling of phenolic compounds from mango (Mangifera indica L.) seed kernel of different cultivars and maturation stages as a preliminary approach to determine functional and nutraceutical value. Food Chem. 2021, 337, 127764. [Google Scholar] [CrossRef] [PubMed]
- Sivasankar, T.; Paunikar, A.W.; Moholkar, V.S. Mechanistic approach to enhancement of the yield of a sonochemical reaction. AIChE J. 2007, 53, 1132–1143. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, F. Effect of ultrasound on structural and physicochemical properties of sweetpotato and wheat flours. Ultrason. Sonochem. 2020, 66, 105118. [Google Scholar] [CrossRef] [PubMed]
- Oladele, A.K.; Duodu, K.G.; Emmambux, N.M. Hydrolysis and antioxidant activity of starch modified with phenolic extracts from grape pomace and sorghum bran under alkaline conditions. Carbohydr. Polym. 2020, 240, 116291. [Google Scholar] [CrossRef]
- Agama-Acevedo, E.; Flores-Silva, P.C.; Bello-Perez, L.A. Chapter 3—Cereal starch production for food applications. In Starches for Food Application; Silva Clerici, M.T.P., Schmiele, M.B.T.-S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 71–102. ISBN 978-0-12-809440-2. [Google Scholar]
- Liang, Q.; Chen, X.; Ren, X.; Yang, X.; Raza, H.; Ma, H. Effects of ultrasound-assisted enzymolysis on the physicochemical properties and structure of arrowhead-derived resistant starch. LWT 2021, 147, 111616. [Google Scholar] [CrossRef]
- Raza, H.; Ameer, K.; Ma, H.; Liang, Q.; Ren, X. Structural and physicochemical characterization of modified starch from arrowhead tuber (Sagittaria sagittifolia L.) using tri-frequency power ultrasound. Ultrason. Sonochem. 2021, 80, 105826. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Liu, Y.; Ouyang, J. Effect of ultrasonic and microwave dual-treatment on the physicochemical properties of chestnut starch. Polymers 2020, 12, 1718. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Observations on the impact of amylopectin and amylose structure on the swelling of starch granules. Food Hydrocoll. 2020, 103, 105663. [Google Scholar] [CrossRef]
- Kaur, H.; Gill, B.S. Effect of high-intensity ultrasound treatment on nutritional, rheological and structural properties of starches obtained from different cereals. Int. J. Biol. Macromol. 2019, 126, 367–375. [Google Scholar] [CrossRef]
- Bernardo, C.O.; Ascheri, J.L.R.; Chávez, D.W.H.; Carvalho, C.W.P. Ultrasound assisted extraction of yam (Dioscorea bulbífera) starch: Effect on morphology and functional properties. Starch-Stärke 2018, 70, 1700185. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Dhull, S.B.; Siroha, A.K.; Purewal, S.S.; Kaur, M.; Kidwai, M.K. Oat starch: Physico-chemical, morphological, rheological characteristics and its applications—A review. Int. J. Biol. Macromol. 2020, 154, 493–498. [Google Scholar] [CrossRef]
- Hu, Y.; Tian, J.; Zou, J.; Yuan, X.; Li, J.; Liang, H.; Zhan, F.; Li, B. Partial removal of acetyl groups in konjac glucomannan significantly improved the rheological properties and texture of konjac glucomannan and κ-carrageenan blends. Int. J. Biol. Macromol. 2019, 123, 1165–1171. [Google Scholar] [CrossRef]
- Soltani-Firouz, M. Application of high-intensity ultrasound in food processing for improvement of food quality. In Design and Optimization of Innovative Food Processing Techniques Assisted by Ultrasound; Academic Press: Cambridge, MA, USA, 2021; pp. 143–167. [Google Scholar]
- Wang, H.; Xu, K.; Ma, Y.; Liang, Y.; Zhang, H.; Chen, L. Impact of ultrasonication on the aggregation structure and physicochemical characteristics of sweet potato starch. Ultrason. Sonochem. 2020, 63, 104868. [Google Scholar] [CrossRef]
- Zabot, G.L.; Silva, E.K.; Emerick, L.B.; Felisberto, M.H.F.; Clerici, M.T.P.S.; Meireles, M.A.A. Physicochemical, morphological, thermal and pasting properties of a novel native starch obtained from annatto seeds. Food Hydrocoll. 2019, 89, 321–329. [Google Scholar] [CrossRef]
- Kumar, R.; Khatkar, B.S. Thermal, pasting and morphological properties of starch granules of wheat (Triticum aestivum L.) varieties. J. Food Sci. Technol. 2017, 54, 2403–2410. [Google Scholar] [CrossRef]
- Yang, W.; Kong, X.; Zheng, Y.; Sun, W.; Chen, S.; Liu, D.; Zhang, H.; Fang, H.; Tian, J.; Ye, X. Controlled ultrasound treatments modify the morphology and physical properties of rice starch rather than the fine structure. Ultrason. Sonochem. 2019, 59, 104709. [Google Scholar] [CrossRef]
- Yang, Q.-Y.; Lu, X.-X.; Chen, Y.-Z.; Luo, Z.-G.; Xiao, Z.-G. Fine structure, crystalline and physicochemical properties of waxy corn starch treated by ultrasound irradiation. Ultrason. Sonochem. 2019, 51, 350–358. [Google Scholar] [CrossRef]
- Al-Attar, H.; Ahmed, J.; Thomas, L. Rheological, pasting and textural properties of corn flour as influenced by the addition of rice and lentil flour. LWT 2022, 160, 113231. [Google Scholar] [CrossRef]
- Bashir, K.; Aggarwal, M. Physicochemical, thermal and functional properties of gamma irradiated chickpea starch. Int. J. Biol. Macromol. 2017, 97, 426–433. [Google Scholar] [CrossRef]
- Bemiller, J.N. Starch modification: Challenges and prospects. Starch-Stärke 1997, 49, 127–131. [Google Scholar] [CrossRef]
- Morrison, W.R.; Laignelet, B. An improved colorimetric procedure for determining apparent and total amylose in cereal and other starches. J. Cereal Sci. 1983, 1, 9–20. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M.B.T. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. ISBN 0076-6879. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Yamazaki, W.T. An alkaline water retention capacity test for the evaluation of cookie baking potentialities of soft winter wheat flours. Cereal Chem. 1953, 30, 242–246. [Google Scholar]
- Medcalf, D.G. Wheat starches I. Comparison of physicochemical properties. Cereal Chem. 1965, 42, 558–568. [Google Scholar]
- Jiang, Q.; Gao, W.; Li, X.; Man, S.; Shi, Y.; Yang, Y.; Huang, L.; Liu, C. Comparative susceptibilities to alkali-treatment of A-, B- and C-type starches of Dioscorea zingiberensis, Dioscorea persimilis and Dioscorea opposita. Food Hydrocoll. 2014, 39, 286–294. [Google Scholar] [CrossRef]
- López-Barraza, D.; Ortega-Ramos, A.; Torregroza-Fuentes, E.; Quintana, S.E.; García-Zapateiro, L.A. Rheological and functional properties of hydrocolloids from Pereskia bleo leaves. Fluids 2021, 6, 349. [Google Scholar] [CrossRef]
 and the loss modulus ()
and the loss modulus ()  of mango kernel starch. (a) MKSU10-300; (b) MKSU20-120; (c) MKSU20-300; (d) MKSU20-480; (e) MKSU30-300; (f) MKS-C.
of mango kernel starch. (a) MKSU10-300; (b) MKSU20-120; (c) MKSU20-300; (d) MKSU20-480; (e) MKSU30-300; (f) MKS-C.
 and the loss modulus ()
and the loss modulus ()  of mango kernel starch. (a) MKSU10-300; (b) MKSU20-120; (c) MKSU20-300; (d) MKSU20-480; (e) MKSU30-300; (f) MKS-C.
of mango kernel starch. (a) MKSU10-300; (b) MKSU20-120; (c) MKSU20-300; (d) MKSU20-480; (e) MKSU30-300; (f) MKS-C.

| Sample Code | Yield Extraction % | Amylose Content g/100 g Starch | TPC mg GAE/g Starch | TEAC µMol Trolox/g Starch |
|---|---|---|---|---|
| MKSU10-300 | 45.20 ± 1.41 a | 35.45 ± 0.87 ab | 84.45 ± 6.21 ab | 12.95 ± 0.31 a |
| MKSU20-120 | 45.70 ± 0.71 a | 32.58 ± 0.28 c | 80.72 ± 5.90 ab | 11.52 ± 1.02 b |
| MKSU20-300 | 47.60 ± 1.70 ab | 36.56 ± 0.32 a | 83.68 ± 3.88 ab | 11.01 ± 0.61 bc |
| MKSU20-480 | 54.00 ± 0.57 c | 36.06 ± 0.42 a | 75.78 ± 3.50 a | 10.01 ± 0.31 c |
| MKSU30-300 | 49.50 ± 2.47 b | 34.35 ± 0.46 b | 89.49 ± 7.76 b | 10.37 ± 0.28 bc |
| MKS-C | 42.05 ± 2.58 d | 28.46 ± 0.93 d | 84.89 ± 1.55 ab | 18.15 ± 1.10 d |
| Sample Code | WHC g/100 g of Starch | OHC g/100 g of Starch | Solubility % | Swelling Power g/g of Starch | ||||
|---|---|---|---|---|---|---|---|---|
| 25 °C | 65 °C | 90 °C | 25 °C | 65 °C | 90 °C | |||
| MKSU10-300 | 91.70 ± 3.51 ac | 82.43 ± 1.28 a | 1.50 ± 0.09 bc | 6.77 ± 0.04 b | 16.31 ± 0.50 b | 2.37 ± 0.10 ab | 6.22 ± 1.26 a | 15.55 ± 0.73 a |
| MKSU20-120 | 87.03 ± 2.71 ab | 87.19 ± 1.92 c | 1.12 ± 0.01 a | 6.21 ± 0.01 a | 14.88 ± 0.58 a | 2.29 ± 0.64 ab | 6.17 ± 0.47 a | 14.84 ± 0.44 a |
| MKSU20-300 | 85.71 ± 3.39 b | 82.72 ± 2.89 ab | 1.49 ± 0.03 b | 7.06 ± 0.25 b | 16.73 ± 0.48 b | 2.54 ± 0.25 ab | 6.89 ± 0.28 a | 17.32 ± 1.22 b |
| MKSU20-480 | 90.71 ± 2.42 ac | 85.76 ± 1.21 bc | 1.61 ± 0.18 bc | 7.12 ± 0.26 b | 16.37 ± 0.17 b | 2.56 ± 0.25 b | 6.89 ± 0.27 a | 17.40 ± 1.24 b |
| MKSU30-300 | 92.85 ± 1.48 c | 87.78 ± 1.69 c | 1.65 ± 0.03 c | 7.05 ± 0.08 b | 17.11 ± 0.51 b | 2.60 ± 0.29 b | 6.22 ± 0.51 a | 18.13 ± 0.62 b |
| MKS-C | 80.48 ± 2.41 d | 76.43 ± 1.63 d | 1.10 ± 0.07 a | 6.20 ± 0.15 a | 15.00 ± 0.58 a | 1.97 ± 0.09 a | 6.17 ± 0.04 a | 14.88 ± 0.47 a |
| Sample Code | PT °C | PV Pa·s | FV Pa·s | TV Pa·s | BD Pa·s | SB Pa·s | Crossover Temperature °C * |
|---|---|---|---|---|---|---|---|
| MKS10-300 | 83.48 ± 1.66 a | 3.50 ± 0.17 ab | 1.95 ± 0.09 ab | 1.24 ± 0.06 a | 2.25 ± 0.11 a | 1.55 ± 0.07 a | 81.20 ± 1.62 ab |
| MKS20-120 | 82.50 ± 1.61 a | 4.65 ± 0.23 d | 2.48 ± 0.12 e | 1.76 ± 0.08 c | 2.90 ± 0.14 c | 2.17 ± 0.10 c | 82.80 ± 1.65 b |
| MKS20-300 | 83.20 ± 1.64 a | 3.19 ± 0.15 b | 2.69 ± 0.13 d | 1.22 ± 0.06 a | 1.97 ± 0.09 b | 0.50 ± 0.02 b | 80.70 ± 1.61 ab |
| MKS20-480 | 82.10 ± 1.64 a | 2.67 ± 0.13 e | 1.88 ± 0.08 a | 0.80 ± 0.04 d | 1.87 ± 0.09 b | 0.79 ± 0.03 d | 78.50 ± 1.57 a |
| MKS30-300 | 84.10 ± 1.68 a | 3.76 ± 0.18 a | 2.27 ± 0.11 c | 1.48 ± 0.07 b | 2.28 ± 0.11 a | 1.49 ± 0.08 a | 80.50 ± 1.59 ab |
| MKS-C | 83.80 ± 1.67 a | 5.17 ± 0.25 f | 2.11 ± 0.10 bc | 1.83 ± 0.09 c | 3.34 ± 0.16 d | 3.06 ± 0.15 e | 81.70 ± 1.63 b |
| Sample Code | Ultrasound Conditions | |
|---|---|---|
| Time min | Power W | |
| MKSU10-300 | 10 | 300 |
| MKSU20-120 | 20 | 120 |
| MKSU20-300 | 20 | 300 |
| MKSU20-480 | 20 | 480 |
| MKSU30-300 | 30 | 300 |
| MKS-C | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mieles-Gómez, L.; Quintana, S.E.; García-Zapateiro, L.A. Ultrasound-Assisted Extraction of Mango (Mangifera indica) Kernel Starch: Chemical, Techno-Functional, and Pasting Properties. Gels 2023, 9, 136. https://doi.org/10.3390/gels9020136
Mieles-Gómez L, Quintana SE, García-Zapateiro LA. Ultrasound-Assisted Extraction of Mango (Mangifera indica) Kernel Starch: Chemical, Techno-Functional, and Pasting Properties. Gels. 2023; 9(2):136. https://doi.org/10.3390/gels9020136
Chicago/Turabian StyleMieles-Gómez, Luis, Somaris E. Quintana, and Luis A. García-Zapateiro. 2023. "Ultrasound-Assisted Extraction of Mango (Mangifera indica) Kernel Starch: Chemical, Techno-Functional, and Pasting Properties" Gels 9, no. 2: 136. https://doi.org/10.3390/gels9020136
APA StyleMieles-Gómez, L., Quintana, S. E., & García-Zapateiro, L. A. (2023). Ultrasound-Assisted Extraction of Mango (Mangifera indica) Kernel Starch: Chemical, Techno-Functional, and Pasting Properties. Gels, 9(2), 136. https://doi.org/10.3390/gels9020136









