Particle Agglomeration and Properties of Pregelatinized Potato Starch Powder
Abstract
:1. Introduction
2. Results and Discussion
2.1. Particle Size Distribution (PSD)
2.2. Flowability, Cohesiveness, and Porosity (ε)
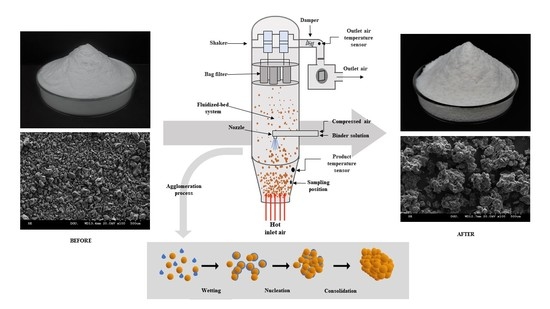
2.3. Morphology of Powder Particle
2.4. Powder Dispersibility and Solubility
2.5. Gel Strength
2.6. Rheological Properties
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. FBAP
4.3. Particle Size Distribution Measurements
4.4. Flowability and Cohesiveness Measurements
4.5. Particle Density (ρparticle) and Porosity (ε) Measurements
4.6. Scanning Electron Microscopy (SEM)
4.7. Powder Dispersibility
4.8. Solubility
4.9. Gel Strength
4.10. Rheological Properties
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.-T.; Wang, R.-S.; Liang, R.-H.; Chen, J.; He, X.-H.; Chen, R.-Y.; Liu, W.; Liu, C.-M. Dynamic High-pressure Microfluidization Assisting Octenyl succinic anhydride Modification of Rice Starch. Carbohydr. Polym. 2018, 193, 336–342. [Google Scholar] [CrossRef]
- Thomas, D.J.; Atwell, W.A. Starches; Eagan Press: St. Paul, MN, USA, 1999; pp. 54–60. [Google Scholar]
- Hedayati, S.; Shahidi, F.; Koocheki, A.; Farahnaky, A.; Majzoobi, M. Physical Properties of Pregelatinized and Granular Cold Water Swelling Maize Starches at Different pH Values. Int. J. Biol. Macromol. 2016, 91, 730–735. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Castanha, N.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E. Starch Modification through Environmentally Friendly Alternatives: A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2482–2505. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, M.; Liang, Y.; Zheng, X.; Sun, L.; Dang, W.; Li, J.; Li, L.; Liu, C. Research Progress on Properties of Pre-gelatinized Starch and its Application in Wheat Flour Products. Grain Oil Sci. Technol. 2022, 5, 87–97. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Zheng, X. A Review of Milling Damaged Starch: Generation, Measurement, Functionality and its Effect on Starch-based Food Systems. Food Chem. 2020, 315, 126267. [Google Scholar] [CrossRef]
- Anastasiades, A.; Thanou, S.; Loulis, D.; Stapatoris, A.; Karapantsios, T.D. Rheological and Physical Characterization of Pregelatinized Maize Starches. J. Food Eng. 2002, 52, 57–66. [Google Scholar] [CrossRef]
- Fitzpatrick, J.J.; Ahrné, L. Food Powder Handling and Processing: Industry Problems, Knowledge Barriers and Research Opportunities. Chem. Eng. Process. 2005, 44, 209–214. [Google Scholar] [CrossRef]
- Barbosa-Cánova, G.V.; Ortega, R.E.; Juliano, P.; Yan, H. Food Powders: Physical Properties, Processing, and Functionality, Kindle ed; Woodhead Publishing: Cambridge, UK, 2005; pp. 175–198. [Google Scholar]
- Ji, J.; Cronin, K.; Fitzpatrick, J.; Fenelon, M.; Miao, S. Effects of Fluid Bed Agglomeration on the Structure Modification and Reconstitution Behaviour of Milk Protein Isolate Powders. J. Food Eng. 2015, 167, 175–182. [Google Scholar] [CrossRef]
- Lee, H.; Yoo, B. Agglomeration of Galactomannan Gum Powders: Physical, Rheological, and Structural Characterizations. Carbohydr. Polym. 2021, 256, 117599. [Google Scholar] [CrossRef]
- Dacanal, G.C.; Feltre, G.; Thomazi, M.G.; Menegalli, F.C. Effects of Pulsating Air Flow in Fluid Bed Agglomeration of Starch Particles. J. Food Eng. 2016, 181, 67–83. [Google Scholar] [CrossRef]
- Barkouti, A.; Turchiuli, C.; Carcel, J.A.; Dumoulin, E. Milk Powder Agglomerate Growth and Properties in Fluidized Bed Agglomeration. Dairy Sci. Technol. 2013, 93, 523–535. [Google Scholar] [CrossRef]
- Lee, H.; Yoo, B. Agglomerated Xanthan Gum Powder Used as a Food Thickener: Effect of Sugar Binders on Physical, Microstructural, and Rheological Properties. Powder Technol. 2020, 362, 301–306. [Google Scholar] [CrossRef]
- Vengateson, U.; Mohan, R. Experimental and Modeling Study of Fluidized Bed Granulation: Effect of Binder Flow Rate and Fluidizing Air Velocity. Resour. Effic. Technol. 2016, 2, S124–S135. [Google Scholar] [CrossRef] [Green Version]
- Rajniak, P.; Mancinelli, C.; Chern, R.; Stepanek, F.; Farber, L.; Hill, B. Experimental Study of Wet Granulation in Fluidized Bed: Impact of the Binder Properties on the Granule Morphology. Int. J. Pharm. 2007, 334, 92–102. [Google Scholar] [CrossRef]
- Terrazas-Velarde, K.; Peglow, M.; Tsotsas, E. Kinetics of Fluidized Bed Spray Agglomeration for Compact and Porous Particles. Chem. Eng. Sci. 2011, 66, 1866–1878. [Google Scholar] [CrossRef]
- Pathare, P.B.; Baş, N.; Fitzpatrick, J.J.; Cronin, K.; Byrne, E.P. Production of Granola Breakfast Cereal by Fluidised Bed Granulation. Food Bioprod. Process 2012, 90, 549–554. [Google Scholar] [CrossRef]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of Instant Soymilk Powders by Ultrafiltration, Spray Drying and Fluidized Bed Agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- Mort, P.R. Scale-up of Binder Agglomeration Processes. Powder Technol. 2005, 150, 86–103. [Google Scholar] [CrossRef]
- Thiengnoi, P.; Suphantharika, M.; Wongkongkatep, P. Influence of Binder Typer and Concentration on Physical Properties of Agglomerated, Spray-dried, and High Oil Loaded Microcapsules. J. Food Agric. Environ. 2012, 10, 141–150. [Google Scholar]
- Li, Q.; Rudolph, V.; Weigl, B.; Earl, A. Interparticle Van der Waals Force in Powder Flowability and Compactibility. Int. J. Pharm. 2004, 280, 77–93. [Google Scholar] [CrossRef]
- Beach, E.; Tormoen, G.; Drelich, J.; Han, R. Pull-off Force Measurements Between Rough Surfaces by Atomic Force Microscopy. J. Colloid Interface Sci. 2002, 247, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.L. Classifying Flow Properties of Solids. Chem. Eng. 1965, 1, 69–72. [Google Scholar]
- Avilés-Avilés, C.; Dumoulin, E.; Turchiuli, C. Fluidised Bed Agglomeration of Particles with Different Glass Transition Temperatures. Powder Technol. 2015, 270, 445–452. [Google Scholar] [CrossRef]
- Nascimento, R.F.; Ávila, M.F.; Taranto, O.P.; Kurozawa, L.E. Agglomeration in Fluidized Bed: Bibliometric Analysis, a Review, and Future Perspectives. Powder Technol. 2022, 406, 117597. [Google Scholar] [CrossRef]
- Gaiani, C.; Schuck, P.; Scher, J.; Desobry, S.; Banon, S. Dairy Powder Rehydration: Influence of Protein State, Incorporation Mode, and Agglomeration. J. Dairy Sci. 2007, 90, 570–581. [Google Scholar] [CrossRef]
- Hafsa, I.; Mandato, S.; Ruiz, T.; Schuck, P.; Jeantet, R.; Mejean, S.; Chevallier, S.; Cuq, B. Impact of the Agglomeration Process on Structure and Functional Properties of the Agglomerates Based on the Durum Wheat Semolina. J. Food Eng. 2015, 145, 25–36. [Google Scholar] [CrossRef]
- BeMiller, J.N. Carbohydrate Chemistry for Food Scientists, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 137–143. [Google Scholar]
- Kyung, J.S.; Yoo, B. Rheological, Thermal, and Gelling Properties of Azuki Bean Starch with Sucrose. Starch-Stärke 2015, 67, 1020–1025. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Williams, P.A. Effect of Sugars on the Thermal and Rheological Properties of Sago Starch. Biopolymers 1999, 50, 401–412. [Google Scholar] [CrossRef]
- Szczesniak, A.S.; Farkas, E. Objective Characterization of the Mouthfeel of Gum Solutions. J. Food Sci. 1962, 27, 381–385. [Google Scholar] [CrossRef]
- Lee, D.; Min, G.; Roh, W.; Yoo, B. Effect of Various Types of Sugar Binder on the Physical Properties of Gum Powders Prepared via Fluidized-bed Agglomeration. Foods 2021, 10, 1387. [Google Scholar] [CrossRef]
- Hausner, H.H. Friction Conditions in a Mass of Metal Powder. Int. J. Powder Met. 1967, 3, 7–13. [Google Scholar]
- Carr, R.L. Evaluating Flow Properties of Solids. Chem. Eng. 1965, 72, 163–168. [Google Scholar]
- Bello-Pérez, L.A.; Aparicio-Saguilán, A.; Méndez-Montealvo, G.; Solorza-Feria, J.; Flores-Huicochea, E. Isolation and Partial Characterization of Mango (Magnifera indica L.) Starch: Morphological, Physicochemical and Functional Studies. Plant Foods Hum. Nutr. 2005, 60, 7–12. [Google Scholar] [CrossRef] [PubMed]






| Sample | MD Concentration (%) | D10 (μm) | D50 (μm) | D90 (μm) | Span |
|---|---|---|---|---|---|
| Raw PPSP | 17.2 ± 0.01 f | 64.8 ± 0.07 e | 165 ± 1.39 f | 2.28 ± 0.02 a | |
| Agglomerated PPSP | 0 (control) | 133 ± 3.00 a | 311 ± 7.93 a | 637 ± 12.5 a | 1.62 ± 0.01 c |
| 10 | 80.0 ± 0.91 e | 191 ± 1.08 c | 395 ± 3.35 b | 1.66 ± 0.02 b | |
| 20 | 104 ± 1.57 c | 195 ± 3.52 c | 331 ± 4.07 d | 1.17 ± 0.02 d | |
| 30 | 116 ± 0.59 b | 234 ± 1.21 b | 380 ± 1.14 c | 1.13 ± 0.00 e | |
| 40 | 88.4 ± 1.30 d | 179 ± 1.39 d | 300 ± 1.89 e | 1.18 ± 0.01 d |
| Sample | MD Concentration (%) | ρbulk (g/cm3) | ρtapped (g/cm3) | ε (%) | CI (%) | HR | Solubility (%) |
|---|---|---|---|---|---|---|---|
| Raw PPSP | 0.62 ± 0.01 a | 0.89 ± 0.02 a | 64.4 ± 0.79 c | 30.8 ± 0.48 a | 1.44 ± 0.00 a | 31.9 ± 1.02 d | |
| Agglomerated PPSP | 0 (control) | 0.22 ± 0.01 e | 0.31 ± 0.00 e | 89.6 ± 0.95 b | 27.4 ± 0.11 b | 1.38 ± 0.00 b | 93.1 ± 0.69 c |
| 10 | 0.33 ± 0.01 bc | 0.46 ± 0.00 b | 91.1 ± 2.31 ab | 27.2 ± 0.31 b | 1.37 ± 0.00 c | 96.0 ± 0.88 b | |
| 20 | 0.29 ± 0.01 d | 0.39 ± 0.01 d | 91.3 ± 0.79 ab | 24.7 ± 0.40 c | 1.33 ± 0.01 d | 97.5 ± 0.40 a | |
| 30 | 0.35 ± 0.01 b | 0.44 ± 0.01 c | 91.3 ± 1.75 ab | 20.8 ± 0.96 d | 1.26 ± 0.01 e | 95.8 ± 0.68 b | |
| 40 | 0.33 ± 0.00 c | 0.43 ± 0.01 c | 92.1 ± 1.51 a | 24.7 ± 0.59 c | 1.33 ± 0.01 d | 96.6 ± 0.51 ab |
| Sample | MD Concentration (%) | Gel Strength (gf) | ||
|---|---|---|---|---|
| 3 Days | 5 Days | 7 Days | ||
| Raw PPSP | 9.67 ± 0.58 d | 40.7 ± 2.31 d | 108 ± 4.16 d | |
| Agglomerated PPSP | 0 (control) | 21.7 ± 1.53 b | 66.3 ± 1.15 b | 161 ± 1.53 b |
| 10 | 32.3 ± 1.15 a | 76.7 ± 0.58 a | 177 ± 3.79 a | |
| 20 | 21.7 ± 2.08 b | 56.3 ± 1.53 c | 143 ± 2.31 c | |
| 30 | 12.0 ± 1.00 c | 43.0 ± 1.00 d | 111 ± 1.73 d | |
| 40 | 9.00 ± 1.00 e | 26.3 ± 0.58 e | 100 ± 1.00 e | |
| Sample | MD Concentration (%) | ηa,50 (Pa·s) | K (Pa·sn) | n |
|---|---|---|---|---|
| Raw PPSP | 1.47 ± 0.02 c | 4.78 ± 0.17 ab | 0.70 ± 0.01 d | |
| Agglomerated PPSP | 0 (control) | 1.67 ± 0.02 a | 4.89 ± 0.01 a | 0.73 ± 0.00 c |
| 10 | 1.58 ± 0.06 b | 4.70 ± 0.02 b | 0.72 ± 0.01 c | |
| 20 | 1.10 ± 0.02 d | 3.18 ± 0.10 c | 0.73 ± 0.00 c | |
| 30 | 0.76 ± 0.01 e | 1.45 ± 0.07 d | 0.77 ± 0.00 b | |
| 40 | 0.59 ± 0.00 f | 1.33 ± 0.07 d | 0.79 ± 0.01 a |
| Sample | MD Concentration (%) | G′ (Pa) | G″ (Pa) | tan δ |
|---|---|---|---|---|
| Raw PPSP | 6.71 ± 0.05 c | 12.1 ± 0.01 c | 1.80 ± 0.02 d | |
| Agglomerated PPSP | 0 (control) | 8.13 ± 0.03 b | 14.3 ± 0.08 b | 1.76 ± 0.00 e |
| 10 | 8.68 ± 0.04 a | 15.8 ± 0.03 a | 1.82 ± 0.01 d | |
| 20 | 5.48 ± 0.03 d | 11.1 ± 0.06 d | 2.02 ± 0.00 c | |
| 30 | 2.27 ± 0.04 e | 6.15 ± 0.04 e | 2.71 ± 0.04 b | |
| 40 | 1.86 ± 0.03 f | 5.70 ± 0.08 f | 3.07 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Yoo, B. Particle Agglomeration and Properties of Pregelatinized Potato Starch Powder. Gels 2023, 9, 93. https://doi.org/10.3390/gels9020093
Lee H, Yoo B. Particle Agglomeration and Properties of Pregelatinized Potato Starch Powder. Gels. 2023; 9(2):93. https://doi.org/10.3390/gels9020093
Chicago/Turabian StyleLee, Hyunwoo, and Byoungseung Yoo. 2023. "Particle Agglomeration and Properties of Pregelatinized Potato Starch Powder" Gels 9, no. 2: 93. https://doi.org/10.3390/gels9020093