Antibacterial Hydrogel Sheet Dressings Composed of Poly(vinyl alcohol) and Silver Nanoparticles by Electron Beam Irradiation
Abstract
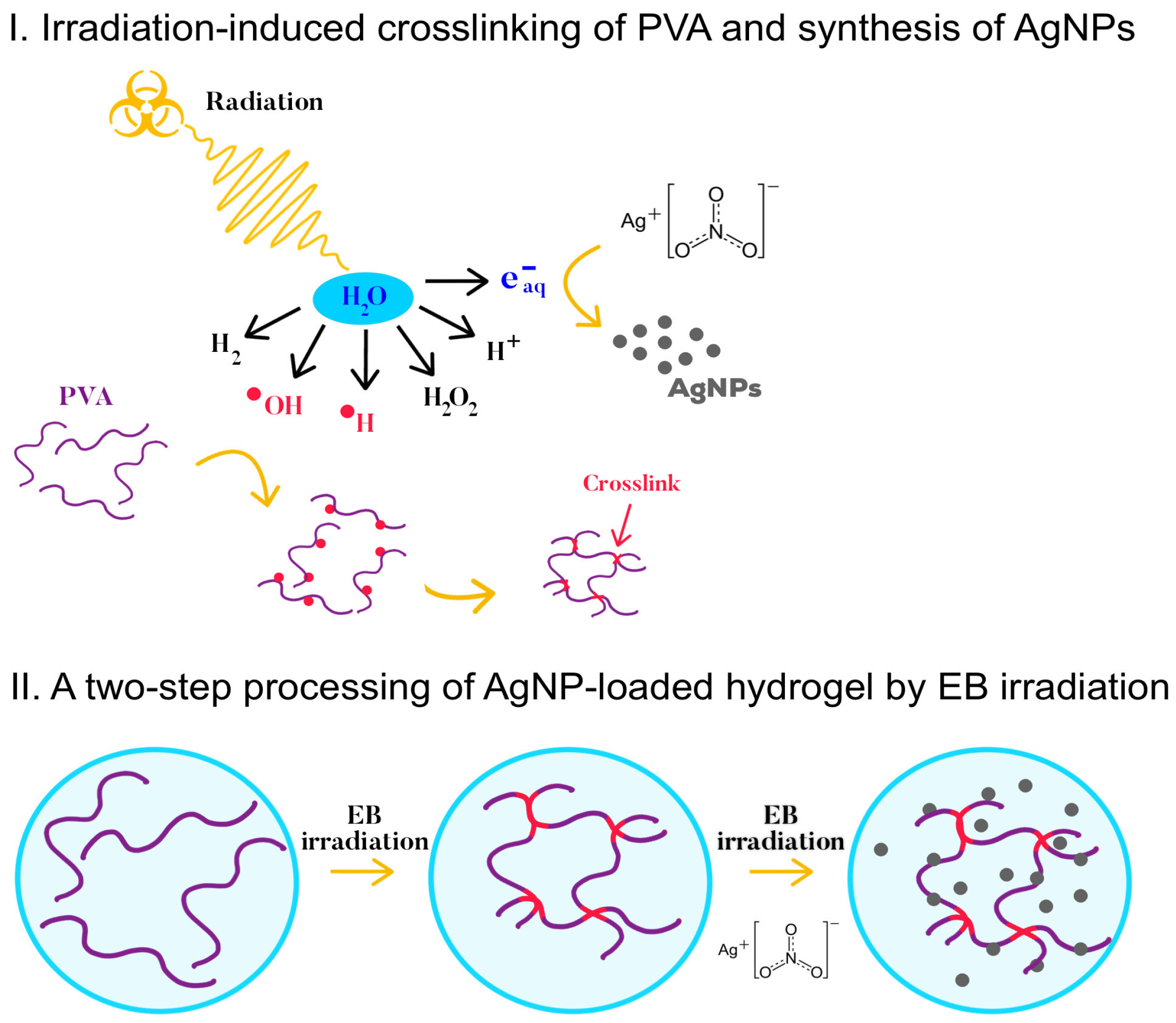
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization of the Hydrogels
2.2. Thermal Properties of the Crosslinked Hydrogels
2.3. Morphology of the Hydrogels
2.4. Effect of Repeated Irradiation on the Sterility, Swelling, and Mechanical Properties of the Hydrogel Sheets
2.5. Antibacterial Properties of the AgNP-Loaded Hydrogel Sheets
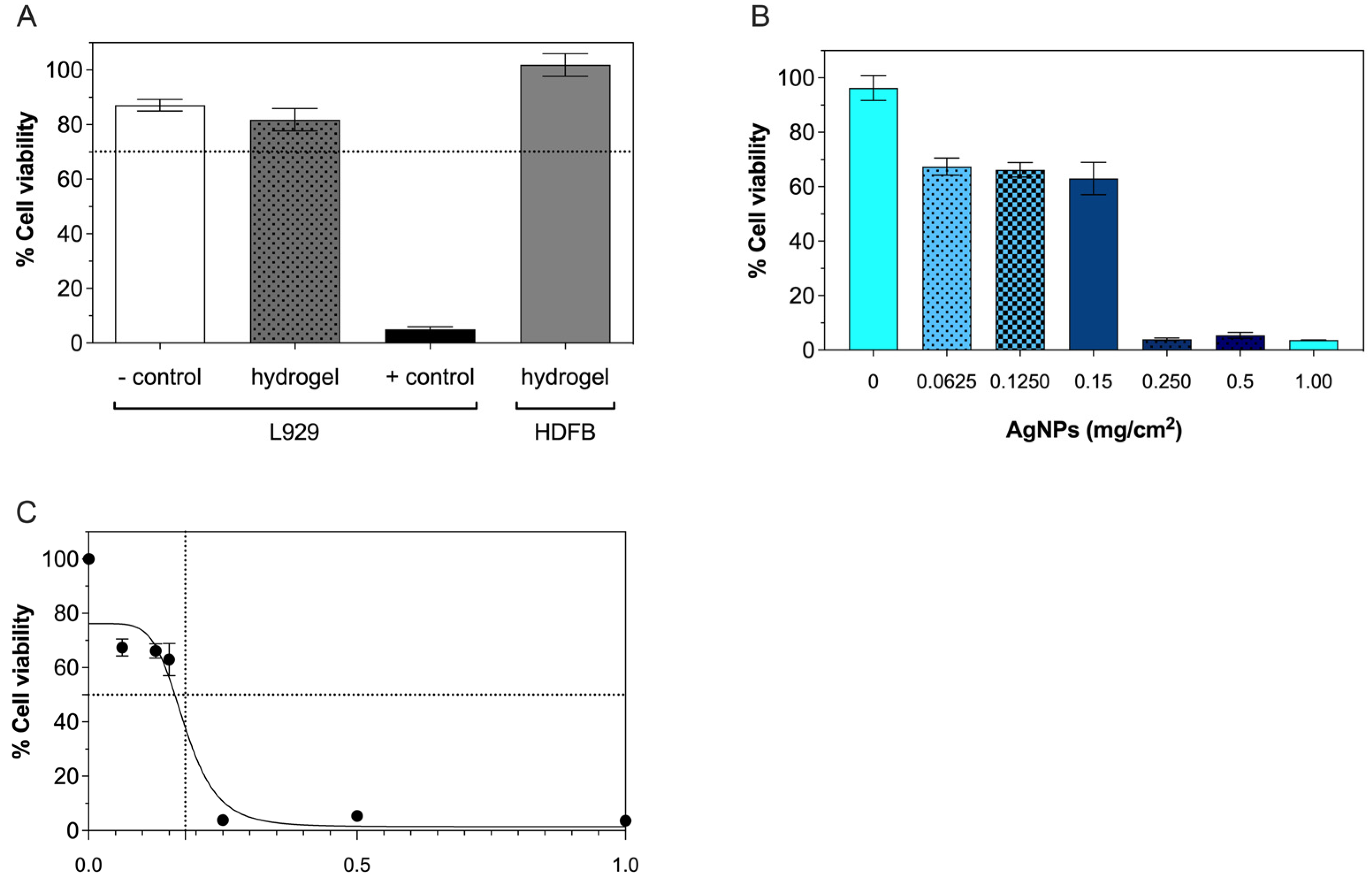
2.6. Cytocompatibility
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the PVA Hydrogels
4.3. Hydrogel Characterization
4.3.1. Swelling
4.3.2. Gel Fraction
4.3.3. Thermal Analysis
4.3.4. Morphology Analysis
4.4. Analysis of the Tensile Properties
4.5. Synthesis of the AgNPs by EB Irradiation on the Hydrogel Sheets
| Solution | Concentration of AgNO3 (mg/mL) |
|---|---|
| A | 80 |
| B | 40 |
| C | 20 |
| D | 10 |
| E | 5 |
4.6. Antibacterial Properties
4.7. In Vitro Cytotoxicity Test
4.8. Inductively Couple Plasma-Mass Spectrometry Analysis
4.9. Test of Sterility
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in Skin Grafting and Treatment of Cutaneous Wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Jiang, N.; Sun, D.; Wang, Y.; Chen, X.; Zhu, S.; Zhang, L. A Fast UV-Curable PU-PAAm Hydrogel with Mechanical Flexibility and Self-Adhesion for Wound Healing. RSC Adv. 2020, 10, 4907–4915. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, F.; Guo, Z.; Xiao, Y.; Zhang, Y.; Sun, X.; Zhe, T.; Cao, Y.; Wang, L.; Lu, Q.; et al. Silver Nanoparticle-Embedded Hydrogel as a Photothermal Platform for Combating Bacterial Infections. Chem. Eng. J. 2020, 382, 122990. [Google Scholar] [CrossRef]
- Massarelli, E.; Silva, D.; Pimenta, A.F.R.; Fernandes, A.I.; Mata, J.L.G.; Armês, H.; Salema-Oom, M.; Saramago, B.; Serro, A.P. Polyvinyl Alcohol/Chitosan Wound Dressings Loaded with Antiseptics. Int. J. Pharm. 2021, 593, 120110. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, X.; Ren, Y.; Xue, W.; Liu, S.; Wang, P.; Zhao, M.; Xu, H.; Chi, B. Injectable Adaptive Self-Healing Hyaluronic Acid/Poly (γ-Glutamic Acid) Hydrogel for Cutaneous Wound Healing. Acta Biomater. 2021, 127, 102–115. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite Hydrogels Based on Gelatin, Chitosan and Polyvinyl Alcohol to Biomedical Applications: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Jaffe, L.; Wu, S.C. Dressings, Topical Therapy, and Negative Pressure Wound Therapy. Clin. Podiatr. Med. Surg. 2019, 36, 397–411. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Ulański, P. Synthesis of Hydrogels by Irradiation of Polymers in Aqueous Solution. Radiat. Phys. Chem. 1999, 55, 139–151. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Yoshii, F. Hydrogels and Their Medical Applications. Nucl. Instrum. Methods Phys. Res. B 1999, 151, 56–64. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Ashfaq, A.; Clochard, M.-C.; Coqueret, X.; Dispenza, C.; Driscoll, M.; Ulański, P.; Al-Sheikhly, M. Polymerization Reactions and Modifications of Polymers by Ionizing Radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef] [PubMed]
- Nešporová, K.; Pavlík, V.; Šafránková, B.; Vágnerová, H.; Odráška, P.; Žídek, O.; Císařová, N.; Skoroplyas, S.; Kubala, L.; Velebný, V. Effects of wound dressings containing silver on skin and immune cells. Sci. Rep. 2020, 10, 15216. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Singh, M.; Mudila, H. Silver nanoparticles as antimicrobial therapeutics: Current perspectives and future challenges. 3 Biotech 2018, 8, 411. [Google Scholar] [CrossRef]
- Leawhiran, N.; Pavasant, P.; Soontornvipart, K.; Supaphol, P. Gamma irradiation synthesis and characterization of AgNP/gelatin/PVA hydrogels for antibacterial wound dressings. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Eid, M.; El-Arnaouty, M.B.; Salah, M.; Soliman, E.-S.; Hegazy, E.-S.A. Radiation synthesis and characterization of poly(vinyl alcohol)/poly(N-vinyl-2-pyrrolidone) based hydrogels containing silver nanoparticles. J. Polym. Res. 2012, 19, 9835. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Ferreira, L.; Figueiredo, M.M.; Gil, M.H.; Ramos, M.A. Structural analysis of dextran-based hydrogels obtained chemoenzymatically. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77, 55–64. [Google Scholar] [CrossRef]
- Ruiz, J.; Mantecón, A.; Cádiz, V. Synthesis and properties of hydrogels from poly (vinyl alcohol) and ethylenediaminetetraacetic dianhydride. Polymer 2001, 42, 6347–6354. [Google Scholar] [CrossRef]
- Benamer, S.; Mahlous, M.; Boukrif, A.; Mansouri, B.; Youcef, S.L. Synthesis and characterisation of hydrogels based on poly(vinyl pyrrolidone). Nucl. Instrum. Methods Phys. Res. B 2006, 248, 284–290. [Google Scholar] [CrossRef]
- Kumar, M.; Varshney, L.; Francis, S. Radiolytic formation of Ag clusters in aqueous polyvinyl alcohol solution and hydrogel matrix. Radiat. Phys. Chem. 2005, 73, 21–27. [Google Scholar] [CrossRef]
- Peppas, N.A.; Merrill, E.W. Poly(vinyl alcohol) hydrogels: Reinforcement of radiation-crosslinked networks by crystallization. J. Polym. Sci. Polym. Chem. Ed. 1976, 14, 441–457. [Google Scholar] [CrossRef]
- Zhao, L.; Mitomo, H.; Zhai, M.; Yoshii, F.; Nagasawa, N.; Kume, T. Synthesis of antibacterial PVA/CM-chitosan blend hydrogels with electron beam irradiation. Carbohydr. Polym. 2003, 53, 439–446. [Google Scholar] [CrossRef]
- Zhai, M.; Yoshii, F.; Kume, T.; Hashim, K. Syntheses of PVA/starch grafted hydrogels by irradiation. Carbohydr. Polym. 2002, 50, 295–303. [Google Scholar] [CrossRef]
- Reinhart, C.T.; Peppas, N.A. Solute diffusion in swollen membranes. Part II. Influence of crosslinking on diffusive properties. J. Membr. Sci. 1984, 18, 227–239. [Google Scholar] [CrossRef]
- Uttayarat, P.; Chiangnoon, R.; Eamsiri, J.; Senawongse, W. Processing and Characterization of Antibacterial Hydrogel Sheet Dressings Composed of Poly(vinyl alcohol) and Silk Fibroin for Wound Healing Application. Walailak J. Sci. Technol. 2019, 16, 349–359. [Google Scholar] [CrossRef]
- Domínguez, A.V.; Algaba, R.A.; Canturri, A.M.; Villodres, R.; Smani, Y. Antibacterial Activity of Colloidal Silver against Gram-Negative and Gram-Positive Bacteria. Antibiotics 2020, 9, 36. [Google Scholar] [CrossRef]
- Alinejad, F.; Momeni, M.; Fatemi, M.J.; Dahmardehei, M.; Naderi, S.; Akhoondinasab, M.R.; Zayedly, M.; Mahboubi, O.; Rahbar, H. Comparing the effect of two types of silver nano-crystalline dressings (acticoat and agcoat) in the treatment of full thickness burn wound. Iran. J. Microbiol. 2018, 10, 378–384. [Google Scholar]
- Ipe, D.S.; Kumar, P.T.S.; Love, R.M.; Hamlet, S.M. Silver Nanoparticles at Biocompatible Dosage Synergistically Increases Bacterial Susceptibility to Antibiotics. Front. Microbiol. 2020, 11, 1074. [Google Scholar] [CrossRef]
- Pimton, P.; Ratphibun, P.; Tassaneesuwan, N.; Chiangnoon, R.; Uttayarat, P. Cytotoxicity Evaluation of Hydrogel Sheet Dressings Fabricated by Gamma Irradiation: Extract and Semi-Direct Contact Tests. Trends Sci. 2022, 19, 4583. [Google Scholar] [CrossRef]
- Srivastava, G.K.; Alonso-Alonso, M.L.; Fernandez-Bueno, I.; Garcia-Gutierrez, M.T.; Rull, F.; Medina, J.; Coco, R.M.; Pastor, J.C. Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation. Sci. Rep. 2018, 8, 1425. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [PubMed]
- Botha, T.L.; Elemike, E.E.; Horn, S.; Onwudiwe, D.C.; Giesy, J.P.; Wepener, V. Cytotoxicity of Ag, Au and Ag-Au bimetallic nanoparticles prepared using golden rod (Solidago canadensis) plant extract. Sci. Rep. 2019, 9, 4169. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Sun, X.; He, Q.-Y. Cytotoxicity of silver nanoparticles against bacteria and tumor cells. Curr. Protein Pept. Sci. 2018, 19, 525–536. [Google Scholar] [CrossRef]
- Varshney, L. Role of natural polysaccharides in radiation formation of PVA–hydrogel wound dressing. Nucl. Instrum. Methods Phys. Res. B 2007, 255, 343–349. [Google Scholar] [CrossRef]





| Dose (kGy) | Gel Fraction (%) | EDS (%) | Mc (g/mol) | ρx (10−4 mol/cm3) | ε (Å) |
|---|---|---|---|---|---|
| 10 | 25.1 ± 16.1 | - | - | - | - |
| 25 | 86.6 ± 5.1 | 2554 ± 294 | 16,240 | 0.8 | 358 |
| 40 | 93.5 ± 2.9 | 1348 ± 222 | 6770 | 1.9 | 173 |
| 60 | 92.0 ± 7.0 | 1311 ± 133 | 6250 | 2.0 | 163 |
| 80 | 93.8 ± 1.0 | 892 ± 17 | 3300 | 3.9 | 130 |
| Sample | Total AgNPs (mg/cm2) | Inhibition Ratio | |
|---|---|---|---|
| E. coli | S. aureus | ||
| Hydrogel A | 1.0 | 1.78 ± 0.09 | 1.50 ± 0.01 |
| Hydrogel B | 0.5 | 1.66 ± 0.01 | 1.26 ± 0.02 |
| Hydrogel C | 0.25 | 1.50 ± 0.17 | - |
| Hydrogel D | 0.125 | - | - |
| Hydrogel E | 0.0625 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiangnoon, R.; Karawak, P.; Eamsiri, J.; Nuchdang, S.; Thamrongsiripak, N.; Neramitmansook, N.; Pummarin, S.; Pimton, P.; Nilgumhang, K.; Uttayarat, P. Antibacterial Hydrogel Sheet Dressings Composed of Poly(vinyl alcohol) and Silver Nanoparticles by Electron Beam Irradiation. Gels 2023, 9, 80. https://doi.org/10.3390/gels9020080
Chiangnoon R, Karawak P, Eamsiri J, Nuchdang S, Thamrongsiripak N, Neramitmansook N, Pummarin S, Pimton P, Nilgumhang K, Uttayarat P. Antibacterial Hydrogel Sheet Dressings Composed of Poly(vinyl alcohol) and Silver Nanoparticles by Electron Beam Irradiation. Gels. 2023; 9(2):80. https://doi.org/10.3390/gels9020080
Chicago/Turabian StyleChiangnoon, Rattanakorn, Pennapa Karawak, Jarurattana Eamsiri, Sasikarn Nuchdang, Nuatawan Thamrongsiripak, Naruemon Neramitmansook, Siwanut Pummarin, Pimchanok Pimton, Kewalee Nilgumhang, and Pimpon Uttayarat. 2023. "Antibacterial Hydrogel Sheet Dressings Composed of Poly(vinyl alcohol) and Silver Nanoparticles by Electron Beam Irradiation" Gels 9, no. 2: 80. https://doi.org/10.3390/gels9020080
APA StyleChiangnoon, R., Karawak, P., Eamsiri, J., Nuchdang, S., Thamrongsiripak, N., Neramitmansook, N., Pummarin, S., Pimton, P., Nilgumhang, K., & Uttayarat, P. (2023). Antibacterial Hydrogel Sheet Dressings Composed of Poly(vinyl alcohol) and Silver Nanoparticles by Electron Beam Irradiation. Gels, 9(2), 80. https://doi.org/10.3390/gels9020080







