Fibrillogenesis and Hydrogel Formation from Fibrinogen Induced by Calcium Salts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Earth Alkaline Metal Chlorides
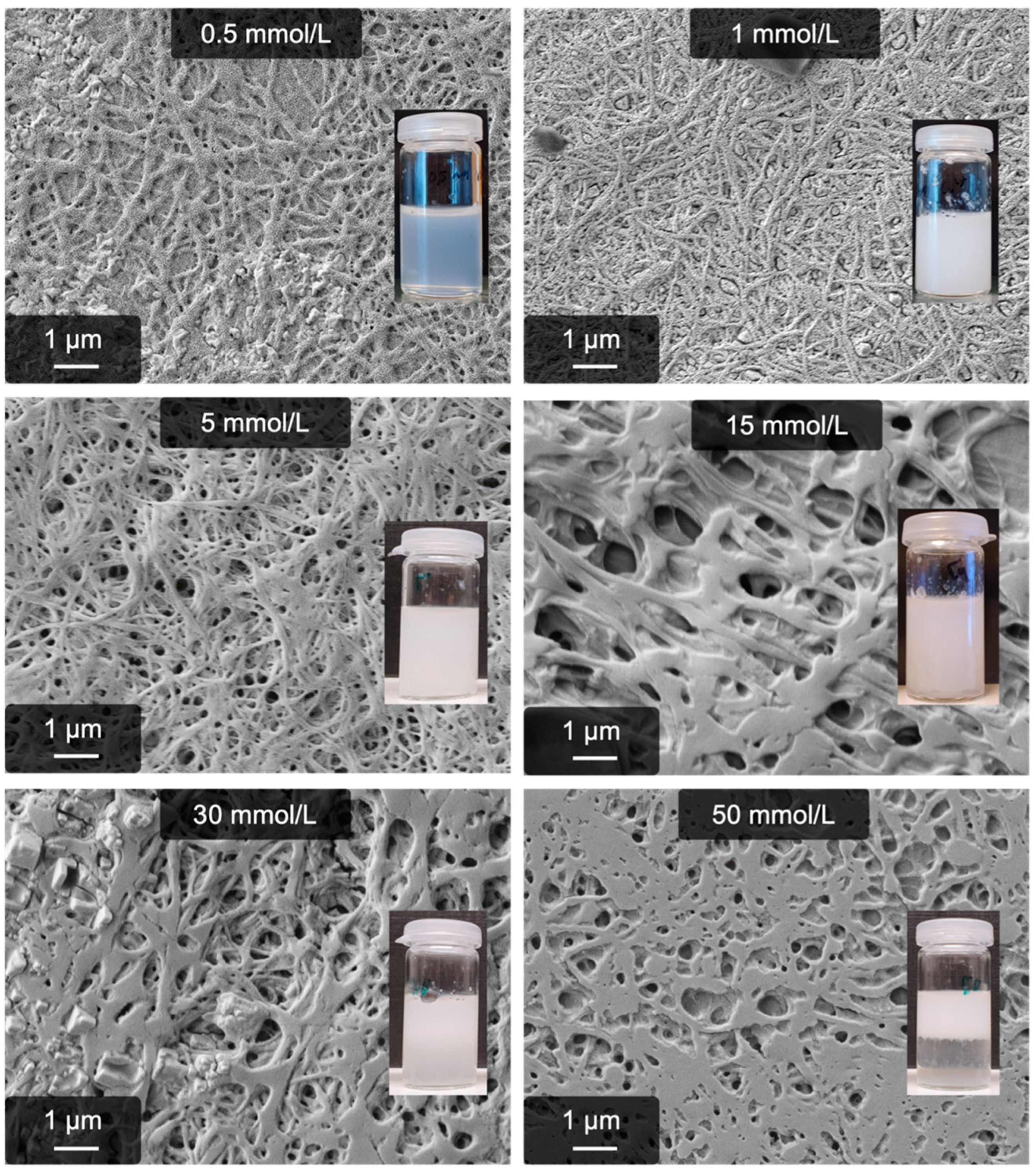
2.2. Calcium-Induced Gelation
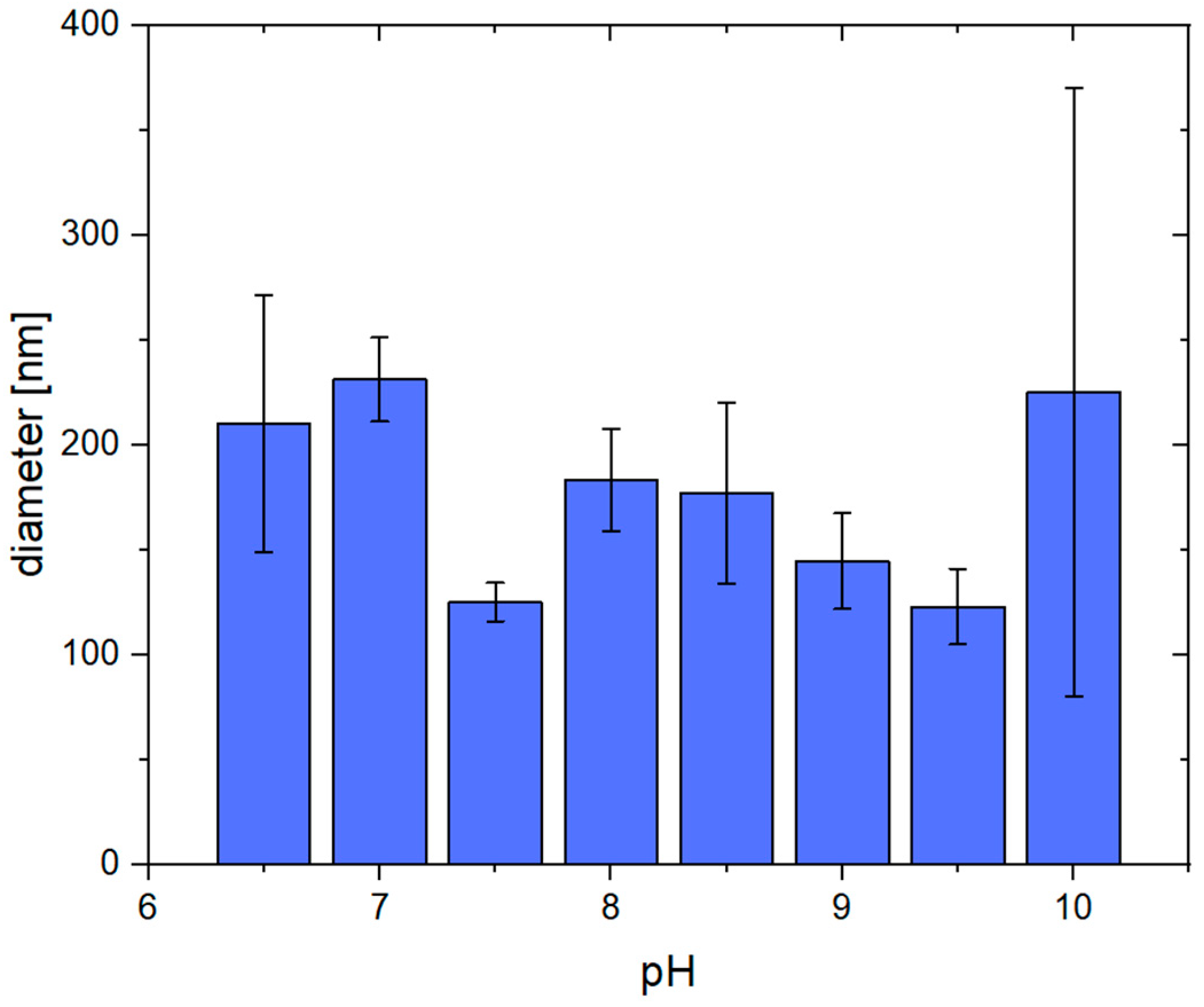
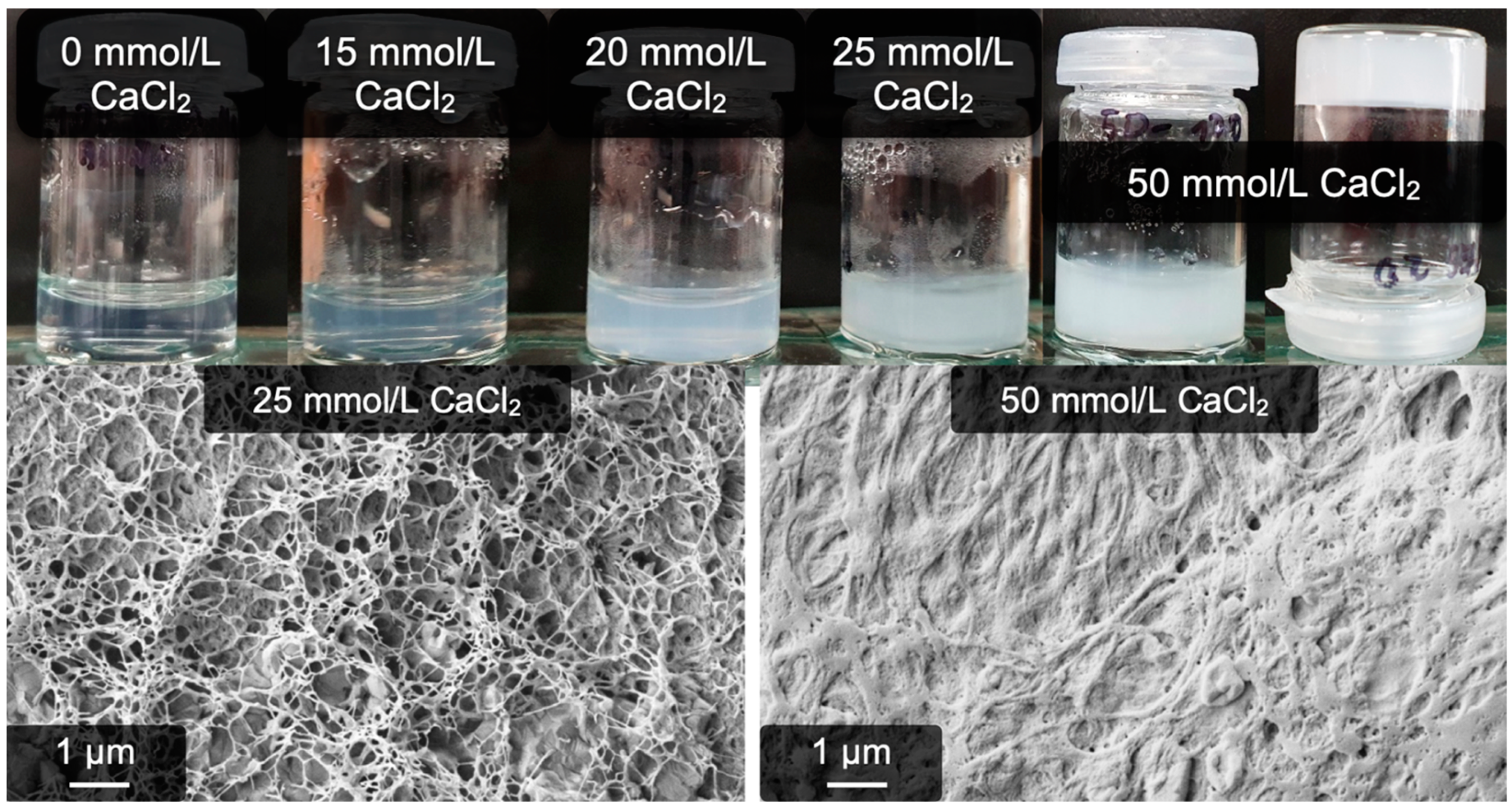
2.3. Calcium-Induced Gelation under Physiological Conditions
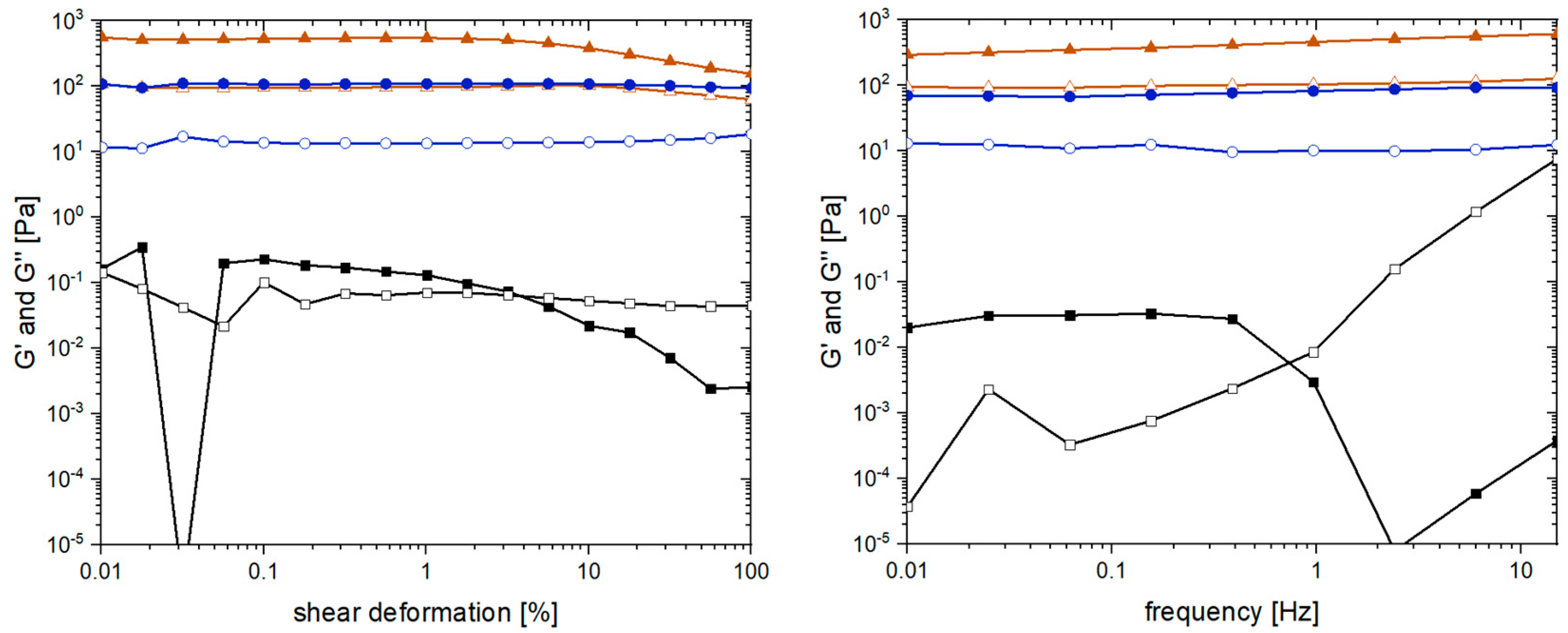
2.4. Yield, Water Content, and Rheological Properties
3. Conclusions
4. Materials and Methods
4.1. Materials
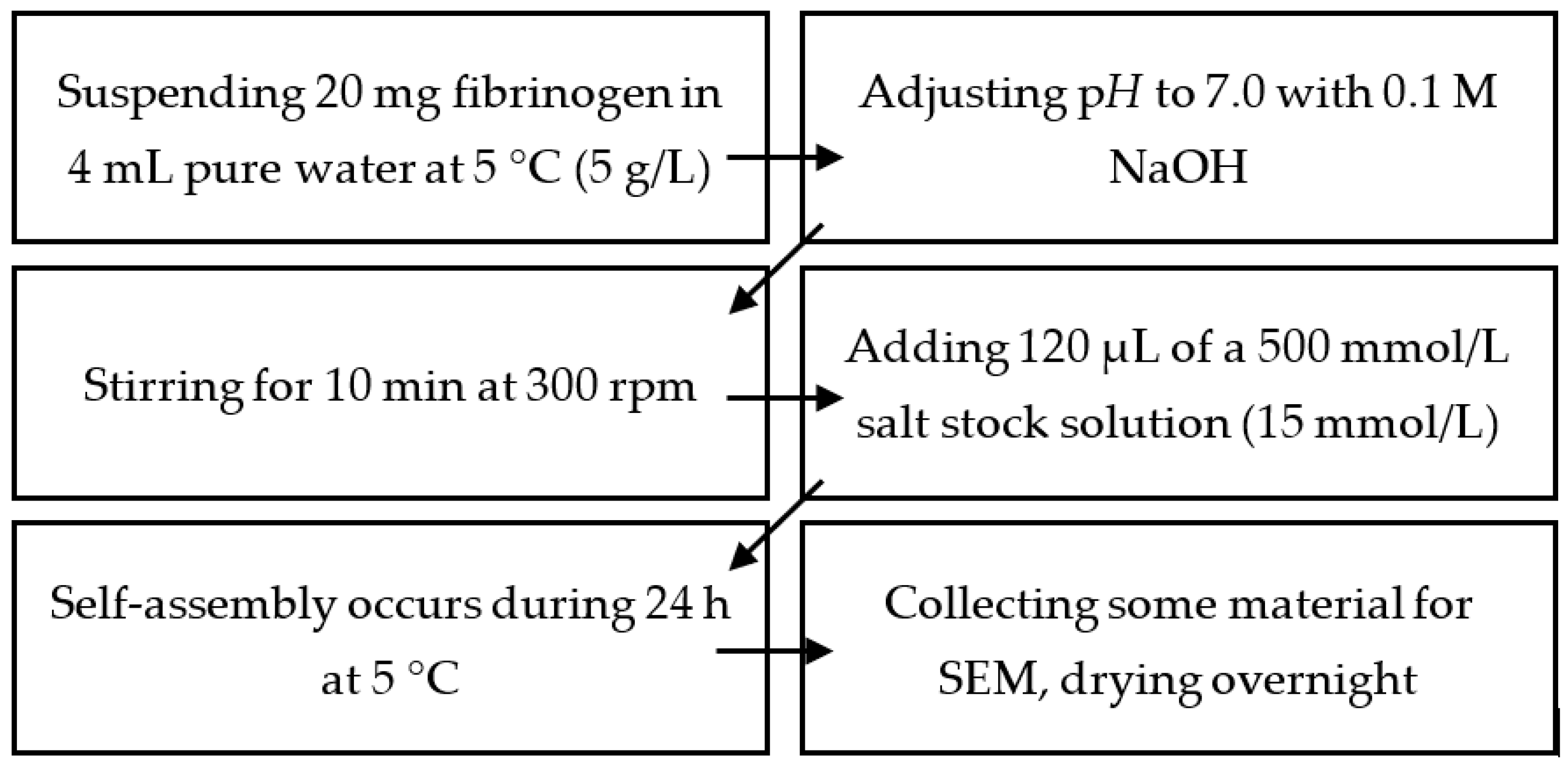
4.2. Production of Mg2+-, Ca2+-,Sr2+-, and Ba2+-Induced Hydrogels
4.3. Production of Fibrinogen Hydrogels under Physiological Conditions
4.4. Inhibition of Thrombin and Factor XIII
4.5. Fibrinogen and Water Content of Calcium-Induced Gels Produced under Physiological Conditions
4.6. Scanning Electron Microscopy (SEM) and Image Analysis
4.7. Rheology
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinney, W.B. Casein Paint and Method of Preparing the Same. U.S. Patent US2212566A, 27 August 1940. [Google Scholar]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A Reversible Wet/Dry Adhesive Inspired by Mussels and Geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef]
- Kopeček, J. Hydrogel Biomaterials: A Smart Future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [Green Version]
- Soorbaghi, F.P.; Isanejad, M.; Salatin, S.; Ghorbani, M.; Jafari, S.; Derakhshankhah, H. Bioaerogels: Synthesis Approaches, Cellular Uptake, and the Biomedical Applications. Biomed. Pharmacother. 2019, 111, 964–975. [Google Scholar] [CrossRef]
- Strube, O.I.; Rüdiger, A.A.; Bremser, W. Buildup of Biobased Adhesive Layers by Enzymatically Controlled Deposition on the Example of Casein. Int. J. Adhes. Adhes. 2015, 63, 9–13. [Google Scholar] [CrossRef]
- Rüdiger, A.A.; Bremser, W.; Strube, O.I. Nanoscaled Biocoatings via Enzyme Mediated Autodeposition of Casein. Macromol. Mater. Eng. 2016, 301, 1181–1190. [Google Scholar] [CrossRef]
- Rüdiger, A.A.; Brassat, K.; Lindner, J.K.N.; Bremser, W.; Strube, O.I. Easily Accessible Protein Nanostructures via Enzyme Mediated Addressing. Langmuir 2018, 34, 4264–4270. [Google Scholar] [CrossRef]
- Strube, O.I.; Büngeler, A.; Bremser, W. Site-Specific In Situ Synthesis of Eumelanin Nanoparticles by an Enzymatic Autodeposition-Like Process. Biomacromolecules 2015, 16, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Strube, O.I.; Büngeler, A.; Bremser, W. Enzyme-Mediated In Situ Synthesis and Deposition of Nonaggregated Melanin Protoparticles. Macromol. Mater. Eng. 2016, 301, 801–804. [Google Scholar] [CrossRef]
- Büngeler, A.; Hämisch, B.; Strube, O.I. The Supramolecular Buildup of Eumelanin: Structures, Mechanisms, Controllability. Int. J. Mol. Sci. 2017, 18, 1901. [Google Scholar] [CrossRef] [Green Version]
- Weisel, J.W. Fibrinogen and Fibrin. Adv. Protein Chem. 2005, 70, 247–299. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, R.F. Fibrinogen and Fibrin. Annu. Rev. Biochem. 1984, 53, 195–229. [Google Scholar] [CrossRef]
- Brennan, M. Fibrin Glue. Blood Rev. 1991, 5, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Sierra, D.H. Fibrin Sealant Adhesive Systems: A Review of Their Chemistry, Material Properties and Clinical Applications. J. Biomater. Appl. 1993, 7, 309–352. [Google Scholar] [CrossRef]
- Linnes, M.P.; Ratner, B.D.; Giachelli, C.M. A Fibrinogen-Based Precision Microporous Scaffold for Tissue Engineering. Biomaterials 2007, 28, 5298–5306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Gregory, C.A.; Molnar, P.; Cui, X.; Jalota, S.; Bhaduri, S.B.; Boland, T. Viability and Electrophysiology of Neural Cell Structures Generated by the Inkjet Printing Method. Biomaterials 2006, 27, 3580–3588. [Google Scholar] [CrossRef] [PubMed]
- Radosevich, M.; Goubran, H.A.; Burnouf, T. Fibrin Sealant: Scientific Rationale, Production Methods, Properties, and Current Clinical Use. Vox Sang. 1997, 72, 133–143. [Google Scholar] [CrossRef]
- Weisel, J.W.; Medved, L. The Structure and Function of the AC Domains of Fibrinogen. Ann. N. Y. Acad. Sci. 2006, 936, 312–327. [Google Scholar] [CrossRef]
- Miyazaki, S.; Hashiguchi, N.; Sugiyama, M.; Takada, M.; Morimoto, Y. Fibrinogen Microspheres as Novel Drug Delivery Systems for Antitumor Drugs. Chem. Pharm. Bull. 1986, 34, 1370–1375. [Google Scholar] [CrossRef] [Green Version]
- Rejinold, N.S.; Muthunarayanan, M.; Deepa, N.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Development of Novel Fibrinogen Nanoparticles by Two-Step Co-Acervation Method. Int. J. Biol. Macromol. 2010, 47, 37–43. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Muthunarayanan, M.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. 5-Fluorouracil Loaded Fibrinogen Nanoparticles for Cancer Drug Delivery Applications. Int. J. Biol. Macromol. 2011, 48, 98–105. [Google Scholar] [CrossRef]
- Vasconcelos, D.M.; Gonçalves, R.M.; Almeida, C.R.; Pereira, I.O.; Oliveira, M.I.; Neves, N.; Silva, A.M.; Ribeiro, A.C.; Cunha, C.; Almeida, A.R.; et al. Fibrinogen Scaffolds with Immunomodulatory Properties Promote in Vivo Bone Regeneration. Biomaterials 2016, 111, 163–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wnek, G.E.; Carr, M.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of Nanofiber Fibrinogen Structures. Nano Lett. 2003, 3, 213–216. [Google Scholar] [CrossRef]
- Carlisle, C.R.; Coulais, C.; Namboothiry, M.; Carroll, D.L.; Hantgan, R.R.; Guthold, M. The Mechanical Properties of Individual, Electrospun Fibrinogen Fibers. Biomaterials 2009, 30, 1205–1213. [Google Scholar] [CrossRef] [Green Version]
- Sell, S.A.; Francis, M.P.; Garg, K.; McClure, M.J.; Simpson, D.G.; Bowlin, G.L. Cross-Linking Methods of Electrospun Fibrinogen Scaffolds for Tissue Engineering Applications. Biomed. Mater. 2008, 3, 045001. [Google Scholar] [CrossRef]
- McManus, M.C.; Sell, S.A.; Bowen, W.C.; Koo, H.P.; Simpson, D.G.; Bowlin, G.L. Electrospun Fibrinogen-Polydioxanone Composite Matrix: Potential for in Situ Urologic Tissue Engineering. J. Eng. Fibers Fabr. 2008, 3, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Rajangam, T.; Paik, H.J.; An, S.S.A. Fabricating Fibrinogen Microfibers with Aligned Nanostructure, as Biodegradable Threads for Tissue Engineering. Bull. Korean Chem. Soc. 2012, 33, 2075–2078. [Google Scholar] [CrossRef] [Green Version]
- Stapelfeldt, K.; Stamboroski, S.; Mednikova, P.; Brüggemann, D. Fabrication of 3D-Nanofibrous Fibrinogen Scaffolds Using Salt-Induced Self Assembly. Biofabrication 2019, 11, 025010. [Google Scholar] [CrossRef] [PubMed]
- Stapelfeldt, K.; Stamboroski, S.; Walter, I.; Suter, N.; Kowalik, T.; Michaelis, M.; Brüggemann, D. Controlling the Multiscale Structure of Nanofibrous Fibrinogen Scaffolds for Wound Healing. Nano Lett. 2019, 19, 6554–6563. [Google Scholar] [CrossRef] [PubMed]
- Stamboroski, S.; Boateng, K.; Lierath, J.; Kowalik, T.; Thiel, K.; Köppen, S.; Noeske, P.-L.M.; Brüggemann, D. Influence of Divalent Metal Ions on the Precipitation of the Plasma Protein Fibrinogen. Biomacromolecules 2021, 22, 4642–4658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Casey, B.; Galanakis, D.K.; Marmorat, C.; Skoog, S.; Vorvolakos, K.; Simon, M.; Rafailovich, M.H. The Influence of Surface Chemistry on Adsorbed Fibrinogen Conformation, Orientation, Fiber Formation and Platelet Adhesion. Acta Biomater. 2017, 54, 164–174. [Google Scholar] [CrossRef]
- Koo, J.; Rafailovich, M.H.; Medved, L.; Tsurupa, G.; Kudryk, B.J.; Liu, Y.; Galanakis, D.K. Evaluation of Fibrinogen Self-Assembly: Role of Its AC Region. J. Thromb. Haemost. 2010, 8, 2727–2735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gollwitzer, R.; Bode, W.; Karges, H.E. On the Aggregation of Fibrinogen Molecules. Thromb. Res. 1983, 29, 41–53. [Google Scholar] [CrossRef]
- Wei, G.; Reichert, J.; Jandt, K.D. Controlled Self-Assembly and Templated Metallization of Fibrinogen Nanofibrils. Chem. Commun. 2008, 33, 3903–3905. [Google Scholar] [CrossRef]
- Wei, G.; Reichert, J.; Bossert, J.; Jandt, K.D. Novel Biopolymeric Template for the Nucleation and Growth of Hydroxyapatite Crystals Based on Self-Assembled Fibrinogen Fibrils. Biomacromolecules 2008, 9, 3258–3267. [Google Scholar] [CrossRef] [PubMed]
- Hense, D.; Büngeler, A.; Kollmann, F.; Hanke, M.; Orive, A.; Keller, A.; Grundmeier, G.; Huber, K.; Strube, O.I. Self-Assembled Fibrinogen Hydro- And Aerogels with Fibrin-like 3D Structures. Biomacromolecules 2021, 22, 4084–4094. [Google Scholar] [CrossRef] [PubMed]
- Marguerie, G. The Binding of Calcium to Fibrinogen: Some Structural Features. Biochim. Biophys. Acta 1977, 494, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Boyer, M.H.; Shainhoff, J.R.; Ratnoff, O.D. Acceleration of Fibrin Polymerization by Calcium Ions. Blood 1972, 39, 382–387. [Google Scholar] [CrossRef]
- Steven, F.S.; Griffin, M.M.; Brown, B.S.; Hulley, T.P. Aggregation of Fibrinogen Molecules by Metal Ions. Int. J. Biol. Macromol. 1982, 4, 367–369. [Google Scholar] [CrossRef]
- Blombäck, B.; Procyk, R.; Adamson, L.; Hessel, B. FXIII Induced Gelation of Human Fibrinogen—An Alternative Thiol Enhanced Thrombin Independent Pathway. Thromb. Res. 1985, 37, 613–628. [Google Scholar] [CrossRef]
- Siebenlist, K.R.; Meh, D.A.; Mosesson, M.W. Protransglutaminase (Factor XIII) Mediated Crosslinking of Fibrinogen and Fibrin. Thromb. Haemost. 2001, 86, 1221–1228. [Google Scholar] [CrossRef] [Green Version]
- Lorand, L. Factor XIII: Structure, Activation, and Interactions with Fibrinogen and Fibrin. Ann. N. Y. Acad. Sci. 2001, 936, 291–311. [Google Scholar] [CrossRef] [PubMed]
- Marx, G. Protofibrin Clots Induced by Calcium and Zinc. Biopolymers 1987, 26, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Marx, G. Divalent Cations Induce Protofibril Gelation. Am. J. Hematol. 1988, 27, 104–109. [Google Scholar] [CrossRef]
- Marx, G. Mechanism of Fibrin Coagulation Based on Selective, Cation-driven, Protofibril Association. Biopolymers 1988, 27, 763–774. [Google Scholar] [CrossRef]
- Davie, E.W. A Brief Historical Review of the Waterfall/Cascade of Blood Coagulation. J. Biol. Chem. 2003, 278, 50819–50832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marguerie, G.; Chagniel, G.; Suscillon, M. The Binding of Calcium to Bovine Fibrinogen. Biochim. Biophys. Acta 1977, 490, 94–103. [Google Scholar] [CrossRef]
- Hämisch, B.; Büngeler, A.; Kielar, C.; Keller, A.; Strube, O.I.; Huber, K. Self-Assembly of Fibrinogen in Aqueous, Thrombin-Free Solutions of Variable Ionic Strengths. Langmuir 2019, 35, 12113–12122. [Google Scholar] [CrossRef]
- Apap-Bologna, A.; Webster, A.; Raitt, F.; Kemp, G. The Influence of Calcium Ions on Fibrinogen Conformation. Biochim. Biophys. Acta 1989, 995, 70–74. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Cichocki, B.; Ekiel-Jezewska, M.L.; Słowicka, A.; Wajnryb, E.; Wasilewska, M. Fibrinogen Conformations and Charge in Electrolyte Solutions Derived from DLS and Dynamic Viscosity Measurements. J. Colloid Interface Sci. 2012, 385, 244–257. [Google Scholar] [CrossRef]
- Credo, R.B.; Curtis, C.G.; Lorand, L. Ca2+-Related Regulatory Function of Fibrinogen. Proc. Natl. Acad. Sci. USA 1978, 75, 4234–4237. [Google Scholar] [CrossRef] [Green Version]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef] [PubMed]





| Initial Gel | Washed Gel | |
|---|---|---|
| Fibrinogen content [mg] | 17.5 ± 0.1 | 10.2 ± 0.7 |
| Water content [mg] | 637.1 ± 23.4 | 471.3 ± 10.2 |
| Water content [mg H2O/mg protein] | 36.4 ± 1.4 | 46.2 ± 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hense, D.; Strube, O.I. Fibrillogenesis and Hydrogel Formation from Fibrinogen Induced by Calcium Salts. Gels 2023, 9, 175. https://doi.org/10.3390/gels9030175
Hense D, Strube OI. Fibrillogenesis and Hydrogel Formation from Fibrinogen Induced by Calcium Salts. Gels. 2023; 9(3):175. https://doi.org/10.3390/gels9030175
Chicago/Turabian StyleHense, Dominik, and Oliver I. Strube. 2023. "Fibrillogenesis and Hydrogel Formation from Fibrinogen Induced by Calcium Salts" Gels 9, no. 3: 175. https://doi.org/10.3390/gels9030175
APA StyleHense, D., & Strube, O. I. (2023). Fibrillogenesis and Hydrogel Formation from Fibrinogen Induced by Calcium Salts. Gels, 9(3), 175. https://doi.org/10.3390/gels9030175