Pluronic® F127 Hydrogel Containing Silver Nanoparticles in Skin Burn Regeneration: An Experimental Approach from Fundamental to Translational Research
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Characterization
2.2. Antimicrobial Preliminary Efficacy Assessment
2.3. In Vitro Permeation Studies
2.4. Physical Characterization of Pluronic® F127 Hydrogel
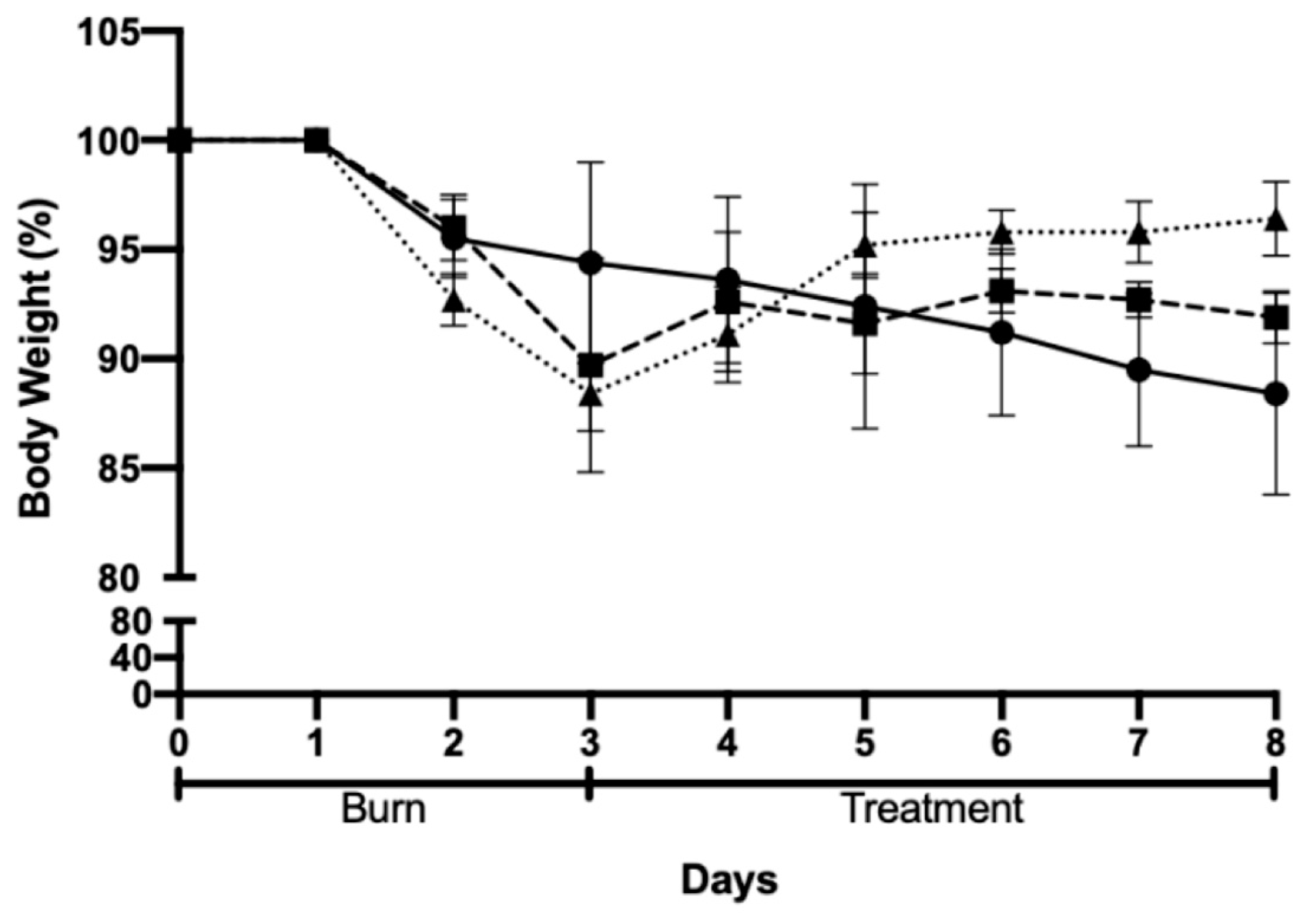
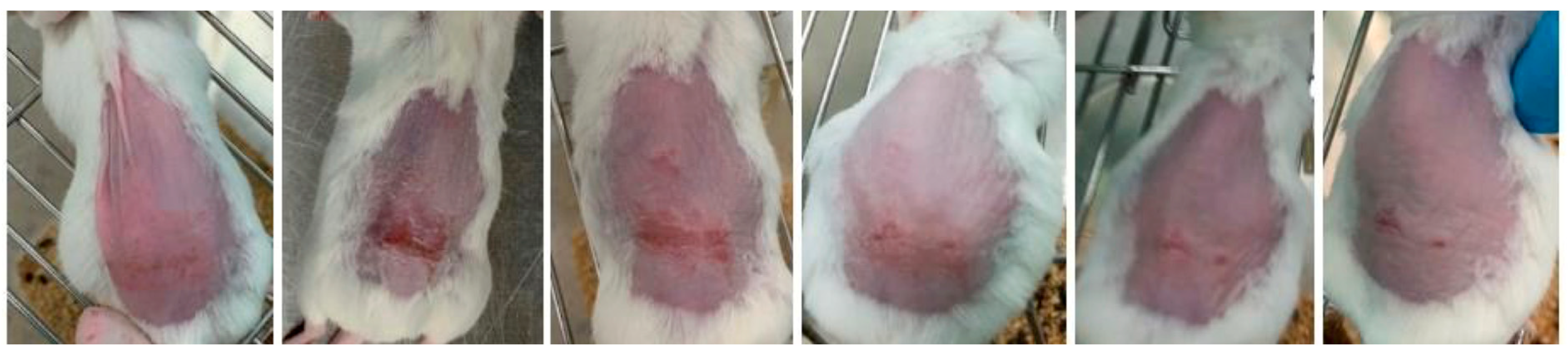
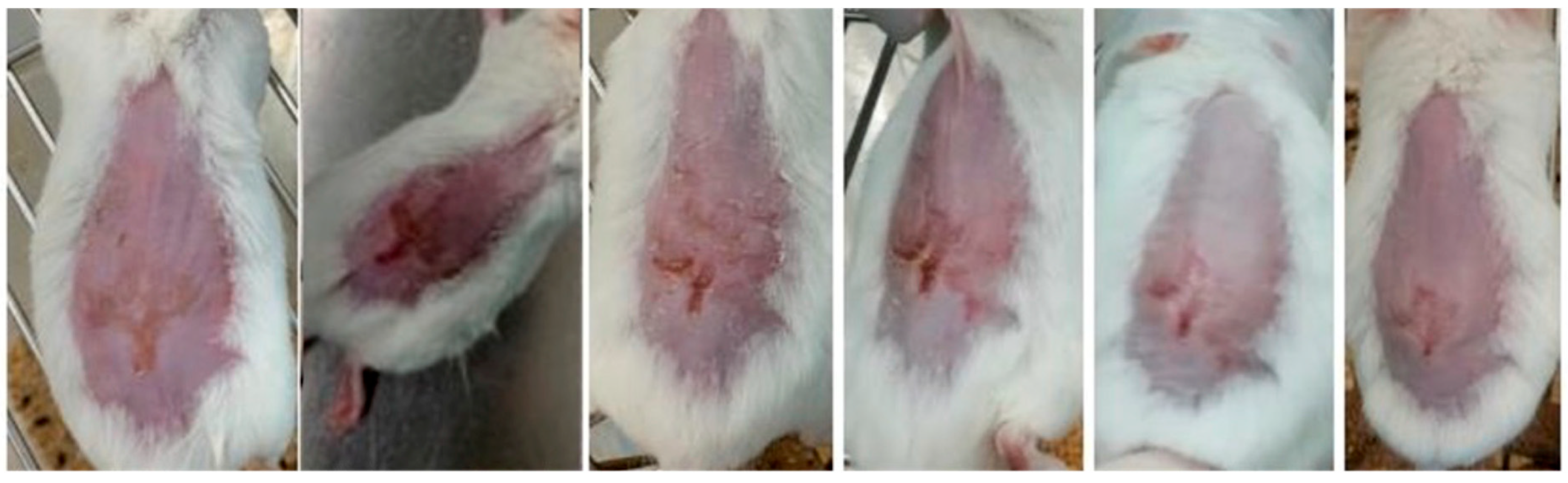
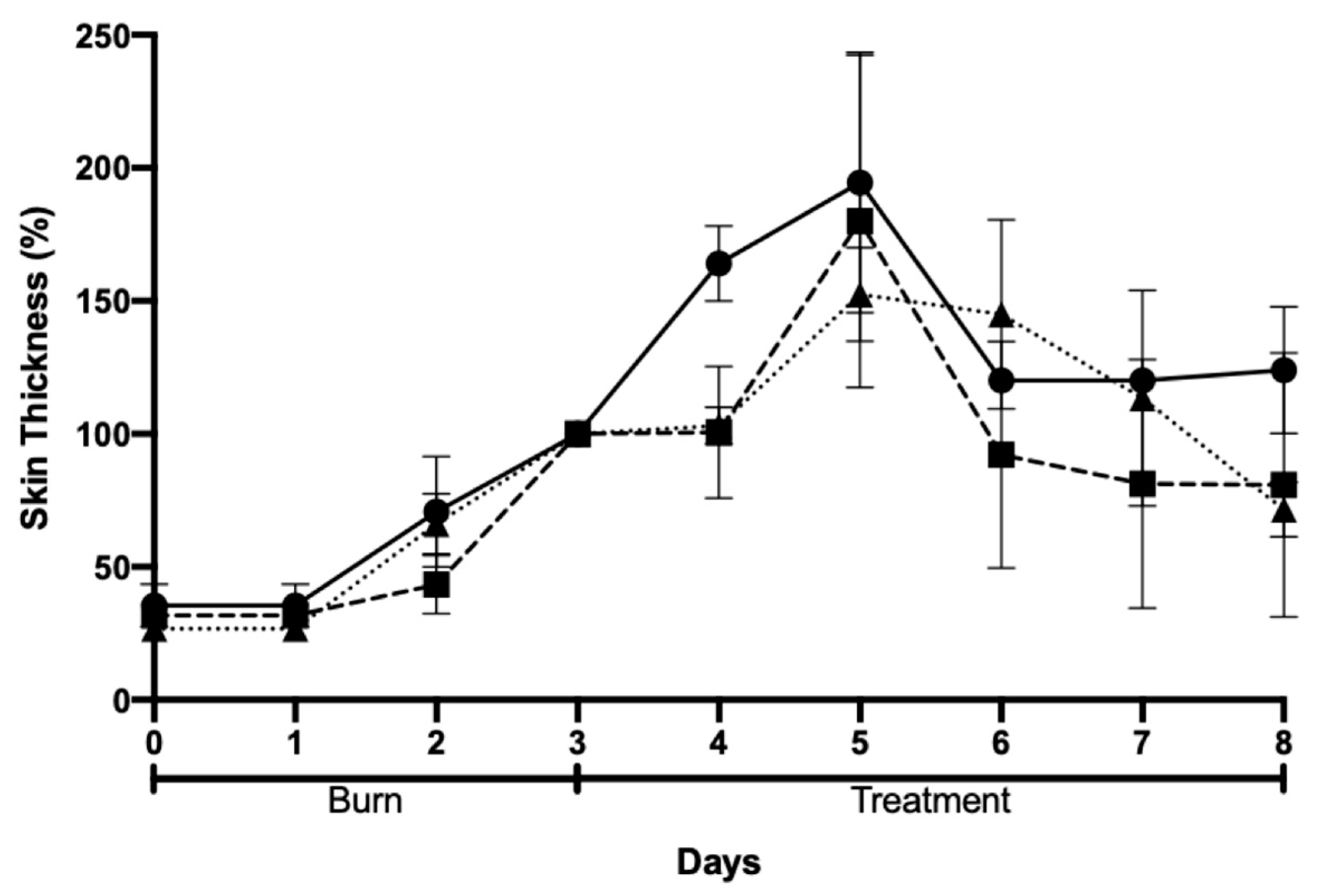
2.5. In Vivo Efficacy and Safety Assessments
2.6. Histopathological Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.1.1. Reagents
5.1.2. Microbial Strains
5.1.3. Animals
5.2. Methods
5.2.1. Preparation of AgNPs
5.2.2. AgNPs Characterization
- Mean Size and Surface Charge
- Morphology Analysis
- Quantification of AgNPs
5.2.3. Antimicrobial Preliminary Efficacy Studies
5.2.4. In Vitro Skin Permeation Studies
5.2.5. Preparation and Physical Characterization of Pluronic® F127 Hydrogel
5.2.6. In Vivo Efficacy and Safety Assays: Proof of Concept
5.2.7. Histology
5.2.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reis, C.P.; Gomes, A.; Rijo, P.; Candeias, S.; Pinto, P.; Baptista, M.; Martinho, N.; Ascensão, L. Development and Evaluation of a Novel Topical Treatment for Acne with Azelaic Acid-Loaded Nanoparticles. Microsc. Microanal. 2013, 19, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Brandner, J.M.; Jensen, J.-M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Mostafavi, E.; Afifi, A.M.; Izadiyan, Z.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Wound dressings functionalized with silver nanoparticles: Promises and pitfalls. Nanoscale 2020, 12, 2268–2291. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.H.; Rijo, P.; Molpeceres, J.; Reis, C.P. Broad overview of engineering of functional nanosystems for skin delivery. Int. J. Pharm. 2017, 532, 710–728. [Google Scholar] [CrossRef]
- Reis, C.P.; Damgé, C. Nanotechnology as a Promising Strategy for Alternative Routes of Insulin Delivery. Methods Enzymol. 2012, 508, 271–294. [Google Scholar]
- Politano, A.D.; Campbell, K.T.; Rosenberger, L.H.; Sawyer, R.G. Use of Silver in the Prevention and Treatment of Infections: Silver Review. Surg. Infect. 2013, 14, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Ovais, M.; Ahmad, I.; Khalil, A.T.; Mukherjee, S.; Javed, R.; Ayaz, M.; Raza, A.; Shinwari, Z.K. Wound healing applications of biogenic colloidal silver and gold nanoparticles: Recent trends and future prospects. Appl. Microbiol. Biotechnol. 2018, 102, 4305–4318. [Google Scholar] [CrossRef]
- Guillamat-Prats, R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef]
- Quitério, M.; Simões, S.; Ascenso, A.; Carvalheiro, M.; Leandro, A.P.; Correia, I.; Viana, A.S.; Faísca, P.; Ascensão, L.; Molpeceres, J.; et al. Development of a Topical Insulin Polymeric Nanoformulation for Skin Burn Regeneration: An Experimental Approach. Int. J. Mol. Sci. 2021, 22, 4087. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. Management of Burns. N. Engl. J. Med. 2019, 380, 2349–2359. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Huang, Y.-C.; Chen, L.Y.; Yu, S.-C.; Yu, H.-Y.; Chuang, S.-S. The skin microbiome of wound scars and unaffected skin in patients with moderate to severe burns in the subacute phase. Wound Repair Regen. 2018, 26, 182–191. [Google Scholar] [CrossRef]
- Abraham, J.P.; Plourde, B.D.; Vallez, L.J.; Nelson-Cheeseman, B.B.; Stark, J.R.; Sparrow, E.M.; Gorman, J.M. Skin Burns. In Theory and Applications of Heat Transfer in Humans; John Wiley & Sons Ltd.: Chichester, UK, 2018; pp. 723–739. [Google Scholar]
- Norman, G.; Christie, J.; Liu, Z.; Westby, M.J.; Jefferies, J.M.; Hudson, T.; Edwards, J.; Mohapatra, D.P.; Hassan, I.A.; Dumville, J.C. Antiseptics for burns. Cochrane Database Syst. Rev. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zou, B.; Liou, Y.-C.; Huang, C. The pathogenesis and diagnosis of sepsis post burn injury. Burn. Trauma 2021, 9, tkaa047. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, Y.; Ohtsuka, M.; Kawaguchi, M.; Sakai, K.; Hashimoto, A.; Hayashi, M.; Madokoro, N.; Asano, Y.; Abe, M.; Ishii, T.; et al. The wound/burn guidelines—6: Guidelines for the management of burns. J. Dermatol. 2016, 43, 989–1010. [Google Scholar] [CrossRef]
- Ahuja, R.B.; Gibran, N.; Greenhalgh, D.; Jeng, J.; Mackie, D.; Moghazy, A.; Moiemen, N.; Palmieri, T.; Peck, M.; et al.; ISBI Practice Guidelines Committee ISBI Practice Guidelines for Burn Care. Burns 2016, 42, 953–1021. [Google Scholar] [CrossRef]
- Wiktor, A.; Richards, D.; Torrey, S.B. Treatment of Minor thermal Burns. 2017. Available online: https://www.medilib.ir/uptodate/show/349 (accessed on 2 March 2023).
- Lloyd, E.C.O.; Rodgers, B.C.; Michener, M.; Williams, M.S. Outpatient burns: Prevention and care. Am. Fam. Physician 2012, 85, 25–32. [Google Scholar]
- Palmieri, T.L. Infection Prevention: Unique Aspects of Burn Units. Surg. Infect. 2019, 20, 111–114. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Burn wound healing: Present concepts, treatment strategies and future directions. J. Wound Care 2017, 26, 5–19. [Google Scholar] [CrossRef]
- Khansa, I.; Schoenbrunner, A.R.; Kraft, C.T.; Janis, J.E. Silver in Wound Care—Friend or Foe? A Comprehensive Review. Plast. Reconstr. Surg.—Glob. Open 2019, 7, e2390. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Grumezescu, V.; Grumezescu, A. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poon, V.K.M.; Burd, A. In vitro cytotoxity of silver: Implication for clinical wound care. Burns 2004, 30, 140–147. [Google Scholar] [CrossRef]
- Abou El-Nour, K.M.M.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Reis, C.P.; Neufeld, R.J.; Veiga, F.; Ribeirod, A.J. Preparation of drug-loaded polymeric nanoparticles. In Nanomedicine in Cancer; Pan Stanford: Singapore, Singapore, 2017; pp. 171–214. [Google Scholar]
- Bastos, V.; Ferreira de Oliveira, J.M.P.; Brown, D.; Johnston, H.; Malheiro, E.; Daniel-da-Silva, A.L.; Duarte, I.F.; Santos, C.; Oliveira, H. Corrigendum to “The influence of Citrate or PEG coating on silver nanoparticle toxicity to a human keratinocyte cell line” [Toxicol. Lett. 249 (2016) 29–41]. Toxicol. Lett. 2016, 257, 97. [Google Scholar] [CrossRef]
- De Matteis, V.; Cascione, M.; Toma, C.; Leporatti, S. Silver Nanoparticles: Synthetic Routes, In Vitro Toxicity and Theranostic Applications for Cancer Disease. Nanomaterials 2018, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016, 42, 46–56. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Li, Y.; Tjong, S. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Cadinoiu, A.N.; Rata, D.M.; Daraba, O.M.; Ichim, D.L.; Popescu, I.; Solcan, C.; Solcan, G. Silver Nanoparticles Biocomposite Films with Antimicrobial Activity: In Vitro and In Vivo Tests. Int. J. Mol. Sci. 2022, 23, 10671. [Google Scholar] [CrossRef]
- Al-Musawi, S.; Albukhaty, S.; Al-Karagoly, H.; Sulaiman, G.M.; Alwahibi, M.S.; Dewir, Y.H.; Soliman, D.A.; Rizwana, H. Antibacterial Activity of Honey/Chitosan Nanofibers Loaded with Capsaicin and Gold Nanoparticles for Wound Dressing. Molecules 2020, 25, 4770. [Google Scholar] [CrossRef]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for Wound Healing and Infection Control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.; Rangasamy, S.; Purushothaman, B.; Song, J.M. The Application of Bactericidal Silver Nanoparticles in Wound Treatment. Nanomater. Nanotechnol. 2015, 5, 23. [Google Scholar] [CrossRef]
- Wong, K.K.Y.; Liu, X. Silver nanoparticles—The real “silver bullet” in clinical medicine? Medchemcomm 2010, 1, 125. [Google Scholar] [CrossRef]
- Bercea, M.; Darie, R.N.; Nit, L.E.; Morariu, S. Temperature Responsive Gels Based on Pluronic F127 and Poly (vinyl alcohol). Ind. Eng. Chem. Res. 2011, 50, 4199–4206. [Google Scholar] [CrossRef]
- Moreno, E.; Schwartz, J.; Larrañeta, E.; Nguewa, P.A.; Sanmartín, C.; Agüeros, M.; Irache, J.M.; Espuelas, S. Thermosensitive hydrogels of poly(methyl vinyl ether-co-maleic anhydride)—Pluronic® F127 copolymers for controlled protein release. Int. J. Pharm. 2014, 459, 1–9. [Google Scholar] [CrossRef]
- Dewan, M.; Sarkar, G.; Bhowmik, M.; Das, B.; Chattoapadhyay, A.K.; Rana, D.; Chattopadhyay, D. Effect of gellan gum on the thermogelation property and drug release profile of Poloxamer 407 based ophthalmic formulation. Int. J. Biol. Macromol. 2017, 102, 258–265. [Google Scholar] [CrossRef]
- Miastkowska, M.; Kulawik-Pióro, A.; Szczurek, M. Nanoemulsion Gel Formulation Optimization for Burn Wounds: Analysis of Rheological and Sensory Properties. Processes 2020, 8, 1416. [Google Scholar] [CrossRef]
- Parhi, R. Cross-linked hydrogel for pharmaceutical applications: A review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Jaquilin, P.J.R.; Oluwafemi, O.S.; Thomas, S.; Oyedeji, A.O. Recent advances in drug delivery nanocarriers incorporated in temperature-sensitive Pluronic F-127–A critical review. J. Drug Deliv. Sci. Technol. 2022, 72, 103390. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; López-Cervantes, M.; Naïk, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar]
- Diniz, I.M.A.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F-127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2015, 26, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.-S.; Pham, C.; Paul, E.; Padiglione, A.; Lo, C.; Cleland, H. Early pathogenic colonisers of acute burn wounds: A retrospective review. Burns 2017, 43, 1757–1765. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Guilger-Casagrande, M.; de Lima, R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef] [Green Version]
- Haider, A.; Kang, I.-K. Preparation of Silver Nanoparticles and Their Industrial and Biomedical Applications: A Comprehensive Review. Adv. Mater. Sci. Eng. 2015, 2015, 165257. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, E.; Milani, M.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A.; Tayefi Nasrabadi, H.; Nikasa, P.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; et al. Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit. Rev. Microbiol. 2016, 42, 173–180. [Google Scholar] [CrossRef]
- Natsuki, J. A Review of Silver Nanoparticles: Synthesis Methods, Properties and Applications. Int. J. Mater. Sci. Appl. 2015, 4, 325. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Martin, G.; León-González, M.E.; Madrid, Y. Simultaneous determination of the size and concentration of AgNPs in water samples by UV–vis spectrophotometry and chemometrics tools. Talanta 2018, 188, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic silver nanoparticles: Synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.P.; Kim, G.-D. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl. Microbiol. Biotechnol. 2017, 101, 79–92. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Bélteky, P.; Rónavári, A.; Zakupszky, D.; Boka, E.; Igaz, N.; Szerencsés, B.; Pfeiffer, I.; Vágvölgyi, C.; Kiricsi, M.; Kónya, Z. Are Smaller Nanoparticles Always Better? Understanding the Biological Effect of Size-Dependent Silver Nanoparticle Aggregation Under Biorelevant Conditions. Int. J. Nanomed. 2021, 16, 3021–3040. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castañón, G.A.; Niño-Martínez, N.; Martínez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanoparticle Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Jeong, Y.; Lim, D.W.; Choi, J. Assessment of Size-Dependent Antimicrobial and Cytotoxic Properties of Silver Nanoparticles. Adv. Mater. Sci. Eng. 2014, 2014, 763807. [Google Scholar] [CrossRef] [Green Version]
- Sonavane, G.; Tomoda, K.; Sano, A.; Ohshima, H.; Terada, H.; Makino, K. In vitro permeation of gold nanoparticles through rat skin and rat intestine: Effect of particle size. Colloids Surfaces B Biointerfaces 2008, 65, 1–10. [Google Scholar] [CrossRef]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Dermatoendocrinol 2009, 1, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Ezealisiji, K.M.; Okorie, H.N. Size-dependent skin penetration of silver nanoparticles: Effect of penetration enhancers. Appl. Nanosci. 2018, 8, 2039–2046. [Google Scholar]
- Tak, Y.K.; Pal, S.; Naoghare, P.K.; Rangasamy, S.; Song, J.M. Shape-Dependent Skin Penetration of Silver Nanoparticles: Does It Really Matter? Sci. Rep. 2015, 5, 16908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraeling, M.E.K.; Topping, V.D.; Keltner, Z.M.; Belgrave, K.R.; Bailey, K.D.; Gao, X.; Yourick, J.J. In vitro percutaneous penetration of silver nanoparticles in pig and human skin. Regul. Toxicol. Pharmacol. 2018, 95, 314–322. [Google Scholar] [PubMed]
- Martínez-Higuera, A.; Rodríguez-Beas, C.; Villalobos-Noriega, J.M.A.; Arizmendi-Grijalva, A.; Ochoa-Sánchez, C.; Larios-Rodríguez, E.; Martínez-Soto, J.M.; Rodríguez-León, E.; Ibarra-Zazueta, C.; Mora-Monroy, R.; et al. Hydrogel with silver nanoparticles synthesized by Mimosa tenuiflora for second-degree burns treatment. Sci. Rep. 2021, 11, 11312. [Google Scholar] [CrossRef] [PubMed]
- Larese, F.F.; D’Agostin, F.; Crosera, M.; Adami, G.; Renzi, N.; Bovenzi, M.; Maina, G. Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology 2009, 255, 33–37. [Google Scholar] [CrossRef]
- Ong, W.T.J.; Nyam, K.L. Evaluation of silver nanoparticles in cosmeceutical and potential biosafety complications. Saudi J. Biol. Sci. 2022, 29, 2085–2094. [Google Scholar] [CrossRef]
- Bianco, C.; Visser, M.J.; Pluut, O.A.; Svetličić, V.; Pletikapić, G.; Jakasa, I.; Riethmuller, C.; Adami, G.; Larese Filon, F.; Schwegler-Berry, D.; et al. Characterization of silver particles in the stratum corneum of healthy subjects and atopic dermatitis patients dermally exposed to a silver-containing garment. Nanotoxicology 2016, 10, 1480–1491. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Marepally, S.K.; Vemula, P.K.; Xu, C. Inorganic Nanoparticles for Transdermal Drug Delivery and Topical Application. In Nanoscience in Dermatology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 57–72. [Google Scholar]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Ahmadi, M.; Adibhesami, M. The Effect of Silver Nanoparticles on Wounds Contaminated with Pseudomonas aeruginosa in Mice: An Experimental Study. Iran. J. Pharm. Res. IJPR 2017, 16, 661–669. [Google Scholar]
- Latifi, N.A.; Karimi, H. Correlation of occurrence of infection in burn patients. Ann. Burn. Fire Disasters 2017, 30, 172–176. [Google Scholar]
- Rigo, C.; Ferroni, L.; Tocco, I.; Roman, M.; Munivrana, I.; Gardin, C.; Cairns, W.; Vindigni, V.; Azzena, B.; Barbante, C.; et al. Active Silver Nanoparticles for Wound Healing. Int. J. Mol. Sci. 2013, 14, 4817–4840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota, A.H.; Prazeres, I.; Mestre, H.; Bento-Silva, A.; Rodrigues, M.J.; Duarte, N.; Serra, A.T.; Bronze, M.R.; Rijo, P.; Gaspar, M.M.; et al. A Newfangled Collagenase Inhibitor Topical Formulation Based on Ethosomes with Sambucus nigra L. Extract. Pharmaceuticals 2021, 14, 467. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Nguyen, T.T.; Ly, K.L.; Tran, A.H.; Nguyen, T.T.N.; Vo, M.T.; Ho, H.M.; Dang, N.T.N.; Vo, V.T.; Nguyen, D.H.; et al. In Vivo Study of the Antibacterial Chitosan/Polyvinyl Alcohol Loaded with Silver Nanoparticle Hydrogel for Wound Healing Applications. Int. J. Polym. Sci. 2019, 2019, 7382717. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A. Therapeutic potential of green synthesized silver nanoparticles loaded PVA hydrogel patches for wound healing. J. Drug Deliv. Sci. Technol. 2019, 54, 101308. [Google Scholar] [CrossRef]
- Xie, Y.; Liao, X.; Zhang, J.; Yang, F.; Fan, Z. Novel chitosan hydrogels reinforced by silver nanoparticles with ultrahigh mechanical and high antibacterial properties for accelerating wound healing. Int. J. Biol. Macromol. 2018, 119, 402–412. [Google Scholar] [CrossRef]
- Badhwar, R.; Mangla, B.; Neupane, Y.R.; Khanna, K.; Popli, H. Quercetin loaded silver nanoparticles in hydrogel matrices for diabetic wound healing. Nanotechnology 2021, 32, 505102. [Google Scholar] [CrossRef] [PubMed]
- Faris Taufeq, F.Y.; Habideen, N.H.; Rao, L.N.; Podder, P.K.; Katas, H. Potential Hemostatic and Wound Healing Effects of Thermoresponsive Wound Dressing Gel Loaded with Lignosus rhinocerotis and Punica granatum Extracts. Gels 2023, 9, 48. [Google Scholar] [CrossRef]
- Shriky, B.; Kelly, A.; Isreb, M.; Babenko, M.; Mahmoudi, N.; Rogers, S.; Shebanova, O.; Snow, T.; Gough, T. Pluronic F127 thermosensitive injectable smart hydrogels for controlled drug delivery system development. J. Colloid Interface Sci. 2020, 565, 119–130. [Google Scholar] [CrossRef]
- Gioffredi, E.; Boffito, M.; Calzone, S.; Giannitelli, S.M.; Rainer, A.; Trombetta, M.; Mozetic, P.; Chiono, V. Pluronic F127 Hydrogel Characterization and Biofabrication in Cellularized Constructs for Tissue Engineering Applications. Procedia CIRP 2016, 49, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Lui, V.C.H.; Chen, Y.; Lok, C.N.; Wong, K.K.Y. Delayed application of silver nanoparticles reveals the role of early inflammation in burn wound healing. Sci. Rep. 2020, 10, 6338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojkovska, J.; Djurdjevic, Z.; Jancic, I.; Bufan, B.; Milenkovic, M.; Jankovic, R.; Miskovic-Stankovic, V.; Obradovic, B. Comparative in vivo evaluation of novel formulations based on alginate and silver nanoparticles for wound treatments. J. Biomater. Appl. 2018, 32, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nie, S.; Hsiao, W.W.; Pam, W. Thermoreversible Pluronic® F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: In vitro drug release, cell cytotoxicity, and uptake studies. Int. J. Nanomed. 2011, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braiman-Wiksman, L.; Solomonik, I.; Spira, R.; Tennenbaum, T. Novel Insights into Wound Healing Sequence of Events. Toxicol. Pathol. 2007, 35, 767–779. [Google Scholar] [CrossRef]

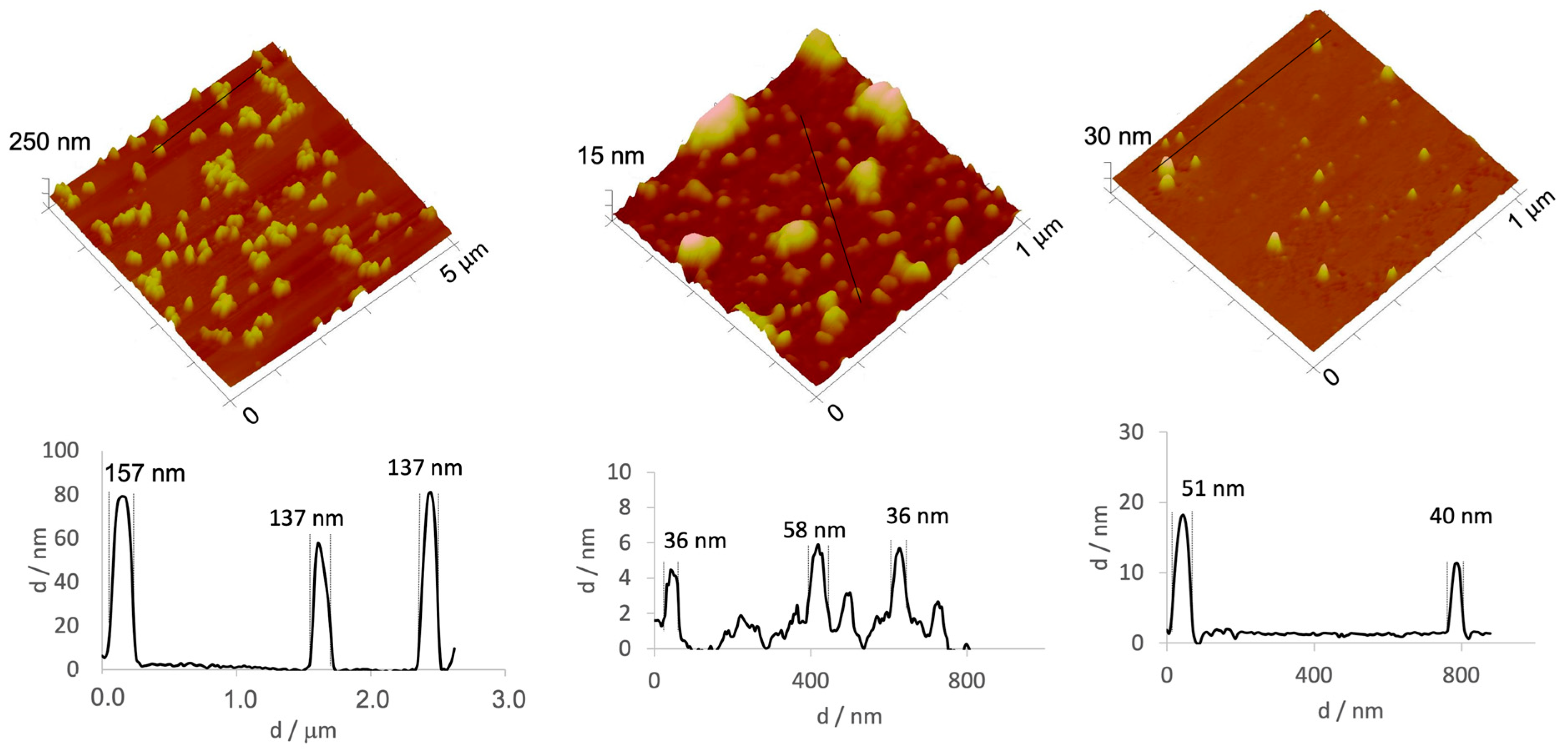

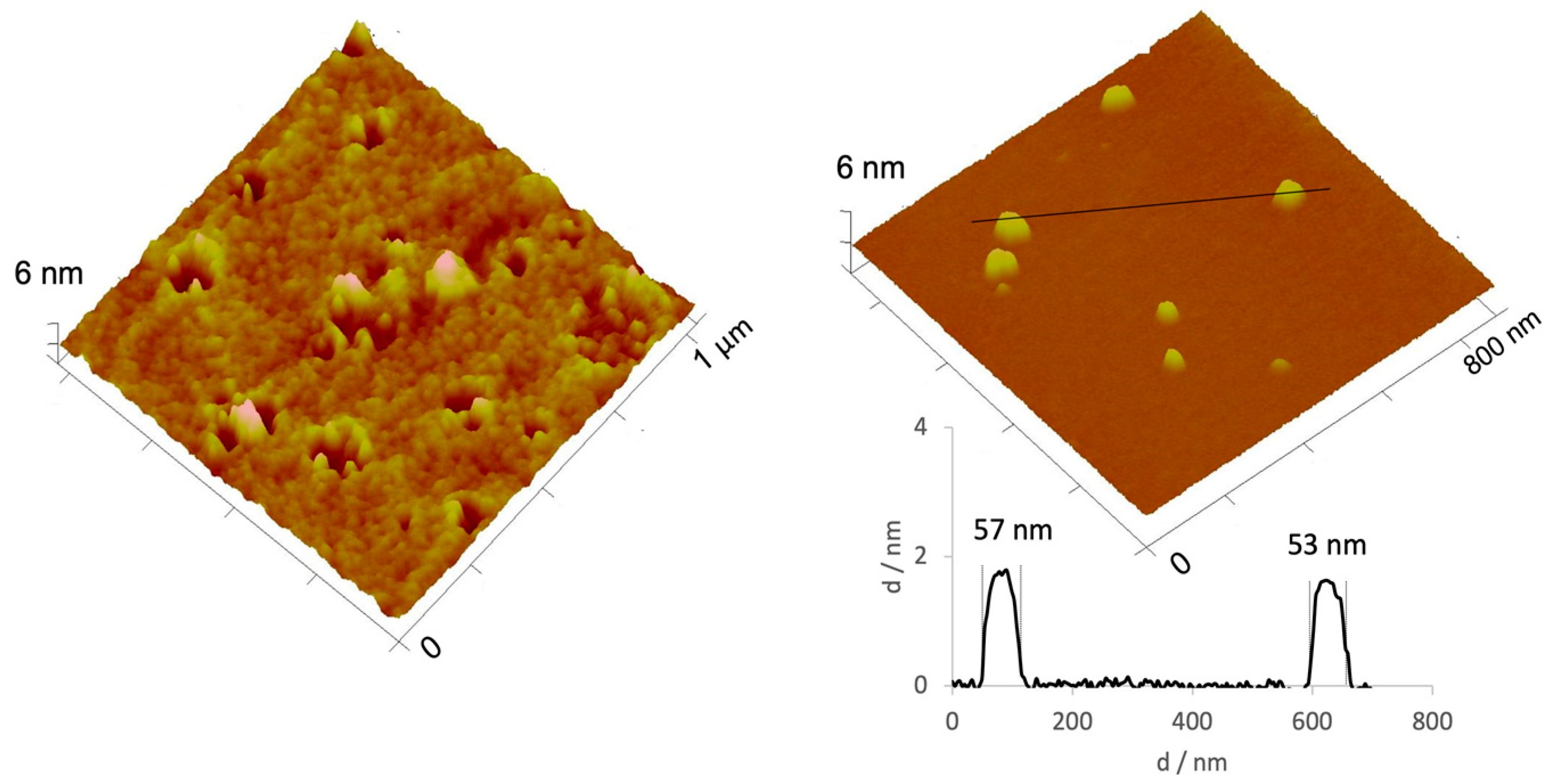


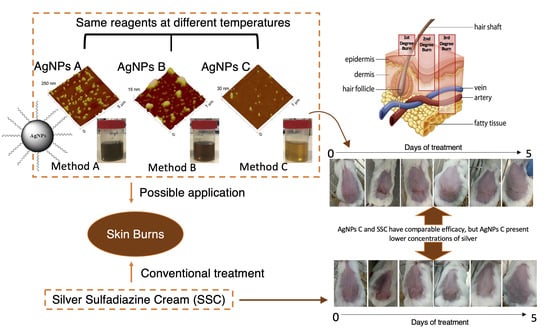
| Preparation Method | Size (nm) | PdI | Zeta Potential (mV) |
|---|---|---|---|
| A | 47.85 ± 1.01 | 0.887 ± 0.003 | −0.06 ± 1.50 |
| B | 18.92 ± 2.61 | 0.628 ± 0.162 | −2.02 ± 0.34 |
| C | 48.04 ± 14.87 | 0.180 ± 0.013 | −0.79 ± 2.17 |
| Sample | MIC (nM) | ||
|---|---|---|---|
| Escherichia coli | Staphylococcus aureus | Pseudomonas aeruginosa | |
| AgNPs | >1.65 | >1.65 | 1.65 |
| Centrifuged AgNPs (66 nM) | >33 | >33 | >33 |
| Lyophilized AgNPs (66 nM) + Pluronic® F127 hydrogel | >33 | >33 | 33 |
| AgNPs concentrated by evaporation (66 nM) | 33 | N/A | 33 |
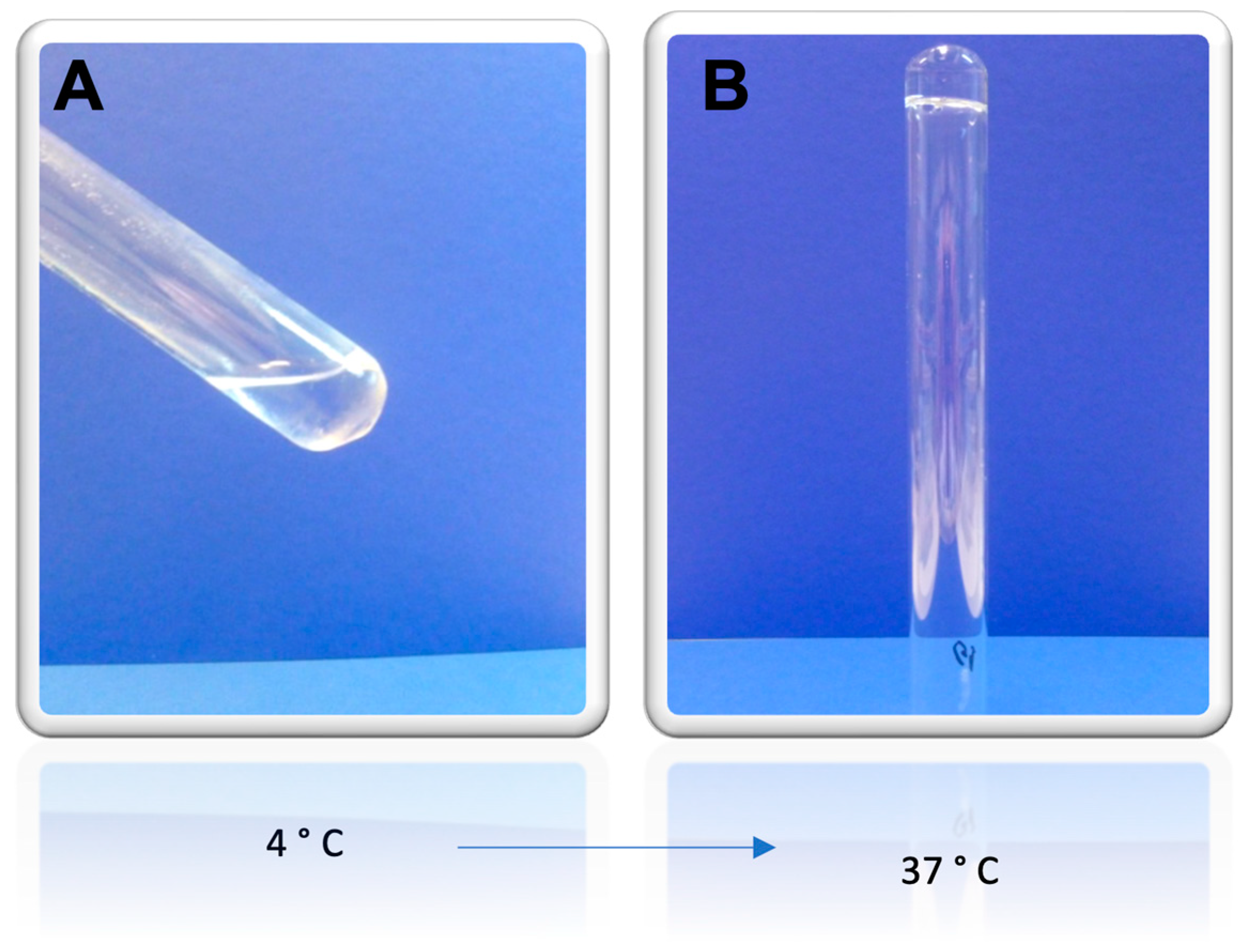
| Time (min) | Viscosity (mPas) | ||
|---|---|---|---|
| 4 °C | 25 °C | 37 °C | |
| 10 | 76.0 | 360.0 | 7320.0 |
| 20 | 76.0 | 380.0 | 7360.0 |
| 30 | 75.2 | 360.0 | 7320.0 |
| Mean ± SD | 75.7 ± 0.5 | 366.7 ± 11.6 | 7333.3 ± 23.1 |
| Epidermal Closure | Epidermal Differentiation | Amount of Granulation Tissue | Inflammatory Infiltrate | Orientation of Collagen Fibers | Collagen Pattern | Total | |
|---|---|---|---|---|---|---|---|
| Commercial formulation of silver sulfadiazine (n = 3) | 0.67 ± 0.58 | 1.33 ± 1.15 | 3.33 ± 1.15 | 2.67 ± 0.58 | 2.33 ± 1.15 | 2.33 ± 1.15 | 12.67 ± 5.77 |
| AgNPs incorporated in Pluronic® F127 hydrogel (n = 4) | 1.0 ± 0.0 | 1.60 ± 0.89 | 3.33 ± 0.89 | 2.80 ± 0.45 | 2.80 ± 0.45 | 2.80 ± 0.45 | 14.60 ± 3.13 |
| Method | Conditions |
|---|---|
| A | AgNO3 was cooled prior to the preparation of the AgNO3 solution, and the solutions used to prepare the AgNPs were at room temperature. |
| B | The AgNO3 and NaBH4 solutions were prepared and stored at 4 °C prior to AgNPs production. |
| C | AgNO3 solution was produced and used at room temperature, while NaBH4 solution was cooled prior to being used in AgNPs production. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francisco, P.; Neves Amaral, M.; Neves, A.; Ferreira-Gonçalves, T.; Viana, A.S.; Catarino, J.; Faísca, P.; Simões, S.; Perdigão, J.; Charmier, A.J.; et al. Pluronic® F127 Hydrogel Containing Silver Nanoparticles in Skin Burn Regeneration: An Experimental Approach from Fundamental to Translational Research. Gels 2023, 9, 200. https://doi.org/10.3390/gels9030200
Francisco P, Neves Amaral M, Neves A, Ferreira-Gonçalves T, Viana AS, Catarino J, Faísca P, Simões S, Perdigão J, Charmier AJ, et al. Pluronic® F127 Hydrogel Containing Silver Nanoparticles in Skin Burn Regeneration: An Experimental Approach from Fundamental to Translational Research. Gels. 2023; 9(3):200. https://doi.org/10.3390/gels9030200
Chicago/Turabian StyleFrancisco, Pedro, Mariana Neves Amaral, Afonso Neves, Tânia Ferreira-Gonçalves, Ana S. Viana, José Catarino, Pedro Faísca, Sandra Simões, João Perdigão, Adília J. Charmier, and et al. 2023. "Pluronic® F127 Hydrogel Containing Silver Nanoparticles in Skin Burn Regeneration: An Experimental Approach from Fundamental to Translational Research" Gels 9, no. 3: 200. https://doi.org/10.3390/gels9030200
APA StyleFrancisco, P., Neves Amaral, M., Neves, A., Ferreira-Gonçalves, T., Viana, A. S., Catarino, J., Faísca, P., Simões, S., Perdigão, J., Charmier, A. J., Gaspar, M. M., & Reis, C. P. (2023). Pluronic® F127 Hydrogel Containing Silver Nanoparticles in Skin Burn Regeneration: An Experimental Approach from Fundamental to Translational Research. Gels, 9(3), 200. https://doi.org/10.3390/gels9030200