Long-Term Performance of Monolithic Silica Aerogel with Different Hydrophobicities: Physical and Color Rendering Properties after an Accelerated Aging Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Samples
2.2. Aging Process
2.3. Characterization before and after Aging
3. Results and Discussion
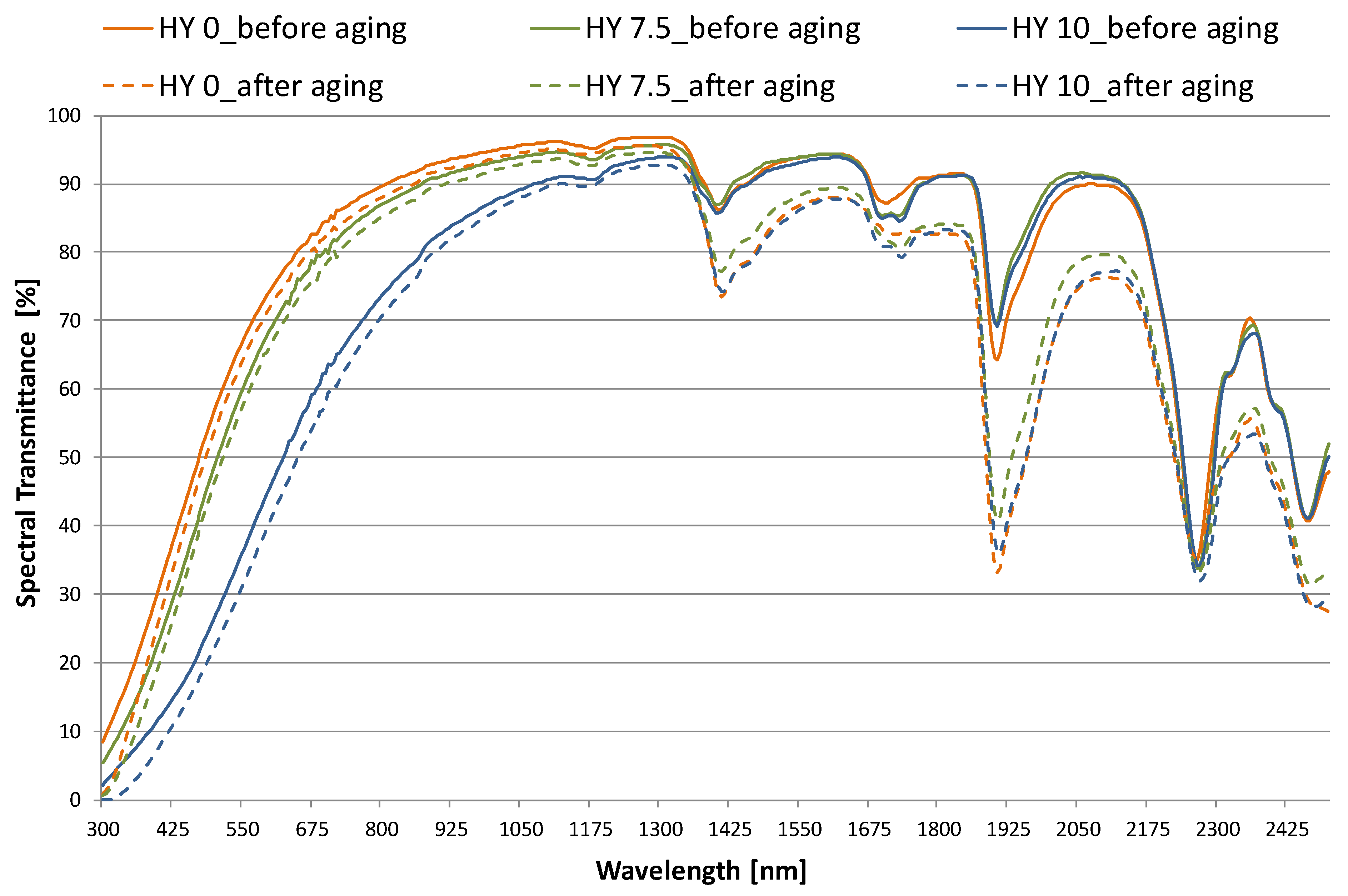
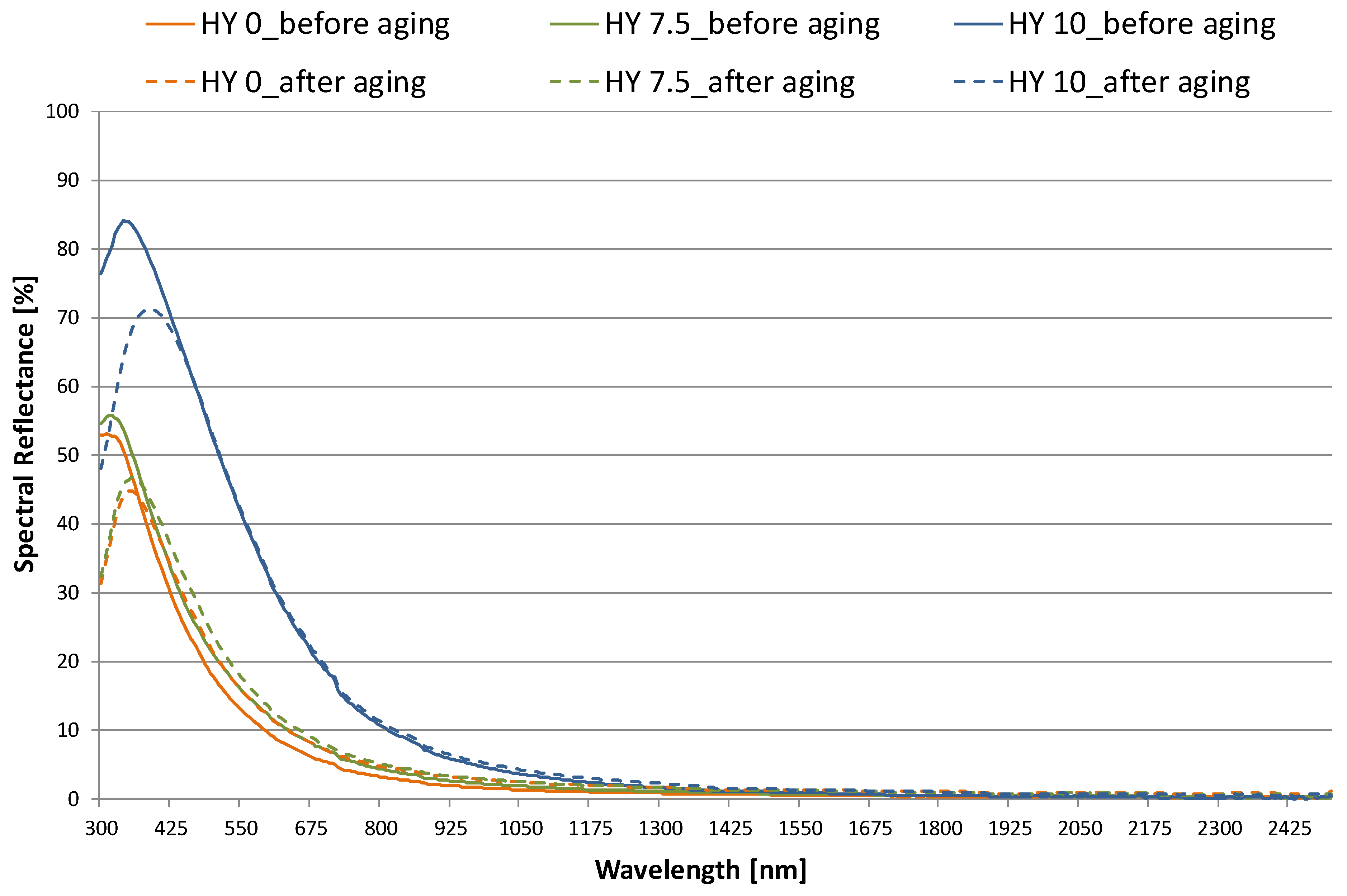
3.1. Optical Properties
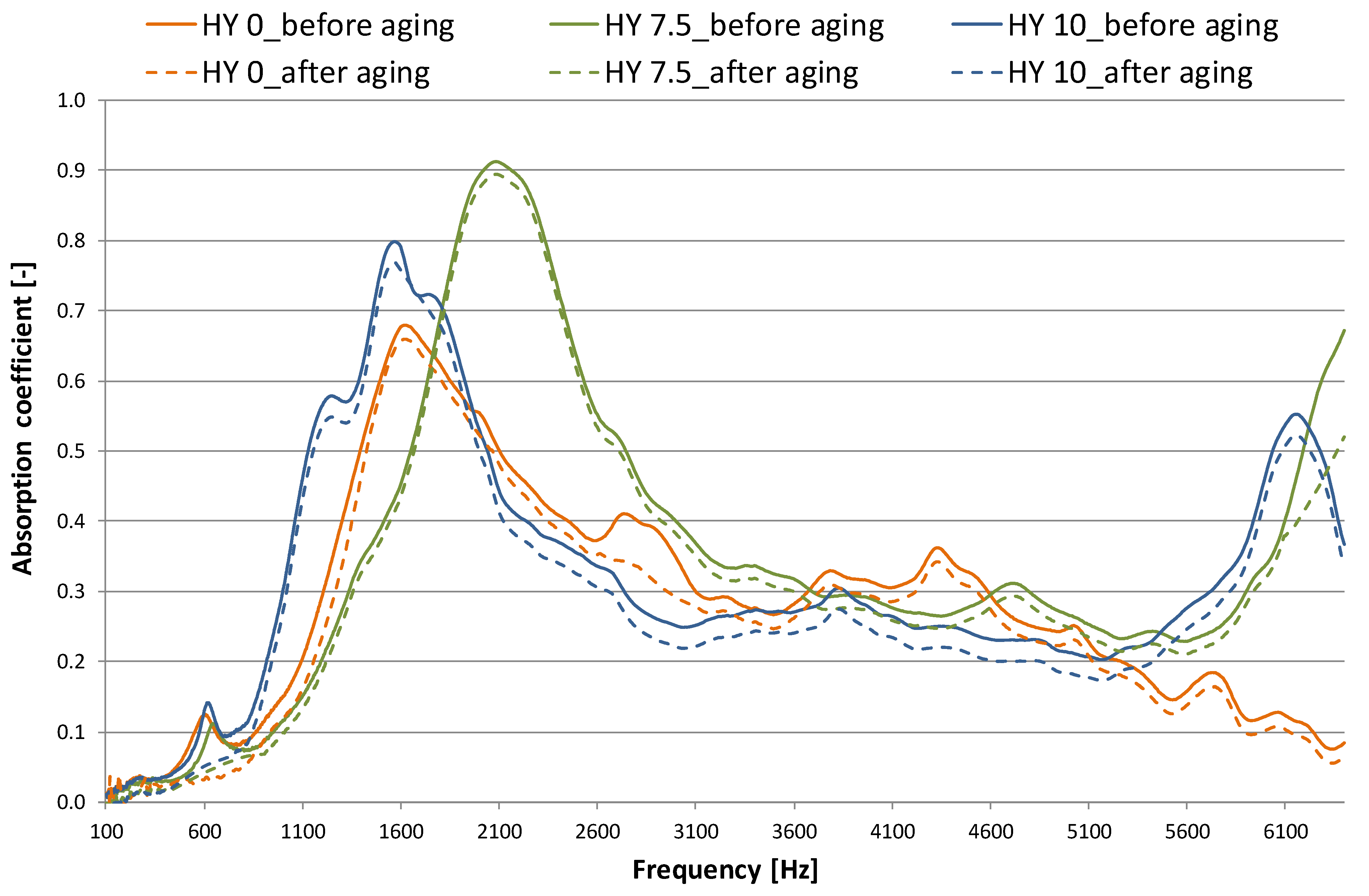
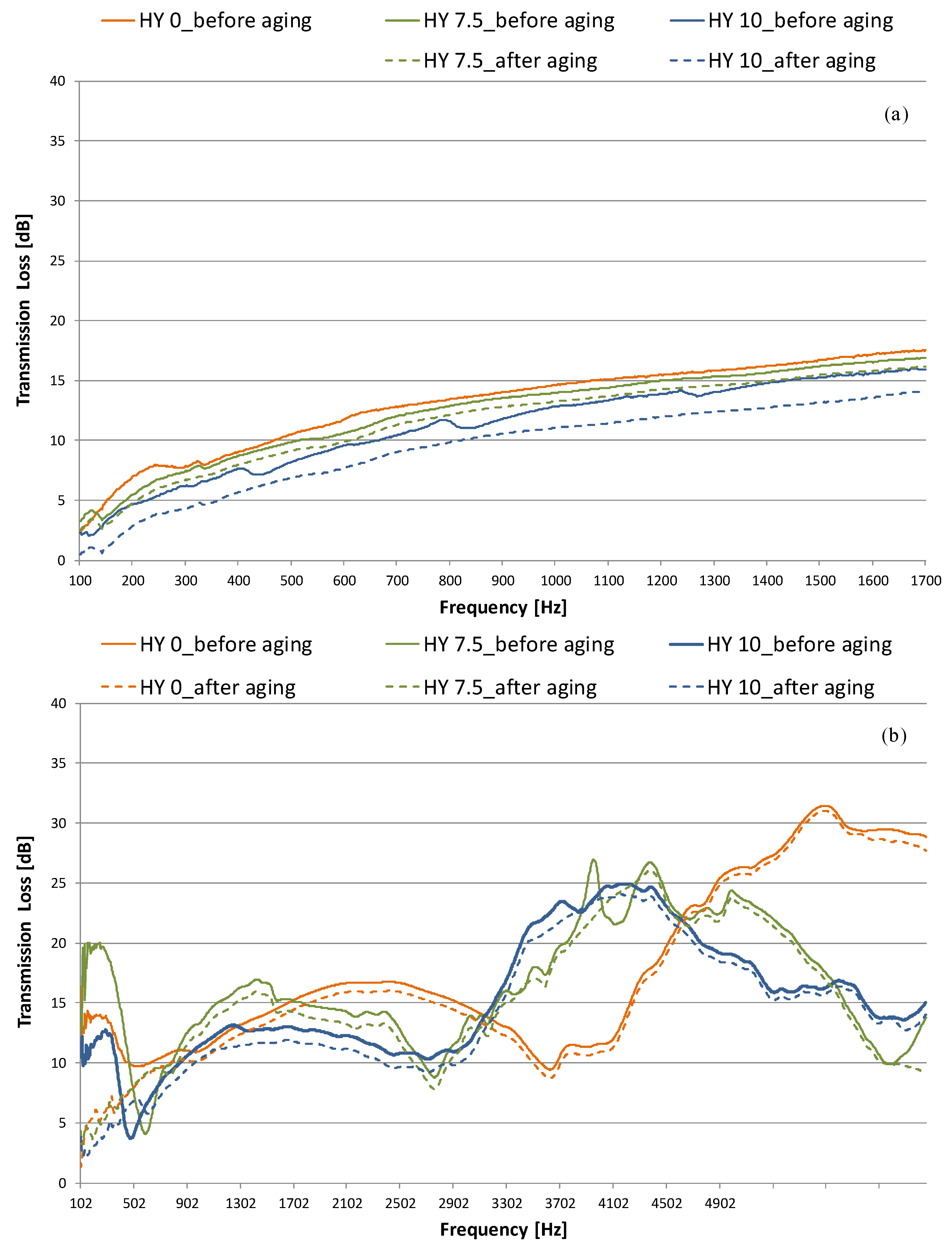
3.2. Acoustic Properties
3.3. Color Rendering
3.4. Physical Properties
4. Conclusions
- -
- high visible transmittance (peak of 0.89 for HY 0) is obtained. The value decreases slightly for HY 7.5 and up to 20% for HY 10 (τv= 0.67, 0.60, 0.37 for HY 0, HY 7.5, and HY 10, respectively). On the contrary, reflectance increases as hydrophobicity increases, especially in the visible range. The aging process results in small but significant changes in the transmittance and reflectance properties;
- -
- when the hydrophobicity increases, a moderate reduction in sound insulation performance (about 1–3 dB) and an improvement in absorption properties (NRC are in the 0.21–0.25 range) are measured. After aging, NRC- and TL-values are reduced up to 0.03 and 2–5 dB for the 10% MTMS sample;
- -
- color shift increases with hydrophobicity. The highest values are obtained for yellow–orange–red tones. The aging worsens the color rendering with HY 0 and HY 7.5 panes (color rendering index Ra decreases by 10 and 14 for HY 0 and HY 7.5, respectively), whereas it is negligible with HY 10 (Ra = 61 before and after aging). The ΔE variations before and after aging are very low in light blue–gray tones. On the contrary, the aging process has a negative effect on light green and azure tones;
- -
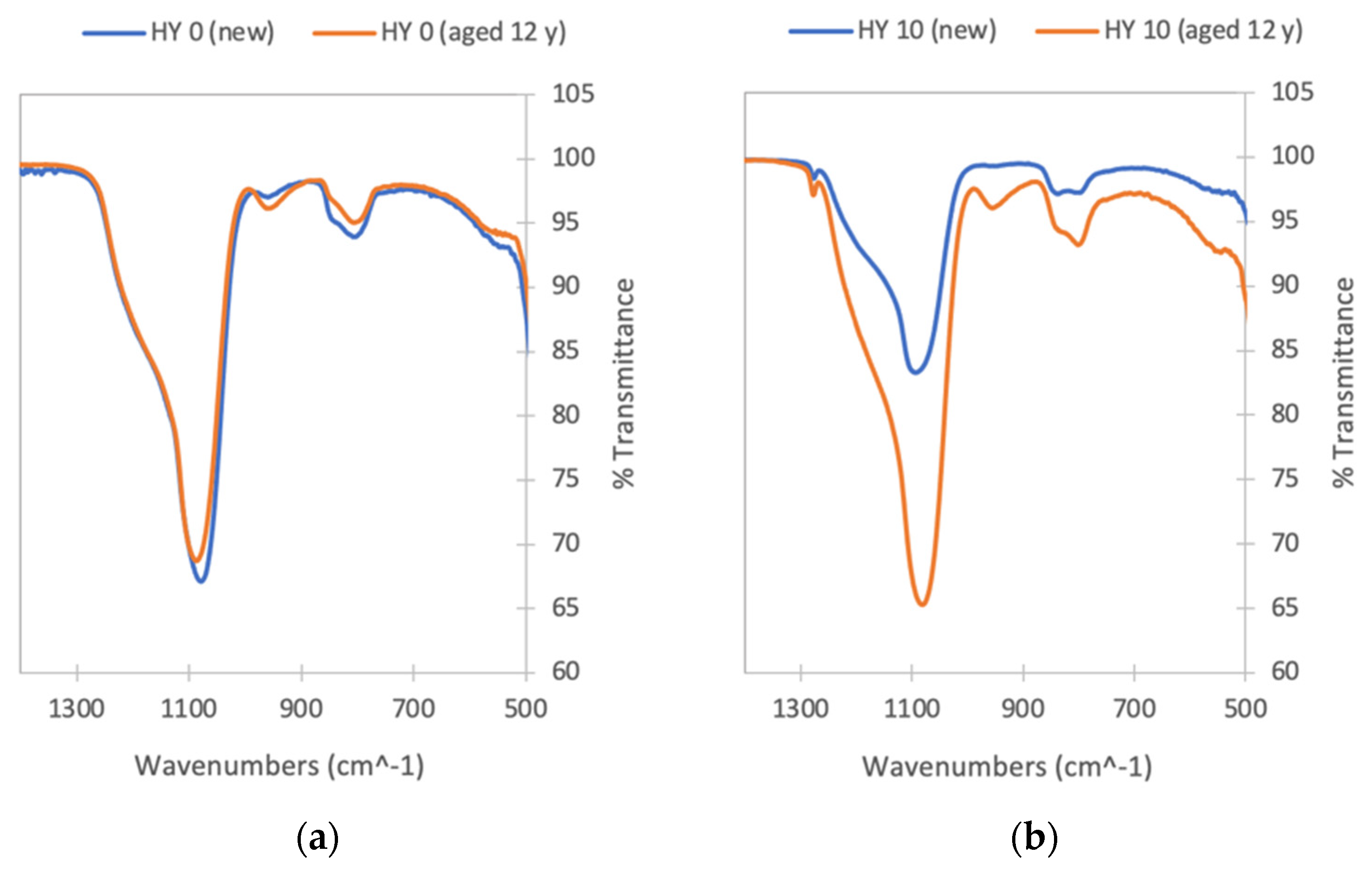
- the increase in the hydrophobicity of the monolithic silica aerogel involves an increase in surface area (reaching 640 m2/g). For hydrophobic samples, the contact angle increases with the amount of hydrophobic precursor employed in aerogel preparation, from 125° for the samples made with 7.5% to 141° for samples with 10% MTMS. Due to the hydrophobicity loss of the samples after aging, the contact angle tests are partially supported by a chemical composition analysis (FTIR). Some change in structure is observed, although the initial chemical composition is not entirely compromised: Si-CH3 groups are still present in the aged HY 7.5 and HY 10 samples.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biswas, K.; Patel, T.; Shrestha, S.; Smith, D.; Desjarlais, A. Whole building retrofit using vacuum insulation panels and energy performance analysis. Energy Build. 2019, 203, 109430. [Google Scholar] [CrossRef]
- Cui, H.; Overend, M. A review of heat transfer characteristics of switchable insulation technologies for thermally adaptive building envelopes. Energy Build. 2019, 199, 427–444. [Google Scholar] [CrossRef]
- Mandilaras, I.; Atsonios, I.; Zannis, G.; Founti, M. Thermal performance of a building envelope incorporating ETICS with vacuum insulation panels and EPS. Energy Build. 2014, 85, 654–665. [Google Scholar] [CrossRef]
- Buratti, C.; Belloni, E.; Merli, F.; Zinzi, M. Aerogel glazing systems for building applications: A review. Energy Build. 2021, 231, 110587. [Google Scholar] [CrossRef]
- Ye, H.; Meng, X.; Long, L.; Xu, B. The route to a perfect window. Renew. Energy 2013, 55, 448–455. [Google Scholar] [CrossRef]
- Jelle, B.P.; Hynd, A.; Gustavsen, A.; Arasteh, D.; Goudey, H.; Hart, R. Fenestration of today and tomorrow: A state-of-the-art review and future research opportunities. Solar. Energy Mater. Solar. Cells. 2012, 96, 1–28. [Google Scholar] [CrossRef]
- Koebel, M.; Rigacci, A.; Achard, P. Aerogel-based thermal superinsulation: An overview. J. Sol. Gel Sci. Technol. 2012, 63, 315–339. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel insulation for building applications: A state-of-the-art review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef]
- Riffat, S.B.; Qiu, G. A review of state-of-the-art aerogel applications in buildings. Int. J. Low Carbon Technol. 2012, 8, 1–6. [Google Scholar] [CrossRef]
- Zinzi, M.; Rossi, G.; Anderson, A.M.; Carroll, M.K.; Moretti, E.; Buratti, C. Optical and visual experimental characterization of a glazing system with monolithic silica aerogel. Sol. Energy 2019, 183, 30–39. [Google Scholar] [CrossRef]
- Ihara, T.; Jelle, B.P.; Gao, T.; Gustavsen, A. Aerogel granule aging driven by moisture and solar radiation. Energy Build. 2015, 103, 238–248. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). Energy in Buildings and Communities (EBC) Annex 65, Long-Term Performance of Super-Insulating-Materials (SIM) in Building Components and Systems—Report of Subtask I: State of the Art and Case Studies; IEA, 3 January 2020. Available online: https://vipa-international.org/ieaebc-annex-65-project (accessed on 15 September 2022).
- International Energy Agency (IEA). Energy in Buildings and Communities (EBC) Annex 65, Long-Term Performance of Super-Insulating-Materials (SIM) in Building Components and Systems—Report of Subtask II: Characterization of materials and Components—Laboratory Scale; IEA, 3 January 2020. Available online: https://vipa-international.org/iea-ebc-annex-65-project (accessed on 15 September 2022).
- Berardi, U.; Nosrati, R.H. Long-term thermal conductivity of aerogel-enhanced insulating materials under different laboratory aging conditions. Energy 2018, 147, 1188–1202. [Google Scholar] [CrossRef]
- Anderson, A.M.; Wattley, C.W.; Carroll, M.K. Silica aerogels prepared via rapid supercritical extraction: Effect of process variables on aerogel properties. J. Non-Cryst. Solids 2009, 355, 101–108. [Google Scholar] [CrossRef]
- Bhuiya, M.M.H.; Anderson, A.M.; Carroll, M.K.; Bruno, B.A.; Ventrella, J.L.; Silberman, B.; Keramati, B. Preparation of monolithic silica aerogel for fenestration applications: Scaling up, reducing cycle time, and improving performance. Ind. Eng. Chem. Res. 2016, 55, 6971–6981. [Google Scholar] [CrossRef]
- Gauthier, B.M.; Bakrania, S.D.; Anderson, A.M.; Carroll, M.K. A fast supercritical extraction technique for aerogel fabrication. J. Non-Cryst. Solids 2004, 350, 238–243. [Google Scholar] [CrossRef]
- Zhao, L.; Strobach, E.; Bhatia, B.; Yang, S.; Leroy, A.; Zhang, L.; Wang, E.N. Theoretical and experimental investigation of haze in transparent aerogels. Opt. Express 2019, 27, A39–A50. [Google Scholar] [CrossRef]
- Buratti, C.; Belloni, E.; Merli, F.; Bianconi, F. Experimental characterization of the color rendering properties of transparent monolithic aerogel. Sol. Energy 2020, 205, 183–191. [Google Scholar] [CrossRef]
- Fiorini, C.V.; Merli, F.; Belloni, E.; Anderson, A.M.; Carroll, M.K.; Buratti, C. Optical and color rendering long-term performance of monolithic aerogel after laboratory accelerated aging: Development of a method and preliminary experimental results. Sol. Energy 2023, in press. [Google Scholar] [CrossRef]
- Jelle, B.P. Accelerated climate aging of building materials components and structures in the laboratory. J. Mater. Sci. 2012, 47, 6475–6496. [Google Scholar] [CrossRef]
- V-Tac Lamps. Available online: https://v-tac.it/ (accessed on 11 January 2021).
- Shimadzu UV/VIS/NIR Spectrophotometer. Available online: https://www.shimadzu.com/an/products/molecular-spectroscopy/uv-vis/uv-vis-nirspectroscopy/solidspec-3700i3700iduv/index.html (accessed on 12 December 2021).
- European Committee for Standardization (CEN). Glass in Building—Determination of Luminous and Solar Characteristics of Glazing; EN 410; CEN: Brussels, Belgium, 2011. [Google Scholar]
- International Organization for Standardization (ISO). Acoustics—Determination of Sound Absorption Coefficient and Impedance in Impedance Tubes—Part 2: Transfer-Function Method; ISO 10534-2; ISO: Geneva, Switzerland, 1998. [Google Scholar]
- Jung, S.S.; Kim, Y.T.; Lee, Y.B.; Cho, S.; Lee, J.K. Measurement of sound transmission loss by using impedance tubes. J. Korean Phys. Soc. 2008, 53, 596–600. [Google Scholar] [CrossRef]
- Barnard, A.R.; Rao, D.M. Measurement of Sound Transmission Loss Using a Modified Four-microphone Impedance Tube. In Proceedings of the 389 NOISE-CON, Baltimore, Maryland, 12–14 July 2004. [Google Scholar]
- Reichenauer, G.; Scherer, G.W. Nitrogen sorption in aerogels. J. Non-Cryst. Solids 2001, 285, 167–174. [Google Scholar]
- Zhao, L.; Yang, S.; Bhatia, B.; Strobach, E.; Wang, E.N. Modelling silica aerogel optical performance by determining its radiative properties. AIP Adv. 2016, 6, 025123. [Google Scholar] [CrossRef]
- Hunt, A.J. Light scattering for aerogel characterization. J. Non-Cryst. Solids 1998, 225, 303–306. [Google Scholar] [CrossRef]
- Buratti, C.; Moretti, E. Experimental performance evaluation of aerogel glazing systems. Appl. Energy 2012, 97, 430–437. [Google Scholar] [CrossRef]
- Duer, K.; Svendsen, S. Monolithic silica aerogel in superinsulating glazings. Sol. Energy 1998, 63, 259–267. [Google Scholar]
- Merli, F.; Anderson, A.M.; Carroll, M.K.; Buratti, C. Acoustic measurements on monolithic aerogel samples and application of the selected solutions to standard window systems. Appl. Acoust. 2018, 142, 123–131. [Google Scholar] [CrossRef]
- Buratti, C.; Merli, F.; Moretti, E. Aerogel-based materials for building applications: Influence of granule size on thermal and acoustic performance. Energy Build. 2017, 152, 472–482. [Google Scholar] [CrossRef]
- Ricciardi, P.; Belloni, E.; Cotana, F. Innovative panels with recycled materials: Thermal and acoustic performance and life cycle assessment. Appl. Energy 2014, 134, 150–162. [Google Scholar] [CrossRef]
- Anderson, A.M.; Carroll, M.K.; Green, E.C.; Melville, J.T.; Bono, M.S. Hydrophobic silica aerogels prepared via rapid supercritical extraction. J. Sol-Gel Sci. Technol. 2010, 53, 199–207. [Google Scholar] [CrossRef]
- Rao, A.V.; Haranath, D. Effect of methyltrimethoxysilane as a synthesis component on the hydrophobicity and some physical properties of silica aerogels. Microporous Mesoporous Mater. 1999, 30, 267–273. [Google Scholar]
- Sert Çok, S.; Koç, F.; Dudás, Z.; Gizli, N. The Methyl Functionality of Monolithic Silica Xerogels Synthesized via the Co-Gelation Approach Combined with Surface Silylation. Gels 2023, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- El Rassy, H.; Pierre, A.C. NMR and IR spectroscopy of silica aerogels with different hydrophobic characteristics. J. Non-Cryst. Solids 2005, 351, 1603–1610. [Google Scholar] [CrossRef]



| Before | After | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | τv | τe | ρv | ρe | τv | τe | ρv | ρe | ∆τv | ∆τe | ∆ρv | ∆ρe |
| HY 0 | 0.67 | 0.74 | 0.13 | 0.11 | 0.64 | 0.71 | 0.16 | 0.12 | −0.03 | −0.03 | 0.03 | 0.01 |
| HY 7.5 | 0.60 | 0.70 | 0.16 | 0.12 | 0.58 | 0.67 | 0.18 | 0.14 | −0.02 | −0.03 | 0.02 | 0.02 |
| HY 10 | 0.37 | 0.57 | 0.41 | 0.28 | 0.32 | 0.53 | 0.41 | 0.27 | −0.05 | −0.04 | 0.00 | −0.01 |
| Sample | NRC Value before Aging | NRC Value after 12 Years Aging |
|---|---|---|
| Granular (15 mm, φ = 0.01–1.2 mm) [34] | 0.27 | - |
| Granular (15 mm, φ = 0.7–2.0 mm) [34] | 0.23 | - |
| Monolithic (12.7 mm) [33] | 0.20 | - |
| Monolithic (19.1 mm) [33] | 0.21 | - |
| Monolithic (25.4 mm) [33] | 0.22 | - |
| Monolithic (29 mm) HY 0 | 0.21 | 0.18 |
| Hydrophobic Monolith (29 mm) HY 7 | 0.23 | 0.21 |
| Hydrophobic Monolith (29 mm) HY 10 | 0.25 | 0.22 |
| Octave Band | αbefore | αafter | ||||
|---|---|---|---|---|---|---|
| HY 0 | HY 7.5 | HY 10 | HY 0 | HY 7.5 | HY 10 | |
| 250 | 0.03 | 0.03 | 0.03 | 0.02 | 0.01 | 0.01 |
| 500 | 0.08 | 0.06 | 0.08 | 0.03 | 0.03 | 0.04 |
| 1000 | 0.23 | 0.16 | 0.37 | 0.18 | 0.15 | 0.34 |
| 2000 | 0.51 | 0.66 | 0.51 | 0.48 | 0.64 | 0.48 |
| Sample | Before | After |
|---|---|---|
| HY 0 | 10.2–59.1 | 8.4–60.7 |
| HY 7.5 | 11.5–64.8 | 7.7–67.0 |
| HY 10 | 12.1–84.0 | 11.6–83.7 |
| Sample | Ra | ||
|---|---|---|---|
| Before Aging | After Aging | Variation | |
| HY 0 | 84 | 74 | −10 |
| HY 7.5 | 89 | 75 | −14 |
| HY 10 | 61 | 61 | 0 |
| Sample | Before Aging | |||
|---|---|---|---|---|
| Surface Area (m2/g) | Peak Pore Size (nm) | Surface Area (m2/g) | Peak Pore Size (nm) | |
| HY 0 | 460 ± 10 | 20–23 | -- | -- |
| HY 7.5 | 600 ± 10 | 25–28 | 680 ± 20 | 27–29 |
| HY 10 | 640 ± 20 | 20–23 | 570 ± 10 | 32–34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorini, C.V.; Merli, F.; Belloni, E.; Carroll, M.K.; Anderson, A.M.; Buratti, C. Long-Term Performance of Monolithic Silica Aerogel with Different Hydrophobicities: Physical and Color Rendering Properties after an Accelerated Aging Process. Gels 2023, 9, 210. https://doi.org/10.3390/gels9030210
Fiorini CV, Merli F, Belloni E, Carroll MK, Anderson AM, Buratti C. Long-Term Performance of Monolithic Silica Aerogel with Different Hydrophobicities: Physical and Color Rendering Properties after an Accelerated Aging Process. Gels. 2023; 9(3):210. https://doi.org/10.3390/gels9030210
Chicago/Turabian StyleFiorini, Costanza Vittoria, Francesca Merli, Elisa Belloni, Mary K. Carroll, Ann M. Anderson, and Cinzia Buratti. 2023. "Long-Term Performance of Monolithic Silica Aerogel with Different Hydrophobicities: Physical and Color Rendering Properties after an Accelerated Aging Process" Gels 9, no. 3: 210. https://doi.org/10.3390/gels9030210
APA StyleFiorini, C. V., Merli, F., Belloni, E., Carroll, M. K., Anderson, A. M., & Buratti, C. (2023). Long-Term Performance of Monolithic Silica Aerogel with Different Hydrophobicities: Physical and Color Rendering Properties after an Accelerated Aging Process. Gels, 9(3), 210. https://doi.org/10.3390/gels9030210










