Effect of Extraction Time on the Extractability and Physicochemical Properties of Pepsin—Soluble Collagen (PCS) from the Skin of Silver Catfish (Pangasius sp.)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yield of Extraction
2.2. Chemical Composition of Extracted Collagen
2.3. Solubility
2.4. Structural Properties
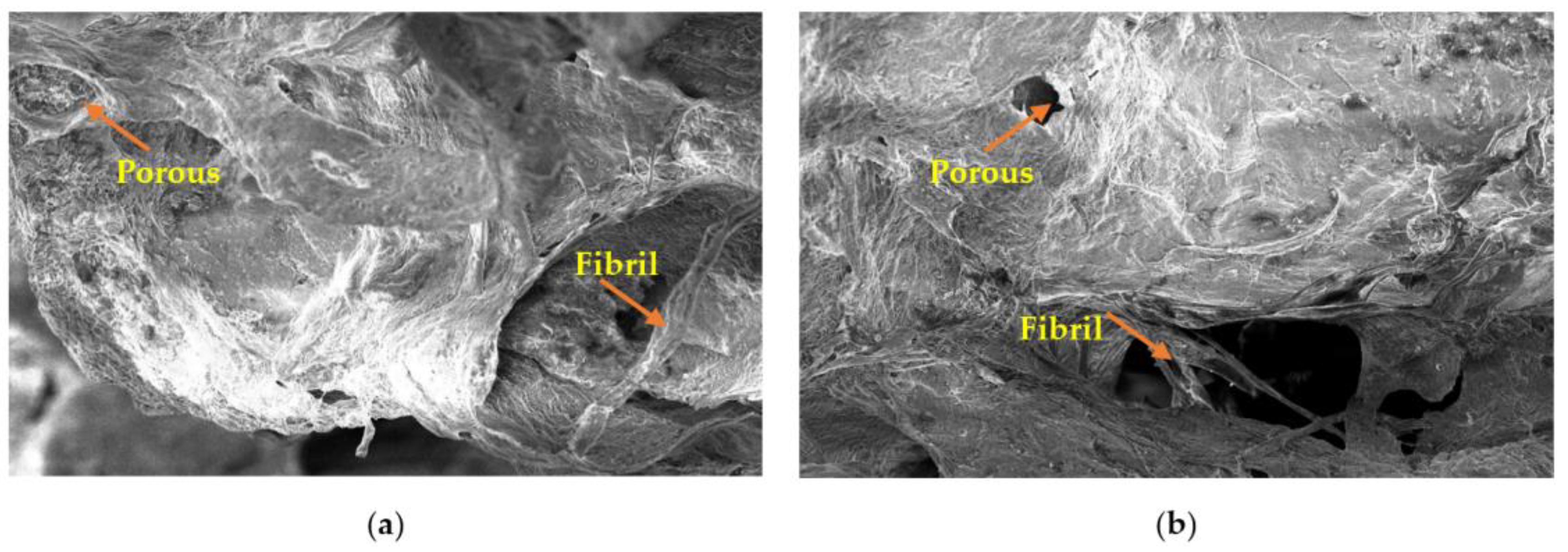
2.5. Microstructure Properties
2.6. Rheological Properties
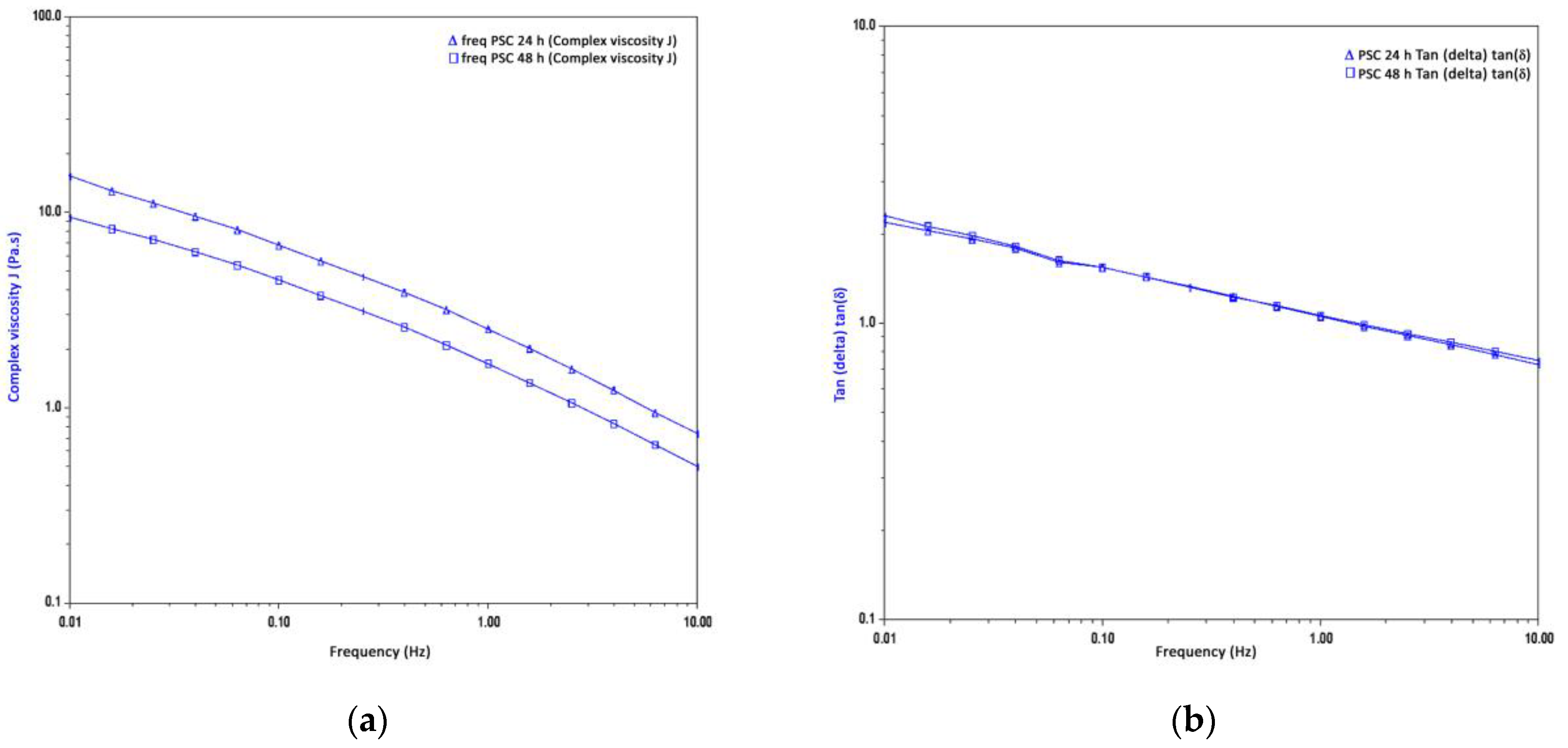
2.6.1. Frequency Sweeps
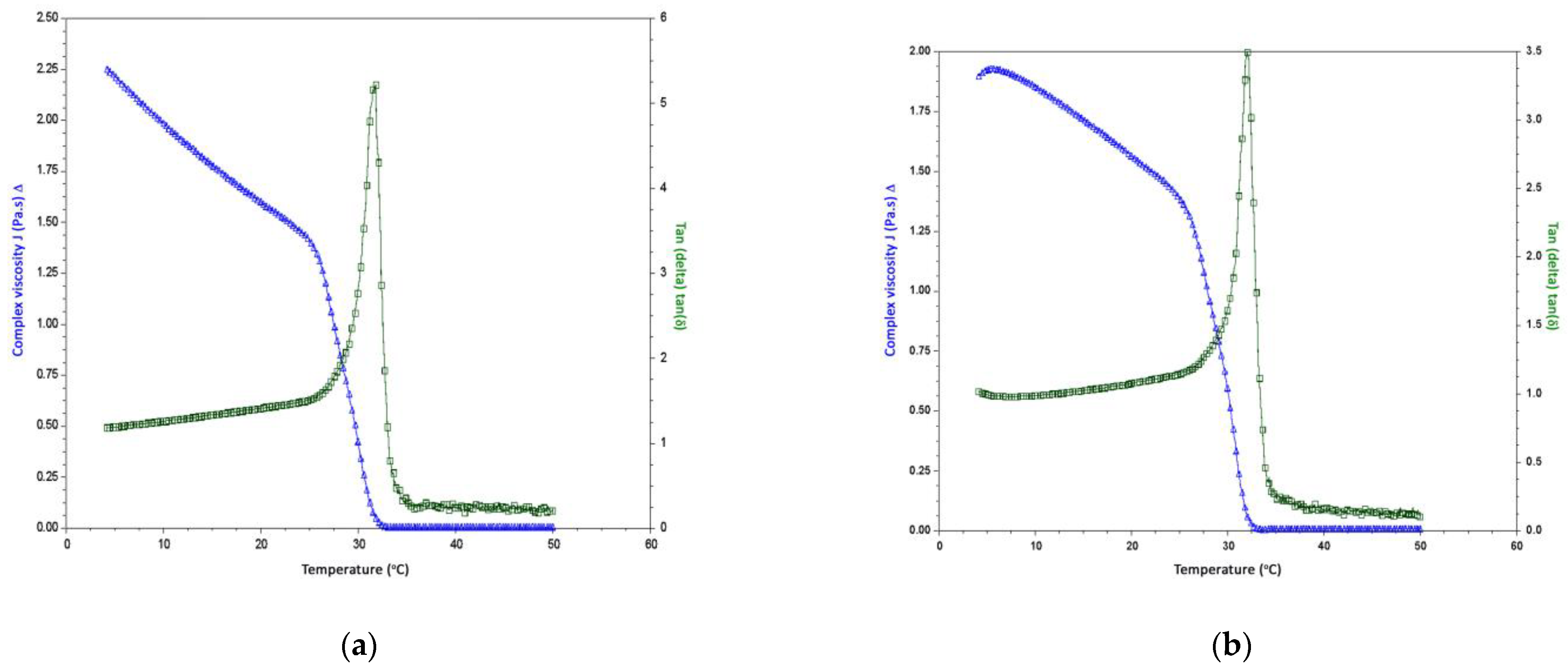
2.6.2. Temperature Sweeps
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Sample Preparation
4.2.2. Pre-Treatment of Sample
4.2.3. Extraction of Pepsin-Soluble Collagen (PSC)
4.2.4. Chemical Composition of Extracted Collagen
4.2.5. Solubility Determination
4.2.6. Structural Properties Determination of Extracted Collagen
4.2.7. Microstructure Determination of Extracted Collagen
4.2.8. Rheological Characterisation of Collagen Solution
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, W.; Chen, W.; Yang, Y.N.; Li, C.; Qian, G. Extraction, optimization, and characterization of collagen from the lung of soft-shelled turtle Pelodiscus sinensis. Int. J. Food Sci. Nutr. 2014, 3, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, A.W.; Sarbon, N.M. Characterization of acid soluble collagen (ASC) and pepsin soluble collagen (PSC) extracted from shortfin scad (Decapterus macrosoma) waste. Food Res. 2020, 4, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Hema, G.; Shyni, K.; Mathew, S.; Anandan, R.; Ninan, G.; Lakshmanan, P. A simple method for isolation of fish skin collagen- biochemical characterization of skin collagen extracted from Albacore Tuna (Thunnus Alalunga), Dog Shark (Scoliodon Sorrakowah), and Rohu (Labeo Rohita). Ann. Biol. Res. 2013, 4, 271–278. [Google Scholar]
- Sukeri, N.; Sampath Kumar, N.S.; Shaik, M.I.; Sarbon, N.M. Extractability and physicochemical properties of cobia (Rachycentron canadum) skin collagen as influenced by lactic acid concentration. J. Food Process. Preserv. 2021, 45, e15080. [Google Scholar] [CrossRef]
- Pati, F.; Dhara, S.; Adhikari, B. Fish collagen: A potential material for biomedical application. In Proceedings of the IEEE Students’ Technology Symposium (TechSym), Kharagpur, India, 3–4 April 2010; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2010; pp. 34–38. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Nalinanon, S. Compositional and physicochemical characteristics of acid-solubilized collagen extracted from the skin of unicorn leatherjacket (Aluterus monoceros). Food Hydrocoll. 2010, 24, 588–594. [Google Scholar] [CrossRef]
- Shaik, M.I.; Chong, J.Y.; Sarbon, N.M. Effect of ultrasound-assisted extraction on the extractability and physico-chemical properties of acid and pepsin soluble collagen derived from Sharpnose stingray (Dasyatis zugei) skin. Biocatal. Agric. Biotechnol. 2021, 38, 102218. [Google Scholar] [CrossRef]
- Sun, L.; Hou, H.; Li, B.; Zhang, Y. Characterization of acid-and pepsin-soluble collagen extracted from the skin of Nile tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2017, 99, 8–14. [Google Scholar] [CrossRef]
- Ong, T.Y.; Shaik, M.I.; Sarbon, N.M. Isolation and characterization of acid and pepsin soluble collagen extracted from sharpnose stingray (Dasyatis zugei) skin. Food Res. 2021, 5, 214–224. [Google Scholar] [CrossRef]
- Krishnamoorthi, J.; Ramasamy, P.; Shanmugam, V.; Shanmugam, A. Isolation and partial characterization of collagen from the outer skin of sepia. Biochem. Biophys. Rep. 2017, 10, 39–45. [Google Scholar] [CrossRef]
- Hamdan, F.S.; Sarbon, N.M. Isolation and characterization of collagen from Fringescale sardinella (Sardinella fimbriata) waste materials. Int. Food Res. J. 2017, 26, 133–140. [Google Scholar]
- Hukmi, N.M.M.; Sarbon, N.M. Isolation and characterization of acid-soluble collagen (ASC) and pepsin soluble collagen (PSC) from the skin of silver catfish (Pangasius sp.). Int. Food Res. J. 2018, 25, 1785–1791. [Google Scholar]
- Yang, H.; Shu, Z. The extraction of collagen protein from pigskin. J. Chem. Pharm. Res. 2014, 6, 683–687. [Google Scholar]
- Schmidt, M.M.; Dornelles, R.C.; Mello, R.O.; Kubota, E.H.; Mazutti, M.; Demiate, I.M. Collagen extraction process. Int. Food Res. J. 2016, 23, 913–922. [Google Scholar]
- Peck Loo, K.; Mashitah, M. Extraction of Acid and Pepsin Soluble Collagen from Selected Malaysian Freshwater Fish Muscles: Modified Lowry’s Measurement Method. J. Agric. Biotechnol. 2013, 4, 17–32. [Google Scholar]
- Skierka, E.; Sadowska, M. The influence of different acids and pepsin on the extractability of collagen from the skin of Baltic cod (Gadus morhua). Food Chem. 2007, 105, 1302–1306. [Google Scholar] [CrossRef]
- Huang, Y.R.; Shiau, C.Y.; Chen, H.H.; Huang, B.C. Isolation and characterization of acid and pepsin-solubilized collagens from the skin of balloon fish (Diodon holocanthus). Food Hydrocoll. 2011, 25, 1507–1513. [Google Scholar] [CrossRef]
- Normah, I.; Nur Ain, H.; Effaniza, E. Physicochemical Properties of Silver Catfish (Pangasius sutchi) Skin Gelatin Produced as Affected by Different Extraction Time. J. Intelek 2014, 9, 7–15. [Google Scholar]
- Normah, I.; Najihah, S.; Zainal, S. Effects of extraction time on the functional properties of silver catfish (Pangasius sutchi) skin gelatin. Sci. Res. J. 2013, 10, 65–82. [Google Scholar] [CrossRef] [Green Version]
- Hashim, R.B.; Jamil, E.F.; Zulkipli, F.H.; Daud, J.M. Fatty acid composition of silver catfish, Pangasius sp. farmed in several rivers of Pahang, Malaysia. J. Oleo Sci. 2015, 64, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Hadfi, N.H.; Sarbon, N.M. Physicochemical properties of silver catfish (Pangasius sp.) skin collagen as influenced by acetic acid concentration. Food Res. 2019, 3, 783–790. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Isolation and properties of acid-and pepsin-soluble collagen from the skin of black tip shark (Carcharhinus limbatus). Eur. Food Res. Technol. 2010, 230, 475–483. [Google Scholar] [CrossRef]
- Veeruraj, A.; Arumugam, M.; Balasubramanian, T. Isolation and characterization of thermostable collagen from the marine eel-fish (Evenchelys macrura). Process Biochem. 2013, 48, 1592–1602. [Google Scholar] [CrossRef]
- Vallejos, N.; González, G.; Troncoso, E.; Zúñiga, R.N. Acid and enzyme-aided collagen extraction from the byssus of Chilean mussels (Mytilus Chilensis): Effect of process parameters on extraction performance. Food Biophys. 2014, 9, 322–331. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Li, G.; Shi, B.; Miao, Y.; Wu, X. Isolation and partial characterization of pepsin-soluble collagen from the skin of grass carp (Ctenopharyngodon idella). Food Chem. 2007, 103, 906–912. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mohammadifar, M.; Mortazavian, A.; Rouhi, M.; Ghasemi, J.; Delshadian, Z. Extraction optimization of pepsin-soluble collagen from eggshell membrane by response surface methodology (RSM). Food Chem. 2016, 190, 186–193. [Google Scholar] [CrossRef]
- Matmaroh, K.; Benjakul, S.; Prodpran, T.; Encarnacion, A.B.; Kishimura, H. Characteristics of acid- soluble collagen and pepsin soluble collagen from the scale of spotted golden goatfish (Parupeneus heptacanthus). Food Chem. 2011, 129, 1179–1186. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Kishimura, H.; Shahidi, F. Isolation and Characterisation of collagen from the skin of brown-banded bamboo shark (Chiloscyllium punctatum). Food Chem. 2010, 119, 1519–1526. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Isolation and characterization of collagen from the cartilages of brown-banded bamboo shark (Chiloscyllium punctatum) and black tip shark (Carcharhinus limbatus). LWT Food Sci. Technol. 2010, 43, 792–800. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Li, G. Isolation and characterisation of collagen from the skin of large fin longbarbel catfish (Mystus macropterus). Food Chem. 2009, 115, 826–831. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Isolation and characterisation of acid and pepsin-solubilised collagen from the skin of Brownstripe red snapper (Lutjanus vitta). Food Chem. 2005, 93, 475–484. [Google Scholar] [CrossRef]
- Bae, I.; Osatomi, K.; Yoshida, A.; Osako, K.; Yamaguchi, A.; Hara, K. Biochemical properties of acid-soluble collagens extracted from the skins of underutilised fishes. Food Chem. 2008, 108, 49–54. [Google Scholar] [CrossRef]
- Nalinanon, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H. Use of pepsin for collagen extraction from the skin of bigeye snapper (Priacanthus tayenus). Food Chem. 2007, 104, 593–601. [Google Scholar] [CrossRef]
- Nalinanon, S.; Benjakul, S.; Kishimura, H. Collagen from the skin of arabesque greenling (Pleurogrammus azonus) solubilized with the aid of acetic acid and pepsin from albacore tuna (Thunnus alalunga) stomach. J. Sci. Food Agric. 2010, 90, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Yi, R.; Xu, N.; Gao, R.; Hong, B. Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis niloticus). LWT Food Sci. Technol. 2016, 66, 453–459. [Google Scholar] [CrossRef]
- Shanmugam, V.; Ramasamy, P.; Subhapradha, N.; Sudharsan, S.; Seedevi, P.; Moovendhan, M.; Krishnamoorthy, J.; Shanmugam, A.; Srinivasan, A. Extraction, structural and physical characterization of type I collagen from the outer skin of Sepiella inermis (Orbigny, 1848). Afr. J. Biotechnol. 2012, 11, 14326–14337. [Google Scholar] [CrossRef]
- Wu, X.; Cai, L.; Cao, A.; Wang, Y.; Li, T.; Li, J. Comparative study on acid-soluble and pepsin-soluble collagens from the skin and swim bladder of grass carp (Ctenopharyngodon idella). J. Sci. Food Agric. 2016, 96, 815–821. [Google Scholar] [CrossRef]
- Sanden, K.W. Development of Vibrational Spectroscopic Techniques for Measuring Quality-Related Parameters of Connective Tissue. Master’s Thesis, Department of Chemistry, Biotechnology and Food Science, Temple University in Philadelphia, Philadelphia, PA, USA, 2011. [Google Scholar]
- Kiew, P.L.; Mat Don, M. Screening and empirical kinetic models of collagen extraction from selected Malaysian freshwater fish. J. Food Eng. 2013, 36, 428–438. [Google Scholar] [CrossRef]
- Duan, R.; Zhang, J.J.; Konno, K.; Wu, M.H.; Li, J.; Chen, Y. Analysis and improvement of stability of pepsin-solubilized collagen from the skin of carp (Cyprinus carpio). Chem Pap. 2012, 66, 636–641. [Google Scholar] [CrossRef]
- Wang, L.; An, X.; Xin, Z.; Zhao, L.; Hu, Q. Isolation and Characterization of Collagen from the Skin of Deep-Sea Redfish (Sebastes mentella). J. Food Sci. 2007, 72, E450–E455. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Lin, S.; Liang, Q.; Shi, Z.; Xu, J.; Ma, H. Isolation, and characterization of collagen from the muscle of Amur sturgeon (Acipenser schrenckii). Biotechnol. Bioprocess Eng. 2014, 19, 935–941. [Google Scholar] [CrossRef]
- Pal, G.; Nidheesh, T.; Suresh, P. Comparative study on the characteristics and in vitro fibril formation ability of acid and pepsin soluble collagen from the skin of catla (Catla catla) and rohu (Labeo rohita). Int. Food Res. J. 2015, 76, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Wang, L.; Sun, W.; Wang, Z.; Xu, J.; Ma, H. Isolation, and characterization of collagen from the cartilage of Amur sturgeon (Acipenser schrenckii). Process Biochem. 2013, 49, 318–323. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Li, G.; Du, Z. Rheological properties of fish skin collagen solution: Effects of temperature and concentration. Korea Aust. Rheol. J. 2010, 22, 119–127. [Google Scholar]
- Ren, G.; Zhang, H.; Guo, J.; Kang, H. Effect of concentration and temperature on the rheological behavior of collagen from swimming bladder of grass carp. Adv. Mater. Res. 2012, 512, 584–588. [Google Scholar] [CrossRef]
- Yang, Y.L.; Kaufman, L. Rheology and Confocal Reflectance Microscopy as Probes of Mechanical Properties and Structure during Collagen and Collagen/Hyaluronan Self-Assembly. Biophys. J. 2009, 96, 1566–1585. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Duan, L.; Tian, Z.; Liu, W.; Li, G.; Huang, X. Rheological behavior of acylated pepsin-solubilized collagen solutions: Effects of concentration. Korea Aust. Rheol. J. 2015, 27, 287–295. [Google Scholar] [CrossRef]
- Moraes, I.; Fasolin, L.; Cunha, R.; Menegalli, F. Dynamic and steady- shear rheological properties of xanthan and guar gums dispersed in yellow passion fruit pulp (Passiflora edulis f. flavicarpa). Braz. J. Chem. Eng. 2001, 28, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Pietrucha, K. Changes in denaturation and rheological properties of collagen–hyaluronic acid scaffolds as a result of temperature dependency. Int. J. Biol. Macromol. 2005, 36, 299–304. [Google Scholar] [CrossRef]
- Lai, G.; Li, Y.; Li, G. Effect of concentration and temperature on the rheological behavior of collagen solution. Int. J. Biol. Macromol. 2008, 42, 285–291. [Google Scholar] [CrossRef]
- Alves, A.; Marques, A.; Martins, E.; Silva, T.; Reis, R. Cosmetic Potential of Marine Fish Skin Collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Analytical Chemists International. Official Methods of Analysis, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]


| Extraction Time (h) | Yield (%) |
|---|---|
| PSC 24 | 23.64 ± 2.86 a |
| PSC 48 | 26.43 ± 2.48 a |
| Sample | Chemical Composition (%) | |||
|---|---|---|---|---|
| Moisture | Ash | Fat | Protein | |
| PSC 24 h | 93.15 ± 0.48 a | 21.86 ± 0.88 b | 2.95 ± 0.31 a | 53.22 ± 2.34 a |
| PSC 48 h | 89.93 ± 0.35 b | 26.69 ± 1.68 a | 2.73 ± 0.81 b | 45.18 ± 1.24 b |
| Functional Group | Mode of Vibration | Wavelength (cm−1) | |
|---|---|---|---|
| 24 h | 48 h | ||
| Amide A | N–H stretching | 3443.27 ± 0.035 a | 3446.19 ± 7.50 a |
| Amide I | C=O stretching | 1636.95 ± 0.15 a | 1634.95 ± 3.12 a |
| Amide II | N–H bending | 1545.58 ± 0.42 a | 1554.59 ± 8.23 a |
| Amide III | N–H bending | 1257.70 ± 10.62 a | 1250.23 ± 12.36 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaik, M.I.; Md Nor, I.N.; Sarbon, N.M. Effect of Extraction Time on the Extractability and Physicochemical Properties of Pepsin—Soluble Collagen (PCS) from the Skin of Silver Catfish (Pangasius sp.). Gels 2023, 9, 300. https://doi.org/10.3390/gels9040300
Shaik MI, Md Nor IN, Sarbon NM. Effect of Extraction Time on the Extractability and Physicochemical Properties of Pepsin—Soluble Collagen (PCS) from the Skin of Silver Catfish (Pangasius sp.). Gels. 2023; 9(4):300. https://doi.org/10.3390/gels9040300
Chicago/Turabian StyleShaik, Mannur Ismail, Intan Nordiana Md Nor, and Norizah Mhd Sarbon. 2023. "Effect of Extraction Time on the Extractability and Physicochemical Properties of Pepsin—Soluble Collagen (PCS) from the Skin of Silver Catfish (Pangasius sp.)" Gels 9, no. 4: 300. https://doi.org/10.3390/gels9040300
APA StyleShaik, M. I., Md Nor, I. N., & Sarbon, N. M. (2023). Effect of Extraction Time on the Extractability and Physicochemical Properties of Pepsin—Soluble Collagen (PCS) from the Skin of Silver Catfish (Pangasius sp.). Gels, 9(4), 300. https://doi.org/10.3390/gels9040300








