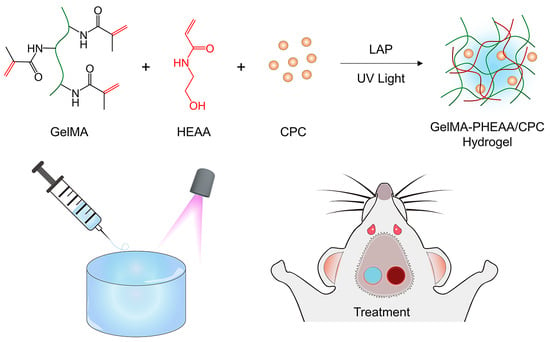
Tough, Injectable Calcium Phosphate Cement Based Composite Hydrogels to Promote Osteogenesis
Abstract
1. Introduction
2. Results and Discussion
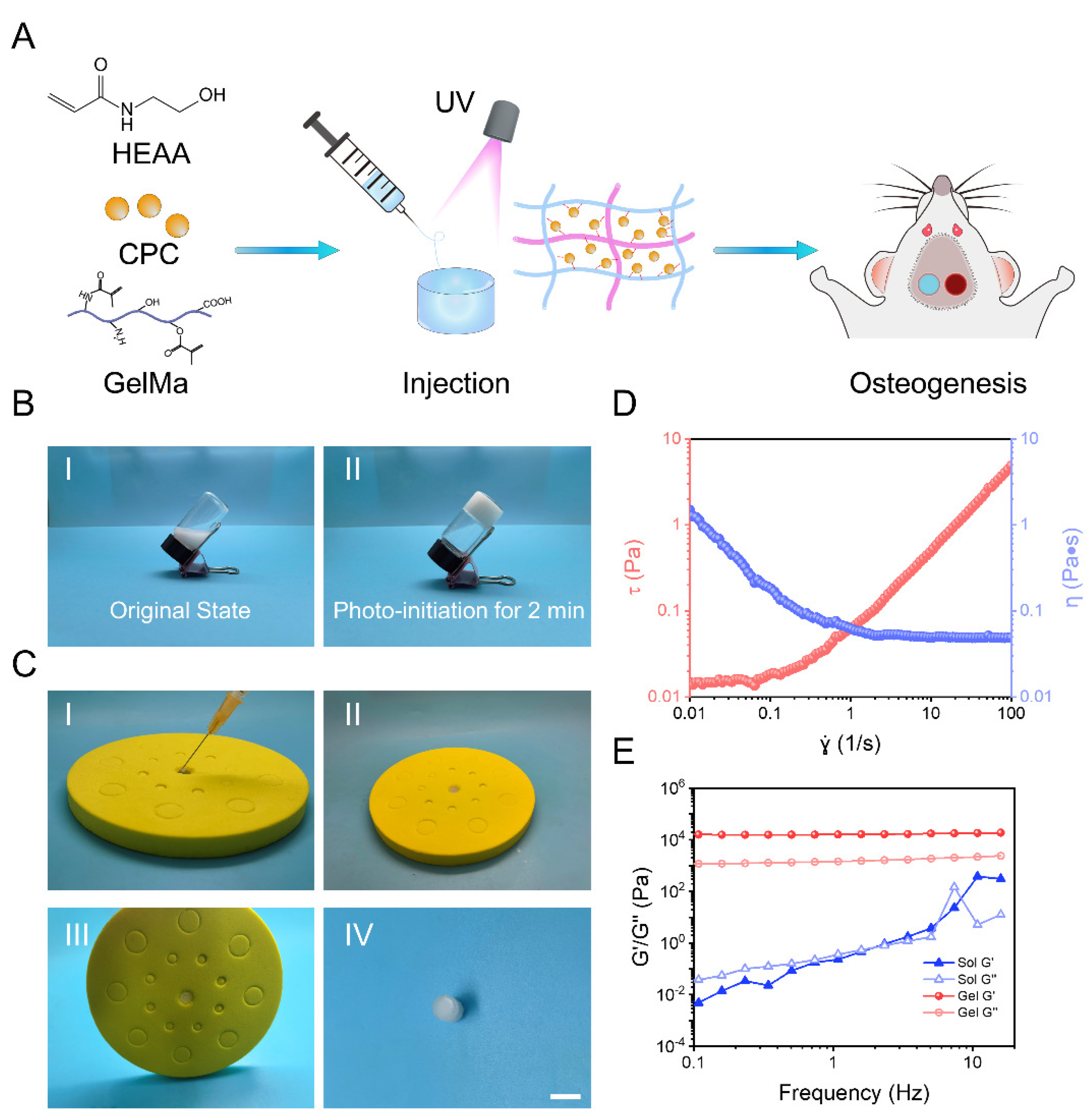
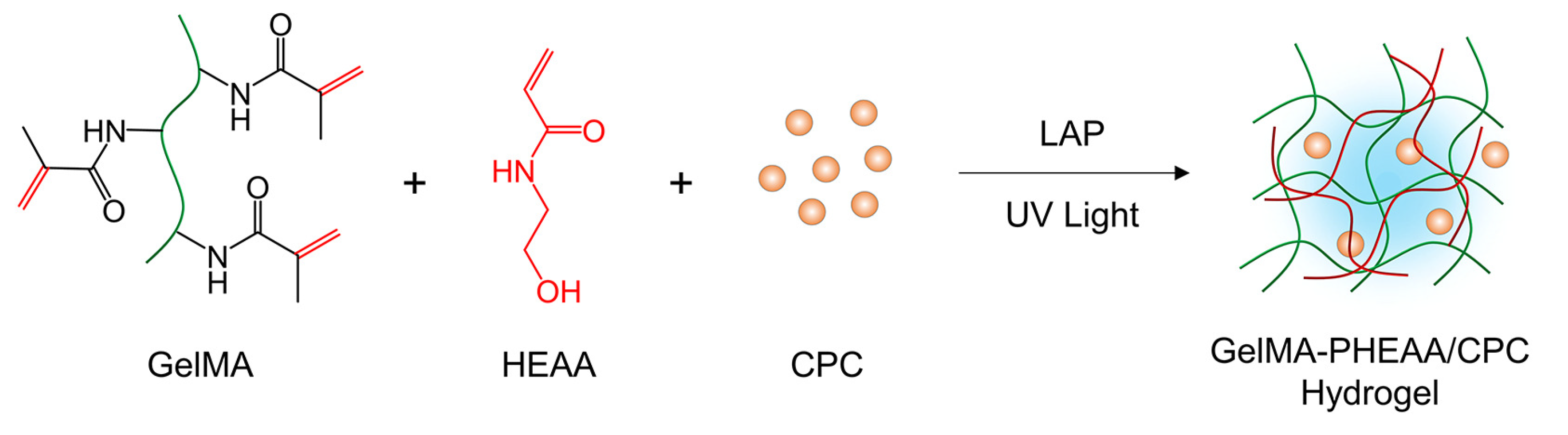
2.1. Synthesis and Characterization of GelMA-PHEAA/CPC Hydrogels
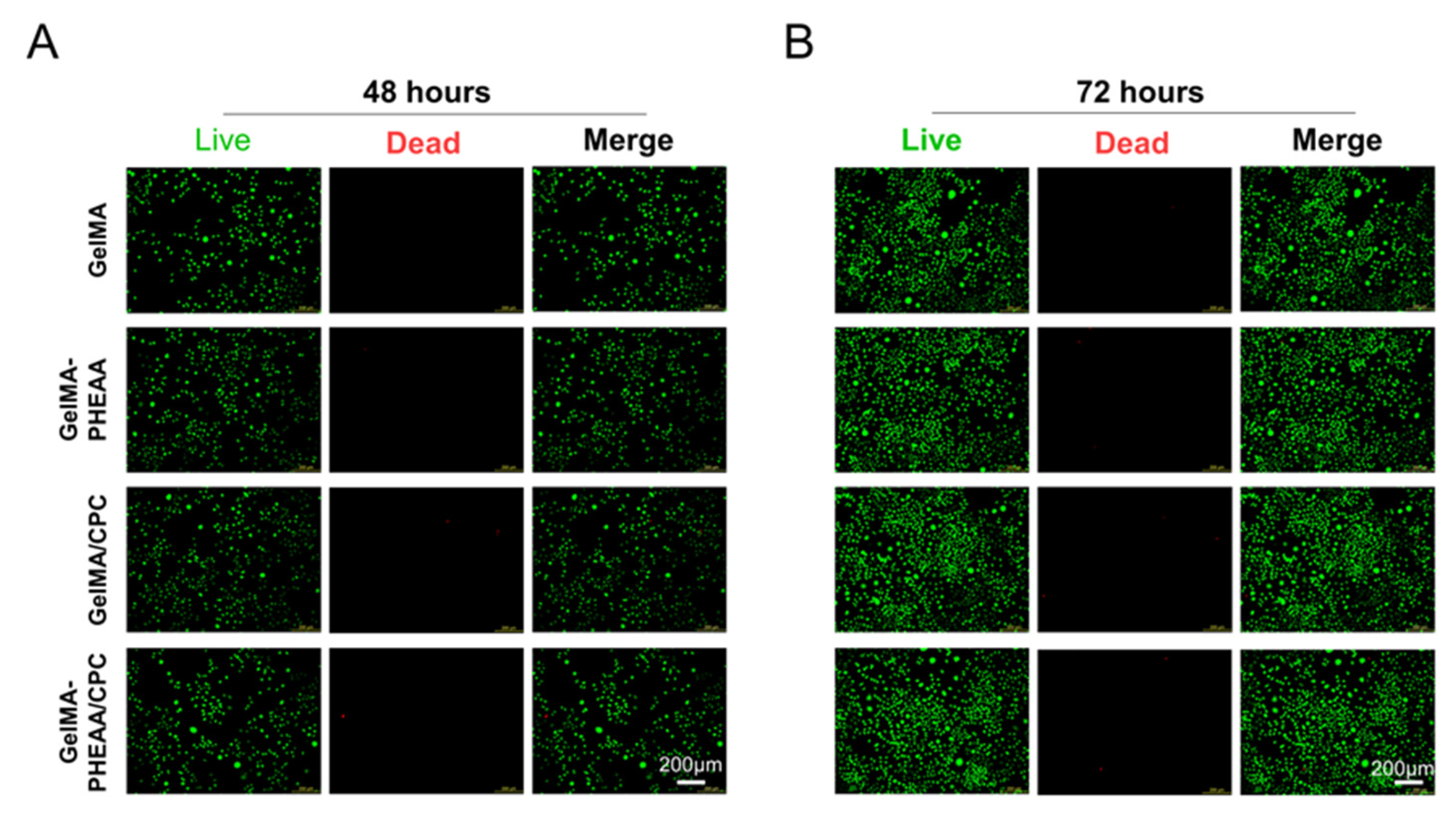
2.2. Cell Proliferation
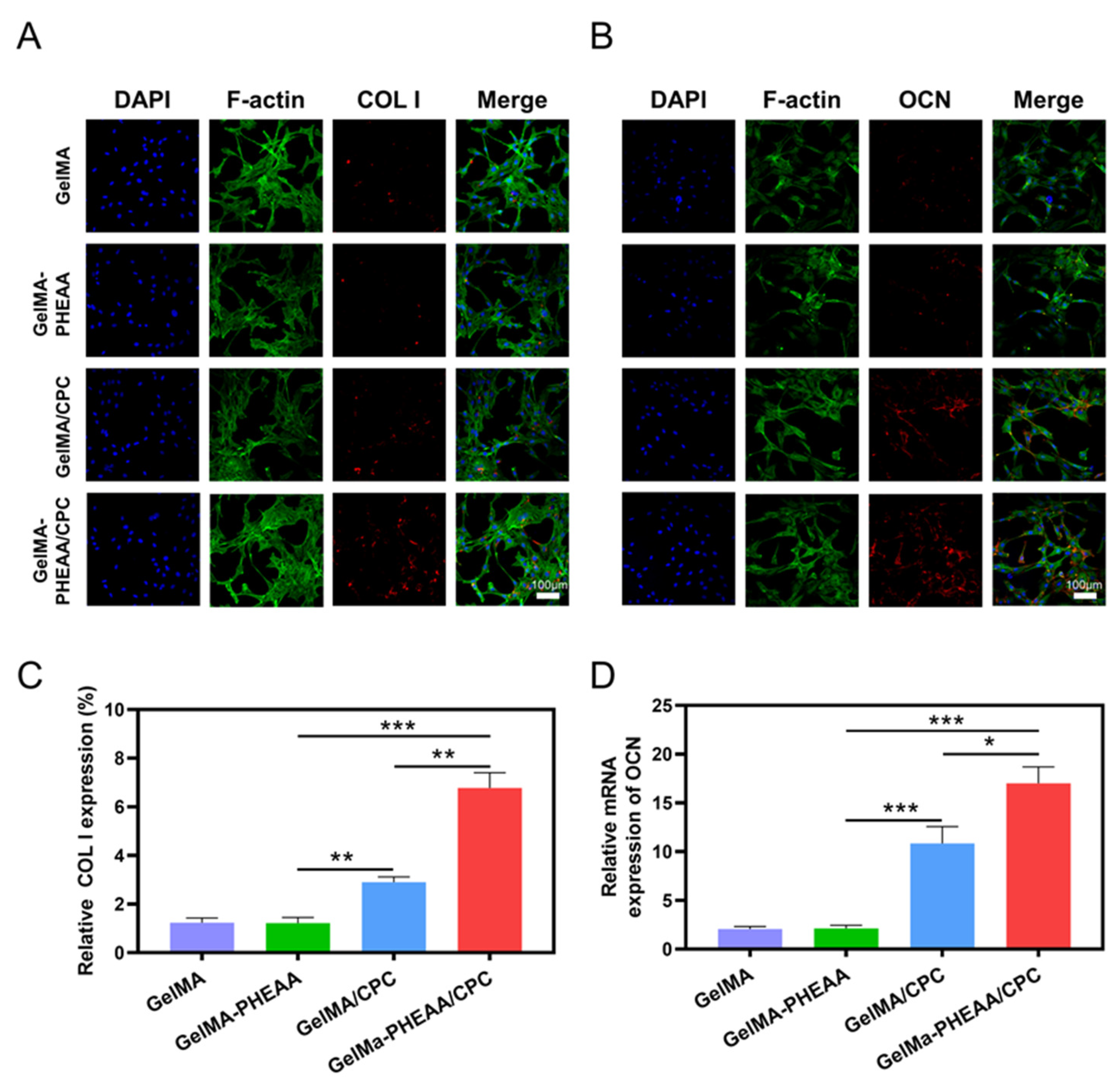
2.3. Osteogenic Activity
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of GelMA-PHEAA/CPC Hydrogels
4.3. Characterization of Hydrogels
4.4. In Vitro Cytocompatibility Evaluation
4.5. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.6. Effects of the Osteogenic Activity of MC3T3
4.7. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Williams, S.A.; Daigle, S.G.; Weiss, R.; Wang, Y.; Arora, T.; Curtis, J.R. Economic Burden of Osteoporosis-Related Fractures in the US Medicare Population. Ann. Pharmacother. 2021, 55, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E.; Giustina, A.; Bilezikian, J.P. Mechanisms of anabolic therapies for osteoporosis. N. Engl. J. Med. 2007, 357, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef]
- van Geel, T.A.; van Helden, S.; Geusens, P.P.; Winkens, B.; Dinant, G.J. Clinical subsequent fractures cluster in time after first fractures. Ann. Rheum. Dis. 2009, 68, 99–102. [Google Scholar] [CrossRef]
- Cummings, S.R.; Lui, L.-Y.; Eastell, R.; Allen, I.E. Association Between Drug Treatments for Patients with Osteoporosis and Overall Mortality Rates: A Meta-analysis. JAMA Intern. Med. 2019, 179, 1491–1500. [Google Scholar] [CrossRef]
- Galbusera, F.; Volkheimer, D.; Reitmaier, S.; Berger-Roscher, N.; Kienle, A.; Wilke, H.-J. Pedicle screw loosening: A clinically relevant complication? Eur. Spine J. 2015, 24, 1005–1016. [Google Scholar] [CrossRef]
- Weiser, L.; Huber, G.; Sellenschloh, K.; Viezens, L.; Püschel, K.; Morlock, M.M.; Lehmann, W. Insufficient stability of pedicle screws in osteoporotic vertebrae: Biomechanical correlation of bone mineral density and pedicle screw fixation strength. Eur. Spine J. 2017, 26, 2891–2897. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, Z.Y.; Zhang, Y.X.; Hu, X.F.; Chen, F.H.; Zhang, L.K.; Zhong, N.; Zhang, J.Y.; Wang, Y.B. Mussel-inspired bioactive 3D-printable poly(styrene-butadiene-styrene) and the in vitro assessment of its potential as cranioplasty implants. J. Mater. Chem. B 2022, 10, 3747–3758. [Google Scholar] [CrossRef]
- Che, L.B.; Wang, Y.; Sha, D.Y.; Li, G.Y.; Wei, Z.H.; Liu, C.S.; Yuan, Y.; Song, D.W. A biomimetic and bioactive scaffold with intelligently pulsatile teriparatide delivery for local and systemic osteoporosis regeneration. Bioact. Mater. 2023, 19, 75–87. [Google Scholar] [CrossRef]
- Che, L.B.; Lei, Z.Y.; Wu, P.Y.; Song, D.W. A 3D Printable and Bioactive Hydrogel Scaffold to Treat Traumatic Brain Injury. Adv. Funct. Mater. 2019, 29, 1904450. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, J.-F.; Fang, X.; Chen, Y. Application and modification of bone cement in vertebroplasty: A literature review. Jt. Dis. Relat. Surg. 2022, 33, 467–478. [Google Scholar] [CrossRef]
- Saadeh, Y.S.; Swong, K.N.; Yee, T.J.; Strong, M.J.; Kashlan, O.N.; Szerlip, N.J.; Oppenlander, M.E.; Park, P. Effect of Fenestrated Pedicle Screws with Cement Augmentation in Osteoporotic Patients Undergoing Spinal Fusion. World Neurosurg. 2020, 143, e351–e361. [Google Scholar] [CrossRef]
- Singh, V.; Mahajan, R.; Das, K.; Chhabra, H.S.; Rustagi, T. Surgical Trend Analysis for Use of Cement Augmented Pedicle Screws in Osteoporosis of Spine: A Systematic Review (2000–2017). Glob. Spine J. 2019, 9, 783–795. [Google Scholar] [CrossRef]
- Raucci, M.G.; D’Amora, U.; Ronca, A.; Ambrosio, L. Injectable Functional Biomaterials for Minimally Invasive Surgery. Adv. Healthc. Mater. 2020, 9, 2000349. [Google Scholar] [CrossRef]
- Zdorovets, M.V.; Kozlovskiy, A.L.; Borgekov, D.B.; Shlimas, D.I. Influence of irradiation with heavy Kr15+ ions on the structural, optical and strength properties of BeO ceramic. J. Mater. Sci. Mater. Electron. 2021, 32, 15375–15385. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Turchenko, V.A.; Kostishyn, V.G.; Panina, L.V.; Kazakevich, I.S.; Balagurov, A.M. Structure and magnetic properties of BaFe11.9In0.1O19 hexaferrite in a wide temperature range. J. Alloys Compd. 2016, 689, 383–393. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Egizbek, K.; Darwish, M.V.; Ibragimova, M.; Shumskaya, A.; Rogachev, A.A.; Ignatovich, Z.V.; Kadyrzhanov, K. Evaluation of the Efficiency of Detection and Capture of Manganese in Aqueous Solutions of FeCeOx Nanocomposites Doped with Nb2O5. Sensors 2020, 20, 4851. [Google Scholar] [CrossRef]
- Darwish, M.A.; Zubar, T.I.; Kanafyev, O.D.; Zhou, D.; Trukhanova, E.L.; Trukhanov, S.V.; Trukhanov, A.V.; Henaish, A.M. Combined Effect of Microstructure, Surface Energy, and Adhesion Force on the Friction of PVA/Ferrite Spinel Nanocomposites. Nanomaterials 2022, 12, 1998. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Zdorovets, M.V. Effect of doping of Ce4+/3+ on optical, strength and shielding properties of (0.5−x)TeO2-0.25MoO-0.25Bi2O3-xCeO2 glasses. Mater. Chem. Phys. 2021, 263, 124444. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Trukhanov, A.V.; Slimani, Y.; You, K.Y.; Trukhanov, S.V.; Trukhanova, E.L.; Esa, F.; Sadaqat, A.; Chaudhary, K.; Zdorovets, M.; et al. Correlation Between Composition and Electrodynamics Properties in Nanocomposites Based on Hard/Soft Ferrimagnetics with Strong Exchange Coupling. Nanomaterials 2019, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Song, Z.; Liu, C. Fast setting and anti-washout injectable calcium–magnesium phosphate cement for minimally invasive treatment of bone defects. J. Mater. Chem. B 2015, 3, 9173–9181. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, W.; Wang, Z.; Wang, Z.; Xie, Q.; Niu, H.; Guo, H.; Yuan, Y.; Liu, C. PEGylated poly(glycerol sebacate)-modified calcium phosphate scaffolds with desirable mechanical behavior and enhanced osteogenic capacity. Acta Biomater. 2016, 44, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, Y.; Ji, L.; Geng, Z.; Wang, J.; Liu, C. Calcium phosphate-based materials regulate osteoclast-mediated osseointegration. Bioact. Mater. 2021, 6, 4517–4530. [Google Scholar] [CrossRef]
- Cai, P.; Lu, S.; Yu, J.; Xiao, L.; Wang, J.; Liang, H.; Huang, L.; Han, G.; Bian, M.; Zhang, S.; et al. Injectable nanofiber-reinforced bone cement with controlled biodegradability for minimally-invasive bone regeneration. Bioact. Mater. 2023, 21, 267–283. [Google Scholar] [CrossRef]
- Diaferia, C.; Rosa, E.; Balasco, N.; Sibillano, T.; Morelli, G.; Giannini, C.; Vitagliano, L.; Accardo, A. The Introduction of a Cysteine Residue Modulates The Mechanical Properties of Aromatic-Based Solid Aggregates and Self-Supporting Hydrogels. Chem. Eur. J. 2021, 27, 14886–14898. [Google Scholar] [CrossRef]
- Rachmiel, D.; Anconina, I.; Rudnick-Glick, S.; Halperin-Sternfeld, M.; Adler-Abramovich, L.; Sitt, A. Hyaluronic Acid and a Short Peptide Improve the Performance of a PCL Electrospun Fibrous Scaffold Designed for Bone Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 2425. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.; Wang, J.; Zhang, W.; Lu, W.; Gao, Y.; Zhang, B.; Guo, Y. Development of a Photo-Crosslinking, Biodegradable GelMA/PEGDA Hydrogel for Guided Bone Regeneration Materials. Materials 2018, 11, 1345. [Google Scholar] [CrossRef]
- Dong, Z.; Yuan, Q.; Huang, K.; Xu, W.; Liu, G.; Gu, Z. Gelatin methacryloyl (GelMA)-based biomaterials for bone regeneration. RSC Adv. 2019, 9, 17737–17744. [Google Scholar] [CrossRef]
- Kurian, A.G.; Singh, R.K.; Patel, K.D.; Lee, J.-H.; Kim, H.-W. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact. Mater. 2022, 8, 267–295. [Google Scholar] [CrossRef]
- Pu, X.; Tong, L.; Wang, X.; Liu, Q.; Chen, M.; Li, X.; Lu, G.; Lan, W.; Li, Q.; Liang, J.; et al. Bioinspired Hydrogel Anchoring 3DP GelMA/HAp Scaffolds Accelerates Bone Reconstruction. ACS Appl. Mater. Interfaces 2022, 14, 20591–20602. [Google Scholar] [CrossRef]
- Song, P.; Li, M.; Zhang, B.; Gui, X.; Han, Y.; Wang, L.; Zhou, W.; Guo, L.; Zhang, Z.; Li, Z.; et al. DLP fabricating of precision GelMA/HAp porous composite scaffold for bone tissue engineering application. Compos. Part B Eng. 2022, 244, 110163. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Ren, B.; Zhang, Y.; Ma, J.; Xu, L.; Chen, Q.; Zheng, J. Super Bulk and Interfacial Toughness of Physically Crosslinked Double-Network Hydrogels. Adv. Funct. Mater. 2017, 27, 1703086. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, D.; Gong, L.; Zhang, Y.; Xie, S.; Ren, B.; Liu, Y.; Yang, F.; Zhou, G.; Chang, Y.; et al. Double-Network Physical Cross-Linking Strategy To Promote Bulk Mechanical and Surface Adhesive Properties of Hydrogels. Macromolecules 2019, 52, 9512–9525. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Zhang, Y.; Yang, F.; Liu, Y.; Wang, X.; Yang, J.; Gong, X.; Zheng, J. Highly stretchable, self-adhesive, biocompatible, conductive hydrogels as fully polymeric strain sensors. J. Mater. Chem. A 2020, 8, 20474–20485. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, F.; He, J.; Xu, L.; Wang, T.; Feng, Z.-Q.; Chang, Y.; Gong, X.; Zhang, G.; Zheng, J. Multiple Physical Bonds to Realize Highly Tough and Self-Adhesive Double-Network Hydrogels. ACS Appl. Polym. Mater. 2020, 2, 1031–1042. [Google Scholar] [CrossRef]
- Sha, D.; Tang, S.; Dong, Z.; Chen, K.; Wang, N.; Liu, C.; Ling, X.; He, H.; Yuan, Y. Wearable, antibacterial, and self-healable modular sensors for monitoring joints movement ultra-sensitively. Eur. Polym. J. 2022, 180, 111617. [Google Scholar] [CrossRef]
- Wu, F.; Wei, J.; Guo, H.; Chen, F.; Hong, H.; Liu, C. Self-setting bioactive calcium–magnesium phosphate cement with high strength and degradability for bone regeneration. Acta Biomater. 2008, 4, 1873–1884. [Google Scholar] [CrossRef]
- Wang, S.G.; Wang, F.; Shi, K.; Yuan, J.F.; Sun, W.L.; Yang, J.T.; Chen, Y.X.; Zhang, D.; Che, L.B. Osteichthyes skin-inspired tough and sticky composite hydrogels for dynamic adhesive dressings. Compos. Part B Eng. 2022, 241, 110010. [Google Scholar] [CrossRef]



| Primers | Sequences | Primers | Sequences | |
|---|---|---|---|---|
| GAPDH | Forward | AGAACATCATCCCTGCATCCAC | GAPDH | Forward |
| Reverse | TCAGATCCACGACGGACACA | Reverse | ||
| RUNX2 | Forward | CCTCGAATGGCAGCACGCTA | RUNX2 | Forward |
| Reverse | GCCGCCAAACAGACTCATCCA | Reverse | ||
| ALP | Forward | CACGGCGTCCATGAGCAGAAC | ALP | Forward |
| Reverse | CAGGCACAGTGGTCAAGGTTGG | Reverse | ||
| COL I | Forward | TGGTCCTGCTGGTCCTGCTG | COL I | Forward |
| Reverse | CTGTCACCTTGTTCGCCTGTCTC | Reverse |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Peng, Z.; Zhang, D.; Song, D. Tough, Injectable Calcium Phosphate Cement Based Composite Hydrogels to Promote Osteogenesis. Gels 2023, 9, 302. https://doi.org/10.3390/gels9040302
Wang Y, Peng Z, Zhang D, Song D. Tough, Injectable Calcium Phosphate Cement Based Composite Hydrogels to Promote Osteogenesis. Gels. 2023; 9(4):302. https://doi.org/10.3390/gels9040302
Chicago/Turabian StyleWang, Yazhou, Zhiwei Peng, Dong Zhang, and Dianwen Song. 2023. "Tough, Injectable Calcium Phosphate Cement Based Composite Hydrogels to Promote Osteogenesis" Gels 9, no. 4: 302. https://doi.org/10.3390/gels9040302
APA StyleWang, Y., Peng, Z., Zhang, D., & Song, D. (2023). Tough, Injectable Calcium Phosphate Cement Based Composite Hydrogels to Promote Osteogenesis. Gels, 9(4), 302. https://doi.org/10.3390/gels9040302