Influence of Different pH Values on Gels Produced from Tea Polyphenols and Low Acyl Gellan Gum
Abstract
:1. Introduction
2. Results and Discussion
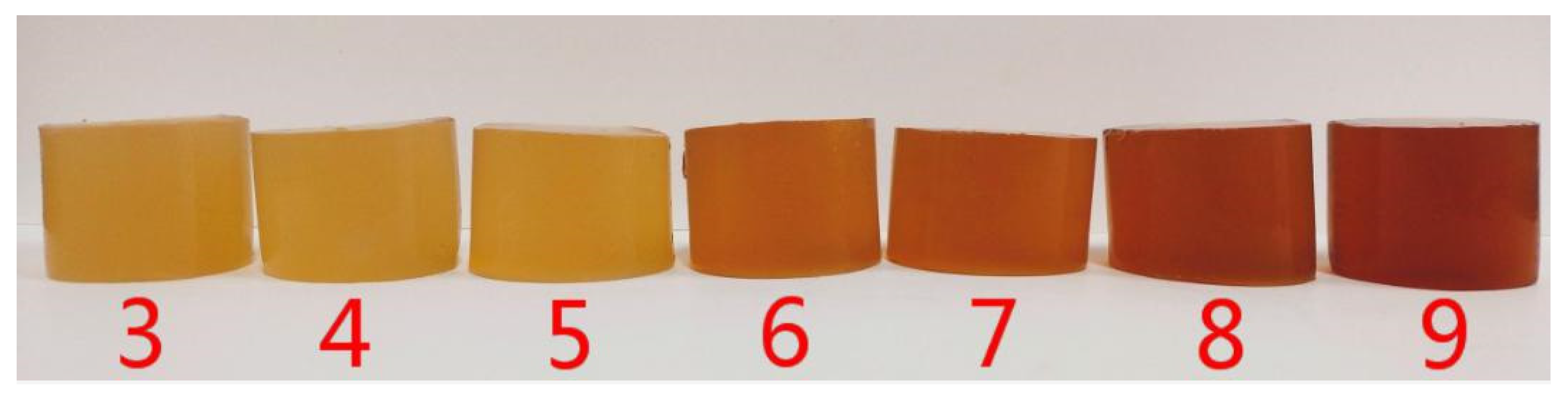
2.1. Surface Color Analysis
2.2. TPA Results
2.3. Rheological Analyses
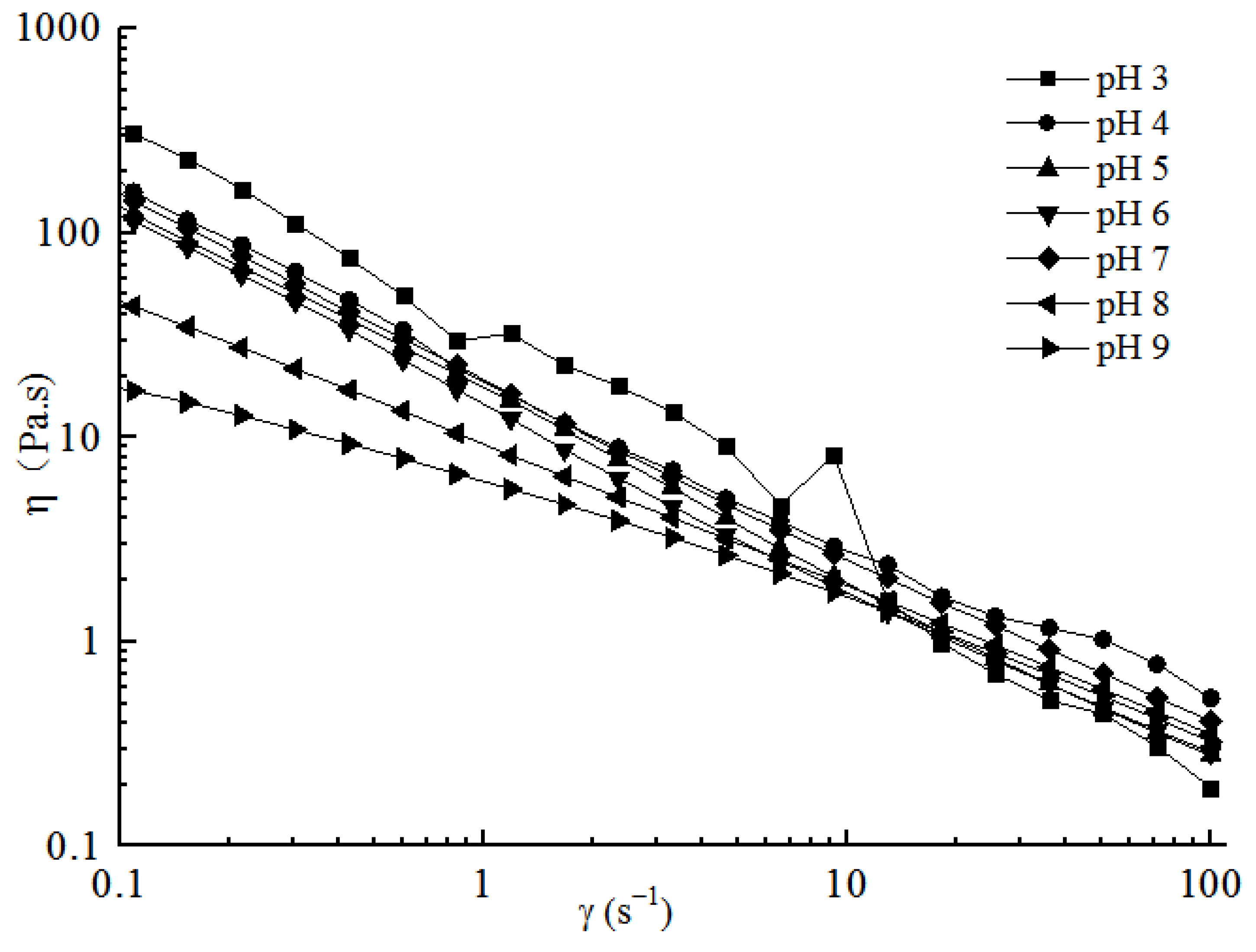
2.3.1. Steady Shear Analyses
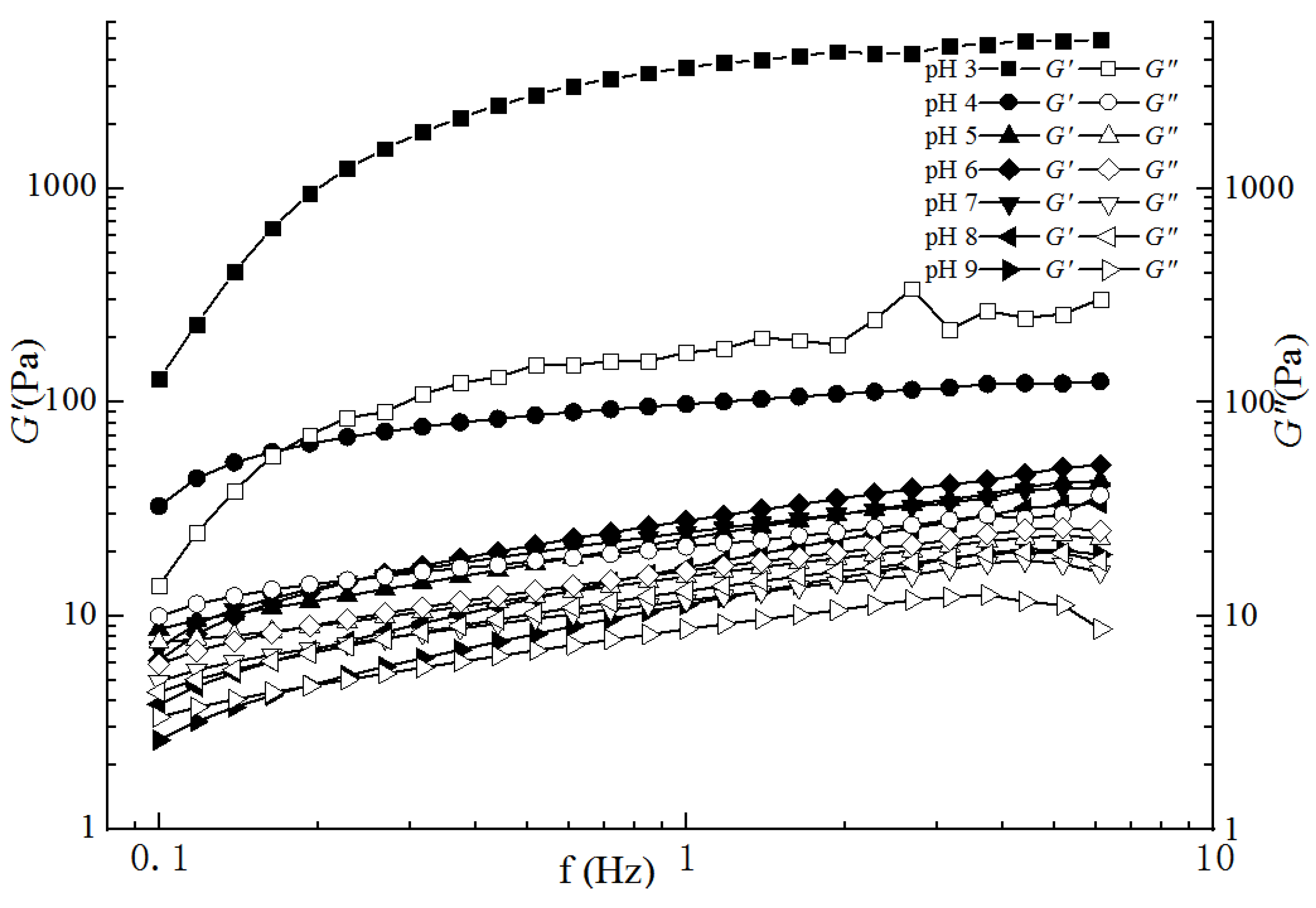
2.3.2. Dynamic Frequency Sweep Analyses
2.3.3. Dynamic Temperature Scanning
2.4. WHC
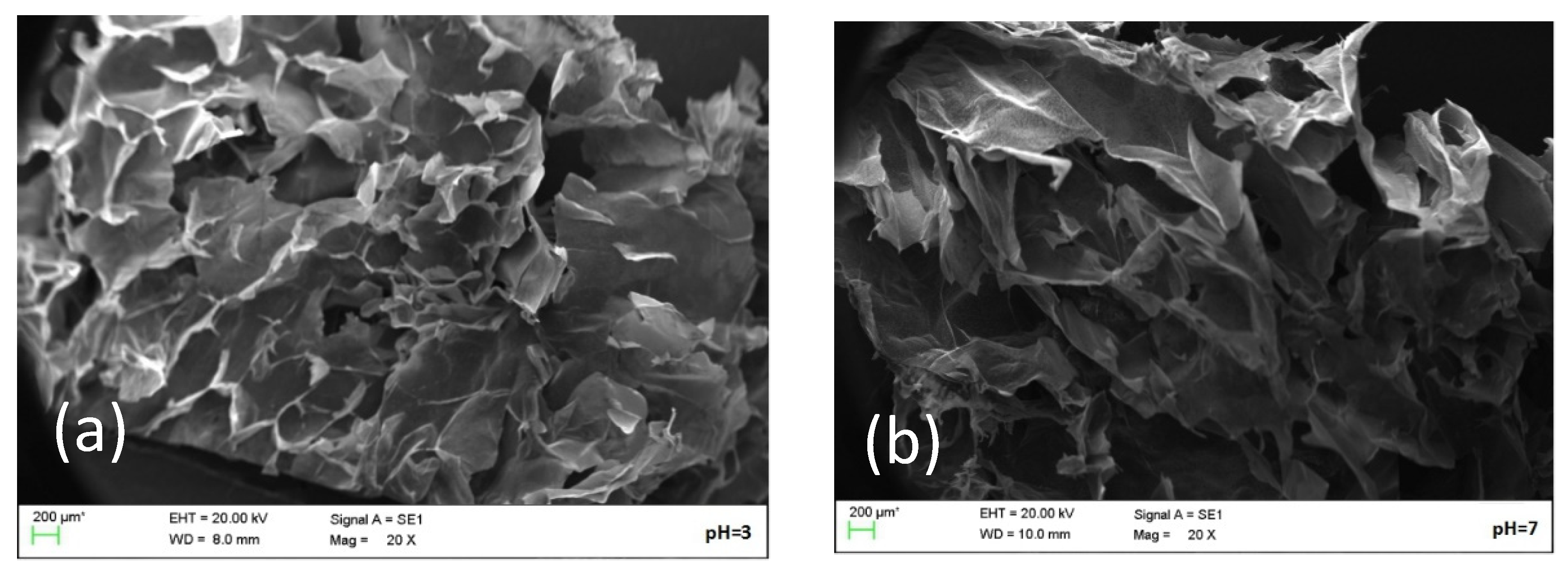
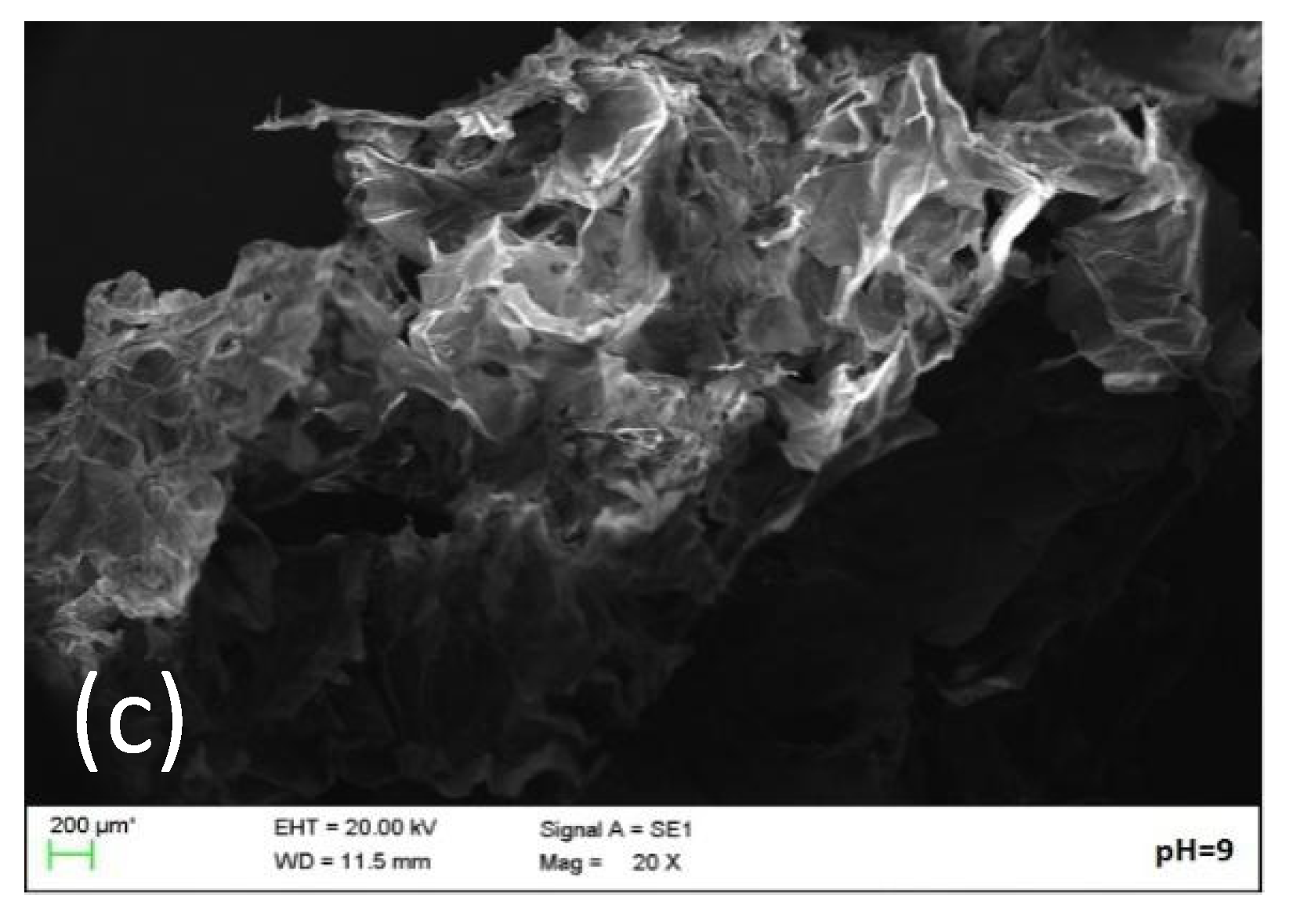
2.5. Microstructure Analysis
2.6. FTIR Spectra Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
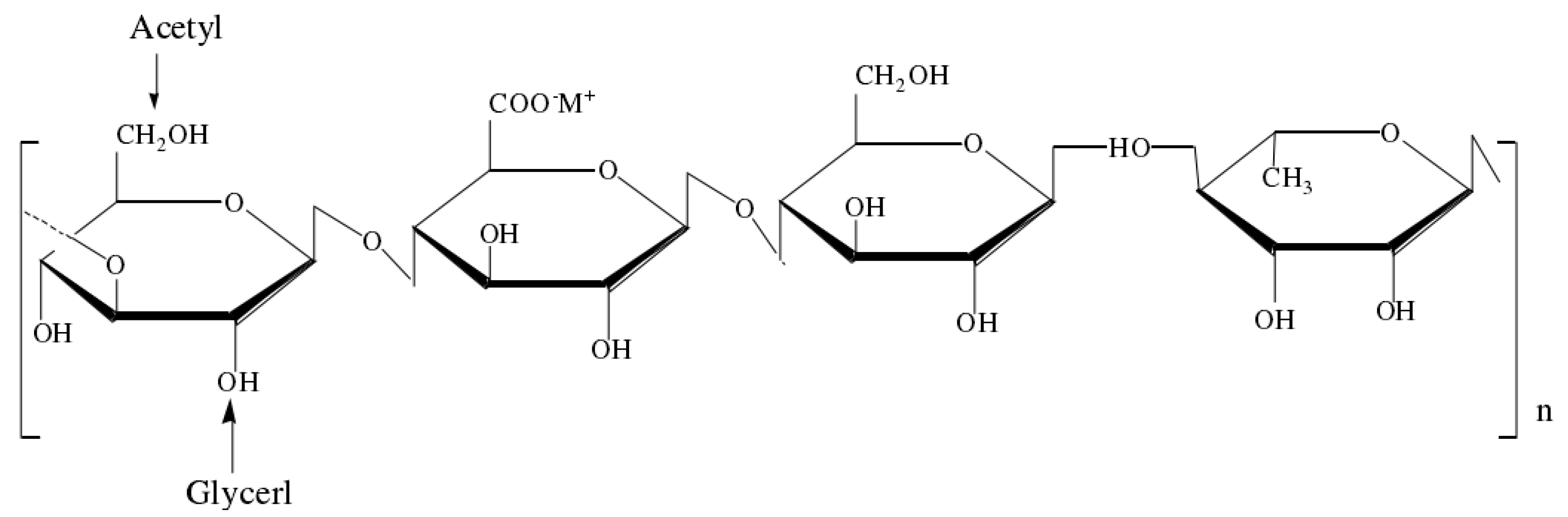
4.2. Preparation of TP-LGG Compound Gels
4.3. Color Measurements
4.4. Texture Profile Analysis (TPA) of TP-LGG Compound Gels
4.5. Rheological Measurements of TP-LGG Compound Gels
4.5.1. Steady Shear Analyses
4.5.2. Dynamic Frequency Sweep Rheological Measurements
4.5.3. Dynamic Temperature Sweep Rheological Measurements
4.6. Water Holding Capacity (WHC)
4.7. Scanning Electron Microscopy (SEM)
4.8. Fourier Transform Infrared Spectroscopy (FTIR)
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Liu, Y.; Zhang, X.; Wu, Z.; Weng, P. Tea polyphenols-loaded nanocarriers: Preparation technology and biological function. Biotechnol. Lett. 2022, 44, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-T.; Cui, W.-Q.; Pan, D.; Jiang, M.; Chang, B.; Sang, L.-X. Tea polyphenols and their chemopreventive and therapeutic effects on colorectal cancer. World J. Gastroenterol. 2020, 26, 562–597. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, N.T. Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sci. 2006, 78, 2073–2080. [Google Scholar] [CrossRef]
- Sun, B.; Wang, W.; He, Z.; Zhang, M.; Kong, F.; Sain, M.; Ni, Y. Improvement of Stability of Tea Polyphenols: A Review. Curr. Pharm. Des. 2018, 24, 3410–3423. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Zhang, A.; Tsang, D.; Huang, Y.; Chen, Z.-Y. Stability of Green Tea Catechins. J. Agric. Food Chem. 1997, 45, 4624–4628. [Google Scholar] [CrossRef]
- Mochizuki, M.; Yamazaki, S.-I.; Kano, K.; Ikeda, T. Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2002, 1569, 35–44. [Google Scholar] [CrossRef]
- Yi, J.J.; Zhao, Y.; BI, J.; Lu, J.; Zhou, M. A review of interactions between cell wall polysaccharides and polyphenols in fruits and vegetables. Food Sci. 2020, 41, 269–275. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Selvendran, R.R.; Morris, V.J. Structure of the acidic extracellular gelling polysaccharide produced by Pseudomonas elodea. Carbohydr. Res. 1983, 124, 123–133. [Google Scholar] [CrossRef]
- Gonçalves, V.M.; Reis, A.; Domingues, M.R.M.; Lopes-Da-Silva, J.A.; Fialho, A.M.; Moreira, L.M.; Sá-Correia, I.; Coimbra, M.A. Structural analysis of gellans produced by Sphingomonas elodea strains by electrospray tandem mass spectrometry. Carbohydr. Polym. 2008, 77, 10–19. [Google Scholar] [CrossRef]
- Li, A.; Gong, T.; Li, X.; Li, X.; Yang, X.; Guo, Y. Preparation of thermally stable emulsion gels based on Glucono-δ-lactone induced gelation of gellan gum. Int. J. Biol. Macromol. 2020, 156, 565–575. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Zala, B.S.; Khutliwala, T.A. An insight into the emerging exopolysaccharide gellan gum as a novel polymer. Carbohydr. Polym. 2013, 93, 670–678. [Google Scholar] [CrossRef]
- Abramovič, H.; Klofutar, C. Water adsorption isotherms of some gellan gum samples. J. Food Eng. 2006, 77, 514–520. [Google Scholar] [CrossRef]
- Chen, Q.; Li, R.; Zhou, T.; Cheng, H.; Han, X. Effect of preparation conditions on fluid gel formation of Gellan gum. Food Sci. 2020, 41, 23–29. [Google Scholar]
- Bradbeer, J.F.; Hancocks, R.; Spyropoulos, F.; Norton, I.T. Low acyl gellan gum fluid gel formation and their subsequent response with acid to impact on satiety. Food Hydrocoll. 2015, 43, 501–509. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, T.; Cheng, H.; Han, X. Gelation properties of low acyl and high acyl mixed acid gellan gels. Trans. Chin. Soc. Agric. Mach. 2020, 51, 360–365. [Google Scholar] [CrossRef]
- Zhu, G.L.; Guo, N.; Ma, M.; Ning, Y.; Ni, Z.H. Study on the properties of gelatin-tea polyphenol compound film. Food Ferment. Ind. 2019, 45, 72–77. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Ming, J.; Zhao, G. Optimization of Adsorption of Tea Polyphenols into Oat β-Glucan Using Response Surface Methodology. J. Agric. Food Chem. 2011, 59, 378–385. [Google Scholar] [CrossRef]
- Phan, A.S.T.; Netzel, G.; Wang, D.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding of dietary polyphenols to cellulose: Structural and nutritional aspects. Food Chem. 2015, 171, 388–396. [Google Scholar] [CrossRef]
- Lin, Z.; Fischer, J.; Wicker, L. Intermolecular binding of blueberry pectin-rich fractions and anthocyanin. Food Chem. 2016, 194, 986–993. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Liang, Y.; Jin, J.; Sun, Q.; Lu, J.; Du, Y.; Lin, C. Impact of heating on chemical compositions of green tea liquor. Food Chem. 2006, 103, 1263–1267. [Google Scholar] [CrossRef]
- Zeng, L.; Ma, M.; Li, C.; Luo, L. Stability of tea polyphenols solution with different pH at different temperatures. Int. J. Food Prop. 2016, 20, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Shpigelman, A.; Israeli, G.; Livney, Y.D. Thermally-induced protein–polyphenol co-assemblies: Beta lactoglobulin-based nanocomplexes as protective nanovehicles for EGCG. Food Hydrocoll. 2010, 24, 735–743. [Google Scholar] [CrossRef]
- Cassanelli, M.; Prosapio, V.; Norton, I.; Mills, T. Acidified/basified gellan gum gels: The role of the structure in drying/rehydration mechanisms. Food Hydrocoll. 2018, 82, 346–354. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Zhou, C.; Wang, C.; Zheng, Y.; Ye, K.; Li, C.; Zhou, G. Effects of gellan gum and inulin on mixed-gel properties and molecular structure of gelatin. Food Sci. Nutr. 2021, 9, 1336–1346. [Google Scholar] [CrossRef]
- Grisel, M.; Muller, G. Rheological Properties of the Schizophyllan−Borax System. Macromolecules 1998, 31, 4277–4281. [Google Scholar] [CrossRef]
- Picone, C.S.F.; Cunha, R.L. Influence of pH on formation and properties of gellan gels. Carbohydr. Polym. 2011, 84, 662–668. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Tan, L.; Ma, H.; Zhu, J.; Zhang, H. Acid gelation properties of low acyl gellan gum. Food Sci. 2017, 15, 51–57. [Google Scholar] [CrossRef]
- Hong, J.; Chen, R.; Zeng, X.-A.; Han, Z. Effect of pulsed electric fields assisted acetylation on morphological, structural and functional characteristics of potato starch. Food Chem. 2016, 192, 15–24. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Z. A pH-sensitive microemulsion-filled gellan gum hydrogel encapsulated apigenin: Characterization and in vitro release kinetics. Colloids Surf. B Biointerfaces 2019, 178, 245–252. [Google Scholar] [CrossRef]
- Shirsath, N.R.; Goswami, A.K. Vildagliptin-loaded gellan gum mucoadhesive beads for sustained drug delivery: Design, optimisation and evaluation. Mater. Technol. 2021, 36, 647–659. [Google Scholar] [CrossRef]
- Lee, H.; Rukmanikrishnan, B.; Lee, J. Rheological, morphological, mechanical, and water-barrier properties of agar/gellan gum/montmorillonite clay composite films. Int. J. Biol. Macromol. 2019, 141, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Sun, H.; Jiang, R.; Yu, Y. Physical and antibacterial properties of corn distarch phosphate/carboxymethyl cellulose composite films containing tea polyphenol. J. Food Process. Preserv. 2020, 44, 14401. [Google Scholar] [CrossRef]
- Mirani, A.; Goli, M. Production of the eggplant-fiber incorporated cupcake and evaluating its chemical, textural and colorimetric properties over a ten-day storage time. J. Food Process. Preserv. 2021, 45, e15311. [Google Scholar] [CrossRef]
- Young, P.; Mills, T.; Norton, I. Influence of pH on fluid gels produced from egg and whey protein isolate. Food Hydrocoll. 2020, 111, 106108. [Google Scholar] [CrossRef]
- Ambebila, E.N.; Santamaría, E.; Maestro, A.; Gutiérrez, J.M.; González, C. Gellan Hydrogels: Preparation, Rheological Characterization and Application in Encapsulation of Curcumin. Food Biophys. 2019, 14, 154–163. [Google Scholar] [CrossRef]
- Majumdar, R.K.; Saha, A.; Dhar, B.; Maurya, P.K.; Roy, D.; Shitole, S.; Balange, A.K. Effect of garlic extract on physical, oxidative and microbial changes during refrigerated storage of restructured product from Thai pangas (pangasianodon hypophthalmus) surimi. J. Food Sci. Technol. 2015, 52, 7994–8003. [Google Scholar] [CrossRef] [Green Version]
- Boni, F.I.; Prezotti, F.G.; Cury, B.S.F. Gellan gum microspheres crosslinked with trivalent ion: Effect of polymer and crosslinker concentrations on drug release and mucoadhesive properties. Drug Dev. Ind. Pharm. 2016, 42, 1283–1290. [Google Scholar] [CrossRef] [Green Version]




| pH | L* | a* | b* | ΔE |
|---|---|---|---|---|
| 3 | 12.28 ± 0.58 b | 2.36 ± 0.09 b | 10.55 ± 1.73 ab | 28.27 ± 0.08 b |
| 4 | 13.97 ± 0.90 ab | 2.35 ± 0.18 b | 11.89 ± 1.92 a | 28.56 ± 1.40 b |
| 5 | 13.95 ± 0.48 ab | 2.82 ± 0.84 ab | 11.32 ± 2.73 ab | 28.74 ± 0.26 b |
| 6 | 14.94 ± 0.58 a | 2.41 ± 0.49 b | 11.61 ± 1.77 a | 28.02 ± 1.41 b |
| 7 | 14.68 ± 0.94 a | 3.48 ± 0.21 a | 8.95 ± 0.32 ab | 28.59 ± 0.35 b |
| 8 | 13.20 ± 1.39 ab | 2.86 ± 0.29 ab | 7.33 ± 0.40 bc | 30.74 ± 0.87 a |
| 9 | 14.88 ± 0.88 a | 2.96 ± 0.06 ab | 5.33 ± 0.95 c | 31.01 ± 0.17 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Wang, X.; Guo, N.; Dai, H.; Wang, Y.; Sun, Y.; Zhu, G. Influence of Different pH Values on Gels Produced from Tea Polyphenols and Low Acyl Gellan Gum. Gels 2023, 9, 368. https://doi.org/10.3390/gels9050368
Zhang F, Wang X, Guo N, Dai H, Wang Y, Sun Y, Zhu G. Influence of Different pH Values on Gels Produced from Tea Polyphenols and Low Acyl Gellan Gum. Gels. 2023; 9(5):368. https://doi.org/10.3390/gels9050368
Chicago/Turabian StyleZhang, Fangyan, Xiangcun Wang, Na Guo, Huanhuan Dai, Yimei Wang, Yiwei Sun, and Guilan Zhu. 2023. "Influence of Different pH Values on Gels Produced from Tea Polyphenols and Low Acyl Gellan Gum" Gels 9, no. 5: 368. https://doi.org/10.3390/gels9050368
APA StyleZhang, F., Wang, X., Guo, N., Dai, H., Wang, Y., Sun, Y., & Zhu, G. (2023). Influence of Different pH Values on Gels Produced from Tea Polyphenols and Low Acyl Gellan Gum. Gels, 9(5), 368. https://doi.org/10.3390/gels9050368







