Effect of Chitosan as Active Bio-colloidal Constituent on the Diffusion of Dyes in Agarose Hydrogel
Abstract
:1. Introduction
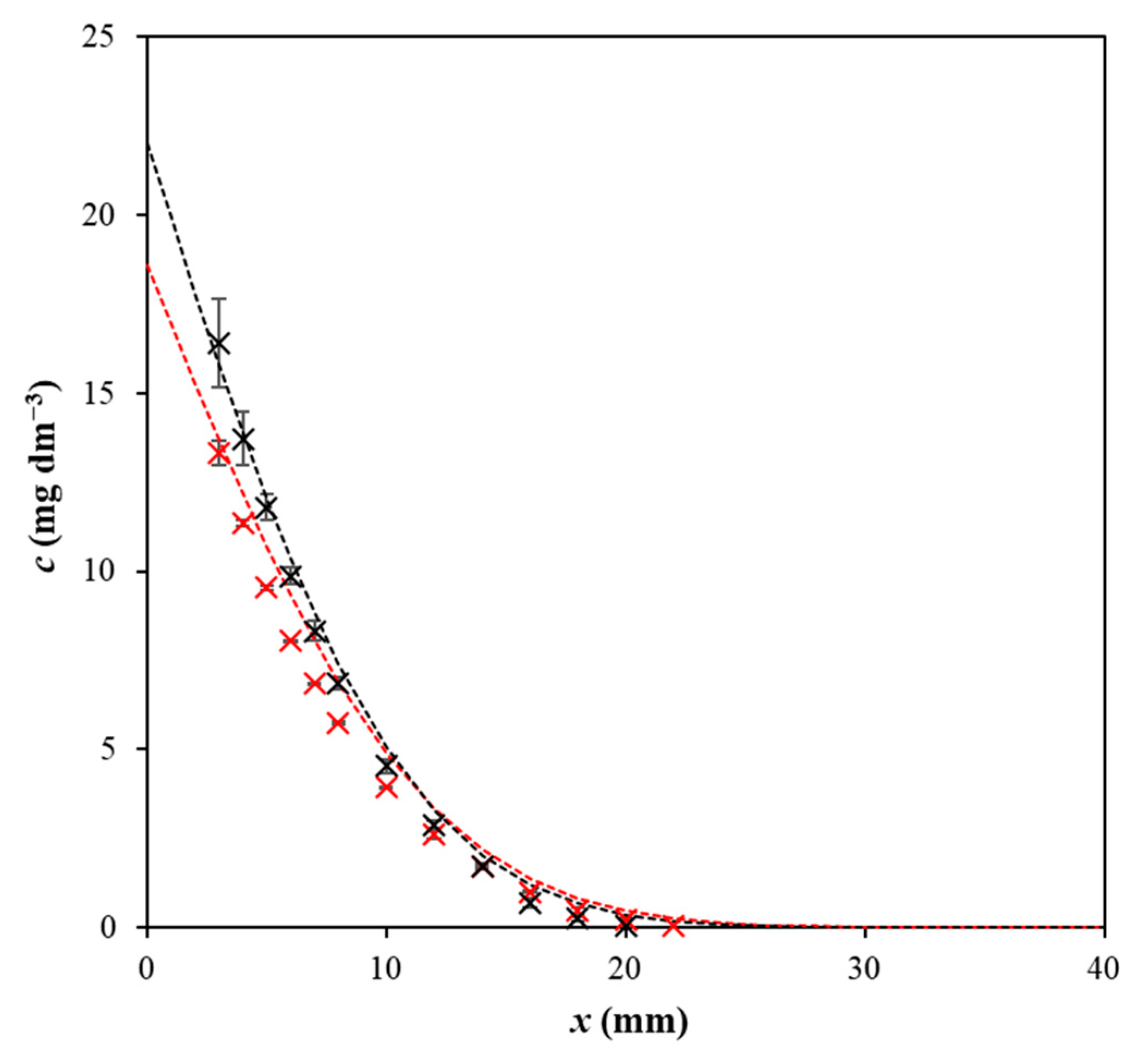
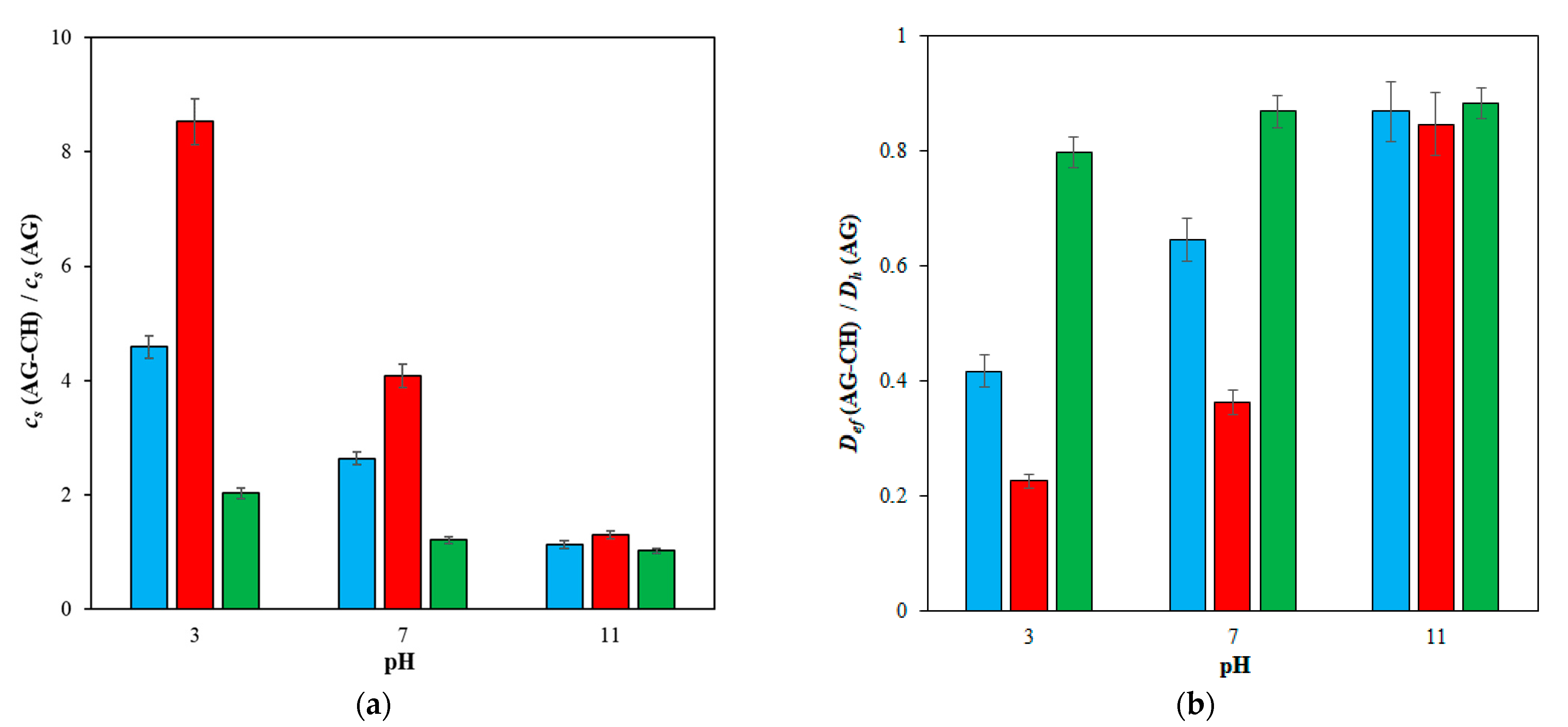
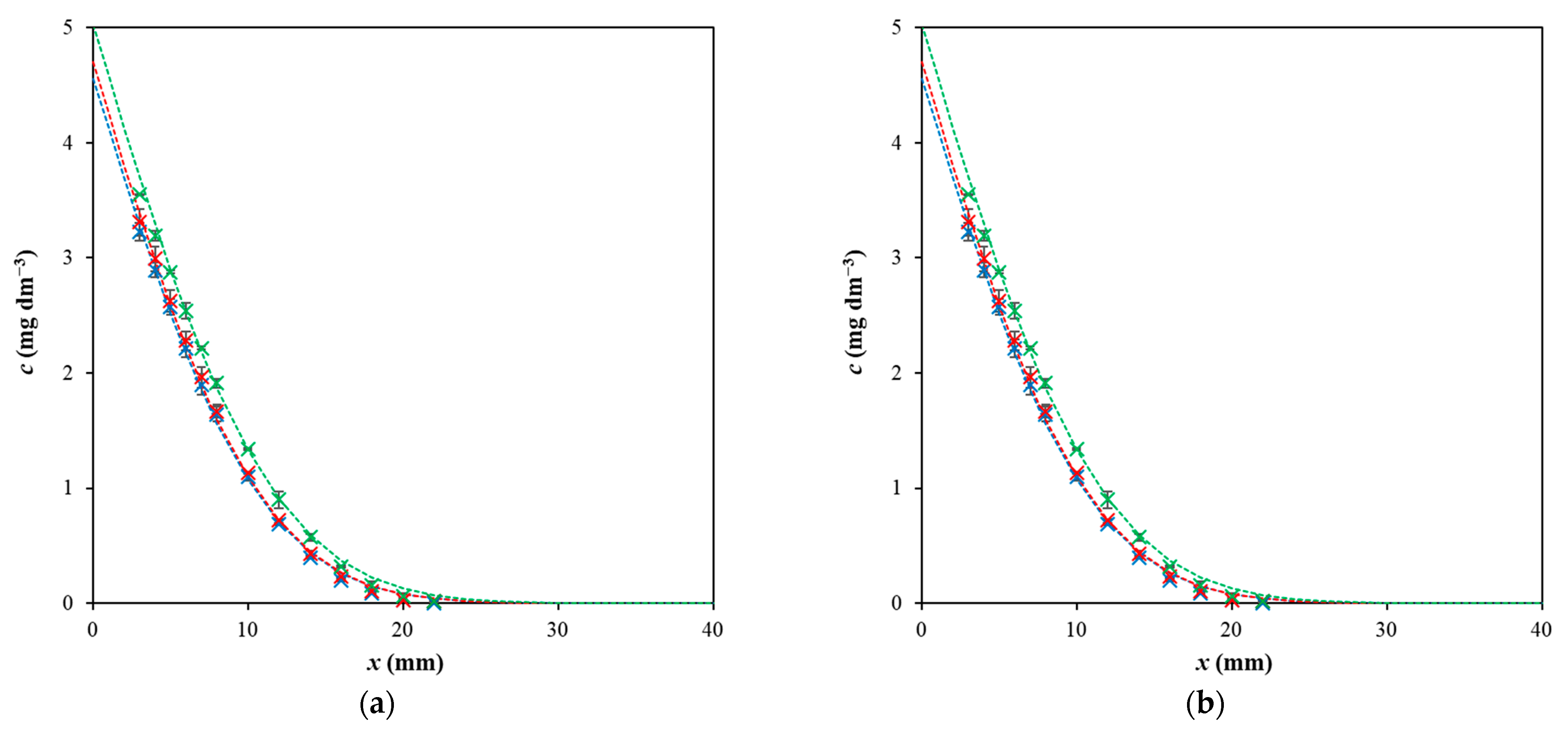
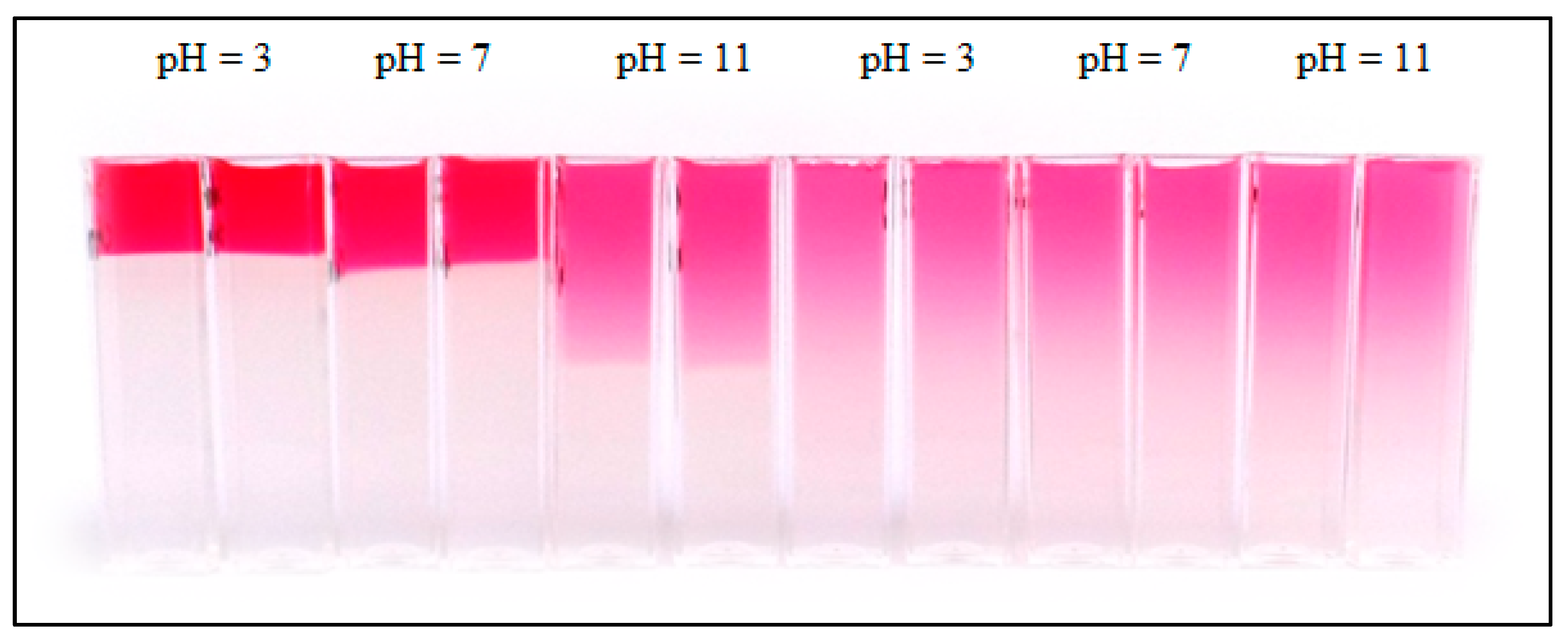
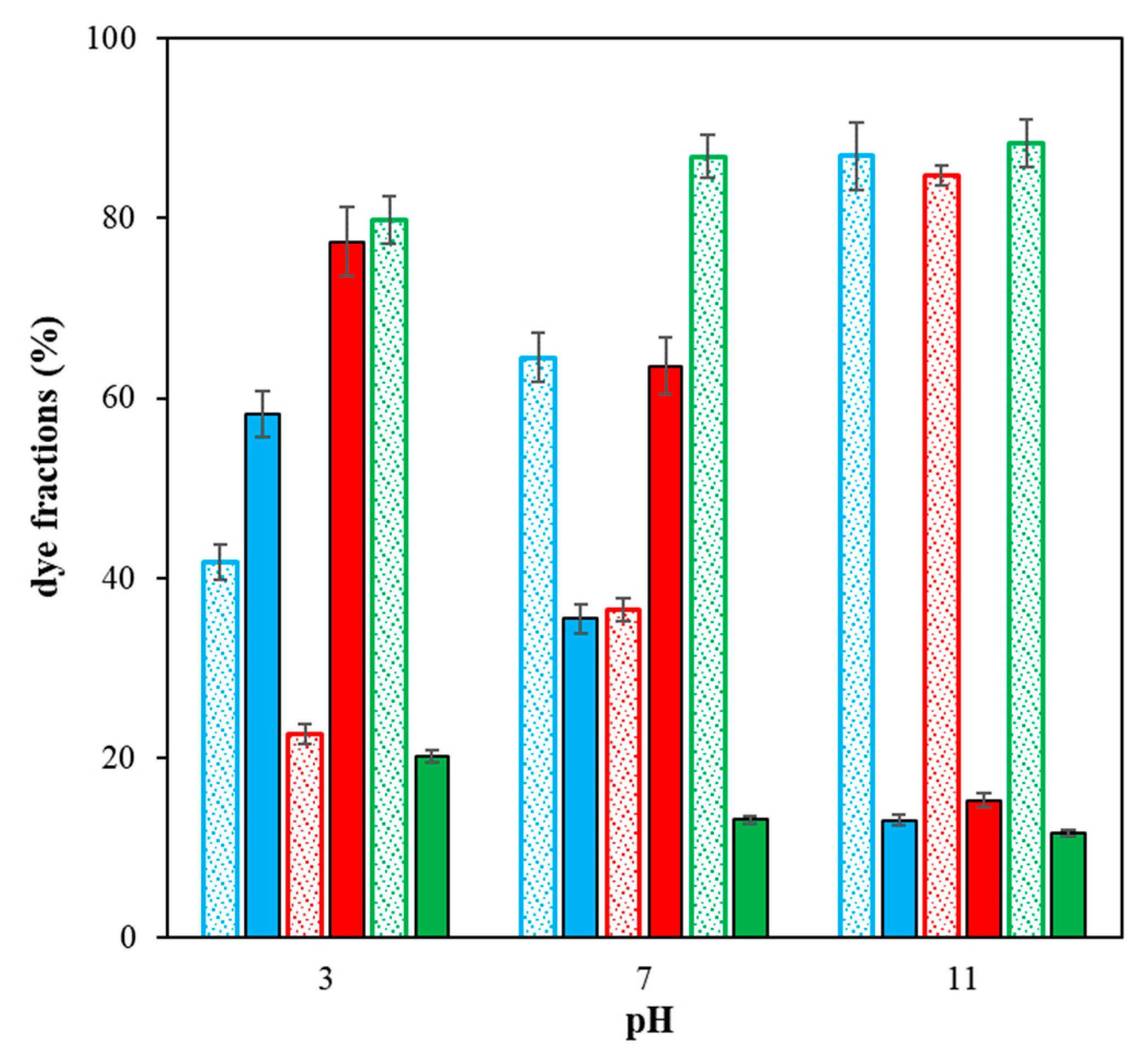
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Hydrogels
4.3. Diffusion Experiments
4.4. Sorption Experiments
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falk, B.; Garramone, S.; Shivkumar, S. Diffusion coefficient of paracetamol in a chitosan hydrogel. Mater. Lett. 2004, 58, 3261–3265. [Google Scholar] [CrossRef]
- Molinaro, G.; Leroux, J.; Damas, J.; Adam, A. Biocompatibility of thermosensitive chitosan-based hydrogels: An in vivo experimental approach to injectable biomaterials. Biomaterials 2002, 23, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Yu, H.; Zain-Ul-Abdin; Chen, Y.; Chen, Q.; Zhou, W.; Zhang, H.; Chen, X. Recent progress on synthesis, property and application of modified chitosan: An overview. Int. J. Biol. Macromol. 2016, 88, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Reis, R.L.; Mano, J.F. Graft copolymerized chitosan—Present status and applications. Carbohydr. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef]
- Ji, J.; Wang, L.; Yu, H.; Chen, Y.; Zhao, Y.; Zhang, H.; Amer, W.A.; Sun, Y.; Huang, L.; Saleem, M. Chemical modifications of chitosan and its applications. Polym.-Plast. Technol. Eng. 2014, 53, 1494–1505. [Google Scholar] [CrossRef]
- An-Chong, C.; Shin-Shing, S.; Yu-Chuang, L.; Fwu-Long., M. Enzymatic grafting of carboxyl groups on to chitosan—To confer on chitosan the property of a cationic dye adsorbent. Bioresour. Technol. 2004, 91, 157–162. [Google Scholar]
- Kyzas, G.Z.; Bikiaris, D.N. Recent modifications of chitosan for adsorption applications: A critical and systematic review. Mar. Drugs 2015, 13, 312–337. [Google Scholar] [CrossRef]
- Kausar, A. Scientific potential of chitosan blending with different polymeric materials: A review. J. Plast. Film Sheeting 2017, 33, 384–412. [Google Scholar] [CrossRef]
- Klučáková, M. How the addition of chitosan affects the transport and rheological properties of agarose hydrogels. Gels 2023, 9, 99. [Google Scholar] [CrossRef]
- Sedláček, P.; Smilek, J.; Klučáková, M. How interactions with polyelectrolytes affect mobility of low molecular ions—Results from diffusion cells. React. Funct. Polym. 2013, 73, 1500–1509. [Google Scholar] [CrossRef]
- Sedláček, P.; Smilek, J.; Klučáková, M. How the interactions with humic acids affect the mobility of ionic dyes in hydrogels—2. Non-stationary diffusion experiments. React. Funct. Polym. 2014, 75, 41–50. [Google Scholar] [CrossRef]
- Xiong, J.Y.; Narayanan, J.; Liu, X.Z.; Chong, T.K.; Chen, S.B.; Chung, T.S. Topology evolution and gelation mechanism of agarose gel. J. Phys. Chem B 2005, 109, 5638–5643. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, L.M.; Daubert, C.R.; Foegeding, E.A. Textural properties of agarose gels. I. Rheological and fracture properties. Food Hydrocoll. 2006, 20, 184–195. [Google Scholar] [CrossRef]
- Barrangou, L.M.; Drake, N.A.; Daubert, C.R.; Foegeding, E.A. Textural properties of agarose gels. II. Relationships between rheological properties and sensory texture. Food Hydrocoll. 2006, 20, 196–203. [Google Scholar] [CrossRef]
- Golmohamadi, M.; Davis, T.A.; Wilkinson, K.J. Diffusion and partitioning of cations in an agarose hydrogel. J. Phys. Chem. A 2012, 116, 6505–6510. [Google Scholar] [CrossRef] [PubMed]
- Lead, J.R.; Starchev, K.; Wilkinson, K.J. Diffusion coefficients of humic substances in agarose gel and in water. Environ. Sci. Technol. 2003, 37, 482–487. [Google Scholar] [CrossRef]
- Gutenwik, J.; Nilson, B.; Axxelson, A. Determination of protein diffusion coefficients in agarose gel with a diffusion cell. Biochem. Eng. J. 2004, 19, 1–7. [Google Scholar] [CrossRef]
- Liang, S.M.; Xu, J.; Weng, L.; Dai, H.; Zhang, X.; Zhang, L. Protein diffusion in agarose hydrogel in situ measured by improved refractive index method. J. Control. Release 2006, 115, 189–196. [Google Scholar] [CrossRef]
- Tan, S.X.; Dai, H.J.; Wu, J.; Zhao, N.; Zhang, X.; Xu, J. Optical investigation of diffusion of levofloxacin mesylate in agarose hydrogel. J. Biomed. Opt. 2009, 14, 050503. [Google Scholar] [CrossRef] [PubMed]
- Labille, J.; Fatin-Rouge, N.; Buffle, J. Local and average diffusion of nanosolutes in agarose gel: The effect of the gel/solution interface structure. Langmuir 2007, 23, 2083–2090. [Google Scholar] [CrossRef]
- Karmaker, S.; Nag, A.J.; Saha, T.K. Adsorption of reactive blue 4 dye onto Chitosan 10B in aqueous solution: Kinetic modeling and isotherm analysis. Russ. J. Phys. Chem. 2020, 94, 2349–2359. [Google Scholar] [CrossRef]
- Liu, D.; Cheng, W.; Yu, J.; Ding, Y. Polyamine chitosan adsorbent for the enhanced adsorption of anionic dyes from water. J. Dispers. Sci. Technol. 2017, 38, 1832–1841. [Google Scholar]
- Qin, Y.; Cai, L.; Feng, D.; Shi.; Liu, J.; Zhang, W.; Shen, Y. Combined use of chitosan and alginate in the treatment of wastewater. J. Appl. Polym. Sci. 2007, 104, 3581–3587. [Google Scholar] [CrossRef]
- Pietrelli, L.; Francolini, I.; Piozzi, A. Dyes adsorption from aqueous solutions by chitosan. Sep. Sci. Technol. 2015, 50, 1101–1107. [Google Scholar] [CrossRef]
- Bekci, Z.; Ozveri, C.; Seki, Y.; Yurdakoc, K. Sorption of malachite green on chitosan bead. J. Hazard. Mater. 2008, 154, 254–261. [Google Scholar] [CrossRef]
- Kekes, T.; Tzia, C. Adsorption of indigo carmine on functional chitosan and β-cyclodextrin/chitosan beads: Equilibrium, kinetics and mechanism studies. J. Environ. Manag. 2020, 262, 110372. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Karim, K.J.A.; Naim, A.A.; Ibrahim, W.A.W. Enhanced removal of Orange G from aqueous solutions by modified chitosan beads: Performance and mechanism. Int. J. Biol. Macromol. 2019, 133, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, X.; Zhao, L.; Li, M.; Yang, W. Double network gelatin/chitosan hydrogel effective removal of dyes from aqueous solutions. J. Polym. Environ. 2022, 30, 2007–2021. [Google Scholar] [CrossRef]
- Cesco, C.T.; Valente, A.J.M.; Paulino, A.T. Methylene blue release from chitosan/pectin and chitosan/DNA blend hydrogels. Pharmaceutics 2021, 13, 842. [Google Scholar] [CrossRef]
- Gonçalves, J.O.; da Silva, K.A.; Rios, E.C.; Crispim, M.M.; Dotto, G.L.; de Almeida Pinto, L.A. Single and binary adsorption of food dyes on chitosan/activated carbon hydrogels. Chem. Eng. Technol. 2019, 42, 454–464. [Google Scholar] [CrossRef]
- Shen, C.; Shen, Y.; Wen, Y.; Wang, H.; Liu, W. Fast and highly efficient removal of dyes under alkaline conditions using magnetic chitosan-Fe(III) hydrogel. Water Res. 2011, 45, 5200–5210. [Google Scholar] [CrossRef]
- Kim, U.J.; Kimura, S.; Wada, M. Characterization of cellulose–chitosan gels prepared using a LiOH/urea aqueous solution. Cellulose 2019, 26, 6189–6199. [Google Scholar] [CrossRef]
- Le, H.Q.; Sekiguchi, Y.; Ardiyanta, D.; Shimoyama, Y. CO2-activated adsorption: A new approach to dye removal by chitosan hydrogel. ACS Omega 2018, 3, 14103–14110. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for developing physical gels of chitosan and of chitosan derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef]
- Barron-Zambrano, J.; Szygula, A.; Ruiz, M.; Sastre, A.M.; Guibal, E. Biosorption of Reactive Black 5 from aqueous solutions by chitosan: Column studies. J. Environ. Manag. 2010, 91, 2669–2675. [Google Scholar] [CrossRef]
- Lazaridis, N.K.; Keenan, H. Chitosan beads as barriers to the transport of azo dye in soil column. J. Hazard. Mater. 2010, 173, 144–150. [Google Scholar] [CrossRef] [PubMed]
- García-Aparicio, C.; Quijada-Garrido, I.; Garrido, L. Diffusion of small molecules in a chitosan/water gel determined by proton localized NMR spectroscopy. J. Colloid Interface Sci. 2012, 268, 14–20. [Google Scholar] [CrossRef]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour. Technol. 2007, 98, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Coura, J.C.; Profeti, D.; Profeti, L.P.R. Eco-friendly chitosan/quartzite composite as adsorbent for dye removal. Mater. Chem. Phys. 2020, 256, 123711. [Google Scholar] [CrossRef]
- Vanamudan, A.; Pamidimukkala, P. Chitosan, nanoclay and chitosan–nanoclay composite as adsorbents for Rhodamine-6G and the resulting optical properties. Int. J. Biol. Macromol. 2015, 74, 127–135. [Google Scholar] [CrossRef]
- Sadiq, A.C.; Rahim, N.Y.; Suah, F.B.M. Adsorption and desorption of malachite green by using chitosan-deep eutectic solvents beads. Int. J. Biol. Macromol. 2020, 164, 3965–3973. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Agarose-chitosan hydrogel-immobilized horseradish peroxidase with sustainable bio-catalytic and dye degradation properties. Int. J. Biol. Macromol. 2019, 124, 742–749. [Google Scholar] [CrossRef]
- Hartig, D.; Hacke, S.; Scholl, S. Concentration-dependent diffusion coefficients for fructose in highly permeable chitosan polymers. Chem. Eng. Technol. 2018, 41, 454–460. [Google Scholar] [CrossRef]
- Yang, J.M.; Su, W.Y.; Leu, T.L.; Yang, M.C. Evaluation of chitosan/PVA blended hydrogel membranes. J. Membr. Sci. 2004, 236, 39–51. [Google Scholar] [CrossRef]
- Waluga, T.; Scholl, S. Diffusion of saccharides and the sugar alcohol sorbitol in chitosan membranes and beads. Chem. Eng. Technol. 2013, 36, 681–686. [Google Scholar] [CrossRef]
- Xu, D.; Loo, L.S.; Wang, K. Characterization and diffusion behavior of chitosan–POSS composite membranes. J. Appl. Polym. Sci. 2011, 122, 427–435. [Google Scholar] [CrossRef]
- Carlough, M.; Hudson, S.; Smith, B.; Spadgenske, D. Diffusion coefficients of direct dyes in chitosan. J. Appl. Polym. Sci. 1991, 42, 3035–3038. [Google Scholar] [CrossRef]
- Klučáková, M.; Smilek, J.; Sedláček, P. How humic acids affect the rheological and transport properties of hydrogels. Molecules 2019, 24, 1545. [Google Scholar] [CrossRef]
- Klučáková, M. Agarose hydrogels enriched by humic acids as complexation agent. Polymers 2020, 12, 687. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 1st ed.; Clarendon Press: Oxford, UK, 1956; pp. 26–41. [Google Scholar]
- Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems, 2nd ed.; Cambridge University Press: Cambridge, MA, USA, 1984; pp. 13–49. [Google Scholar]
- Klučáková, M.; Pekař, M. Study of structure and properties of humic and fulvic acids. IV. Study of interactions of Cu2+ ions with humic gels and final comparison. J. Polym. Mater. 2003, 20, 155–162. [Google Scholar]
- Klučáková, M.; Pekař, M. Study of diffusion of metal cations in humic gels. In Humic Substances: Nature’s Most Versatile Materials, 1st ed.; Ghabbour, E.A., Davies, G., Eds.; Taylor & Francis: New York, NY, USA, 2004; pp. 263–273. [Google Scholar]
- Maekawa, M.; Kamada, C. Mixture diffusion of sulfonated dyes into cellulose membrane: IV. Effects of complex formation between a couple of dyes. Colloids Surf. A Physicochem. Eng. Asp. 2003, 216, 83–90. [Google Scholar] [CrossRef]
- Mansurov, R.R.; Zverev, V.S.; Safronov, A.P. Dynamics of diffusion-limited photocatalytic degradation of dye by polymeric hydrogel with embedded TiO2 nanoparticles. J. Catal. 2022, 406, 9–18. [Google Scholar] [CrossRef]
- Şolpan, D.; Duran, S.; Torun, M. Removal of cationic dyes by poly(acrylamide-co-acrylic acid) hydrogels in aqueous solutions. Radiat. Phys. Chem. 2008, 77, 447–452. [Google Scholar] [CrossRef]
- Abdel-Aal, S.E. Synthesis of copolymeric hydrogels using gamma radiation and their utilization in the removal of some dyes in wastewater. J. Appl. Polym. Sci. 2006, 102, 3720–3731. [Google Scholar] [CrossRef]
- Al-Mubaddel, F.S.; Haider, S.; Aijaz, M.O.; Haider, A.; Kamal, T.; Almasry, W.; Javid, M.; Khan, S.U.-D. Preparation of the chitosan/polyacrylonitrile semi-IPN hydrogel via glutaraldehyde vapors for the removal of Rhodamine B dye. Polym. Bull. 2017, 74, 1535–1551. [Google Scholar] [CrossRef]
- Sandrin, D.; Wagner, D.; Sitta, C.E.; Thoma, R.; Felekyan, S.; Hermes, H.E.; Janiak, C.; de Sousa Amadeu, N.; Kühnemuth, R.; Löwen, H.; et al. Diffusion of macromolecules in a polymer hydrogel: From microscopic to macroscopic scales. Phys. Chem. Chem. Phys. 2016, 18, 12860–12876. [Google Scholar] [CrossRef] [PubMed]
- Roussy, J.; Van Vooren, M.; Dempsey, B.A.; Guibal, E. Influence of chitosan characteristics on the coagulation and the flocculation of bentonite suspensions. Water Res. 2005, 39, 3247–3258. [Google Scholar] [CrossRef]
- Chen, K.; Muthukamar, M. Entropic barrier of topologically immobilized DNA in hydrogels. Proc. Natl. Acad. Sci. USA 2021, 118, e210638011. [Google Scholar] [CrossRef]
- Garcia, L.G.S.; de Melo Guedes, G.M.; da Silva, M.L.Q.; Castelo-Branco, D.S.C.M.; Sidrim, J.J.C.; de Aguiar Cordeiro, R.; Rocha, M.F.G.; Vieira, R.S.; Brilhante, R.S.N. Effect of the molecular weight of chitosan on its antifungal activity against Candida spp. in planktonic cells and biofilm. Carbohydr. Polym. 2018, 195, 662–669. [Google Scholar] [CrossRef]


| Time t | Distance x | Concentration c |
|---|---|---|
| t = 0 | x > 0 | c = 0 |
| t > 0 | x = 0 | c = cs |
| t > 0 | x → ∞ | c = 0 |
| Dye | Dh (pH 3) (m2 s−1) | Dh (pH 7) (m2 s−1) | Dh (pH 11) (m2 s−1) |
|---|---|---|---|
| Direct blue 1 | (1.51 ± 0.05) × 10−10 | (1.55 ± 0.07) × 10−10 | (1.54 ± 0.10) × 10−10 |
| Sirius red F3B | (2.05 ± 0.12) × 10−10 | (2.04 ± 0.10) × 10−10 | (2.03 ± 0.12) × 10−10 |
| Reactive blue 49 | (2.98 ± 0.09) × 10−10 | (2.90 ± 0.13) × 10−10 | (3.02 ± 0.07) × 10−10 |
| Dye | Def (pH 3) (m2 s−1) | Def (pH 7) (m2 s−1) | Def (pH 11) (m2 s−1) |
|---|---|---|---|
| Direct blue 1 | (6.32 ± 0.37) × 10−11 | (1.00 ± 0.05) × 10−10 | (1.34 ± 0.11) × 10−10 |
| Sirius red F3B | (4.64 ± 0.22) × 10−11 | (7.42 ± 0.37) × 10−11 | (1.72 ± 0.13) × 10−10 |
| Reactive blue 49 | (2.36 ± 0.13) × 10−10 | (2.52 ± 0.05) × 10−10 | (2.67 ± 0.08) × 10−10 |
| Dh (m2 s−1) | Def (m2 s−1) | ||
|---|---|---|---|
| Without Chitosan | 0.2 mg g−1 | 0.5 mg g−1 | 1 mg g−1 |
| (1.51 ± 0.05) × 10−10 | (1.32 ± 0.03) × 10−10 | (9.83 ± 0.16) × 10−11 | (6.32 ± 0.37) × 10−11 |
| Dye | K (pH 3) | K (pH 7) | K (pH 11) |
|---|---|---|---|
| Direct blue 1 | 1.39 ± 0.08 | 0.55 ± 0.03 | 0.15 ± 0.01 |
| Sirius red F3B | 3.42 ± 0.23 | 1.75 ± 0.12 | 0.18 ± 0.01 |
| Reactive blue 49 | 0.25 ± 0.01 | 0.15 ± 0.01 | 0.13 ± 0.01 |
| Dye | Pure Agarose Hydrogels | Enriched Hydrogels |
|---|---|---|
| Direct blue 1 | (0.84 ± 0.02)% | (21.26 ± 0.61)% |
| Sirius red F3B | (1.14 ± 0.03)% | (24.48 ± 0.44)% |
| Reactive blue 49 | (3.37 ± 0.06)% | (12.70 ± 0.17)% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klučáková, M. Effect of Chitosan as Active Bio-colloidal Constituent on the Diffusion of Dyes in Agarose Hydrogel. Gels 2023, 9, 395. https://doi.org/10.3390/gels9050395
Klučáková M. Effect of Chitosan as Active Bio-colloidal Constituent on the Diffusion of Dyes in Agarose Hydrogel. Gels. 2023; 9(5):395. https://doi.org/10.3390/gels9050395
Chicago/Turabian StyleKlučáková, Martina. 2023. "Effect of Chitosan as Active Bio-colloidal Constituent on the Diffusion of Dyes in Agarose Hydrogel" Gels 9, no. 5: 395. https://doi.org/10.3390/gels9050395
APA StyleKlučáková, M. (2023). Effect of Chitosan as Active Bio-colloidal Constituent on the Diffusion of Dyes in Agarose Hydrogel. Gels, 9(5), 395. https://doi.org/10.3390/gels9050395








