Synthesis and Characterisation of Hydrogels Based on Poly (N-Vinylcaprolactam) with Diethylene Glycol Diacrylate
Abstract
:1. Introduction
2. Results and Discussion
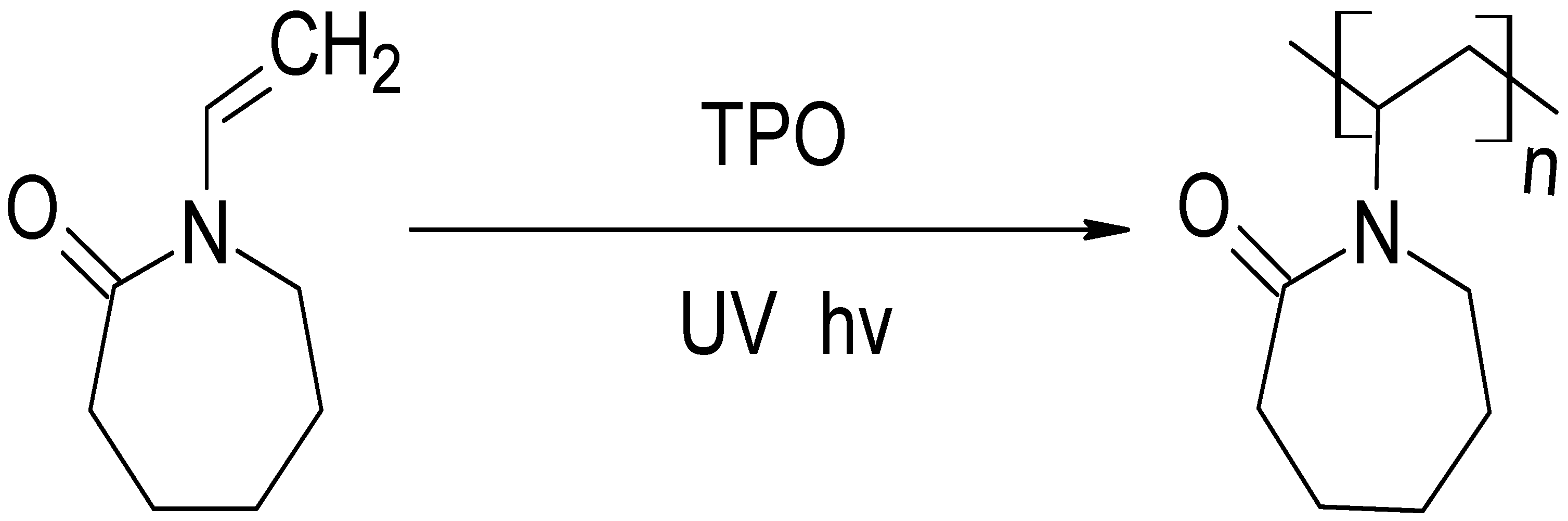
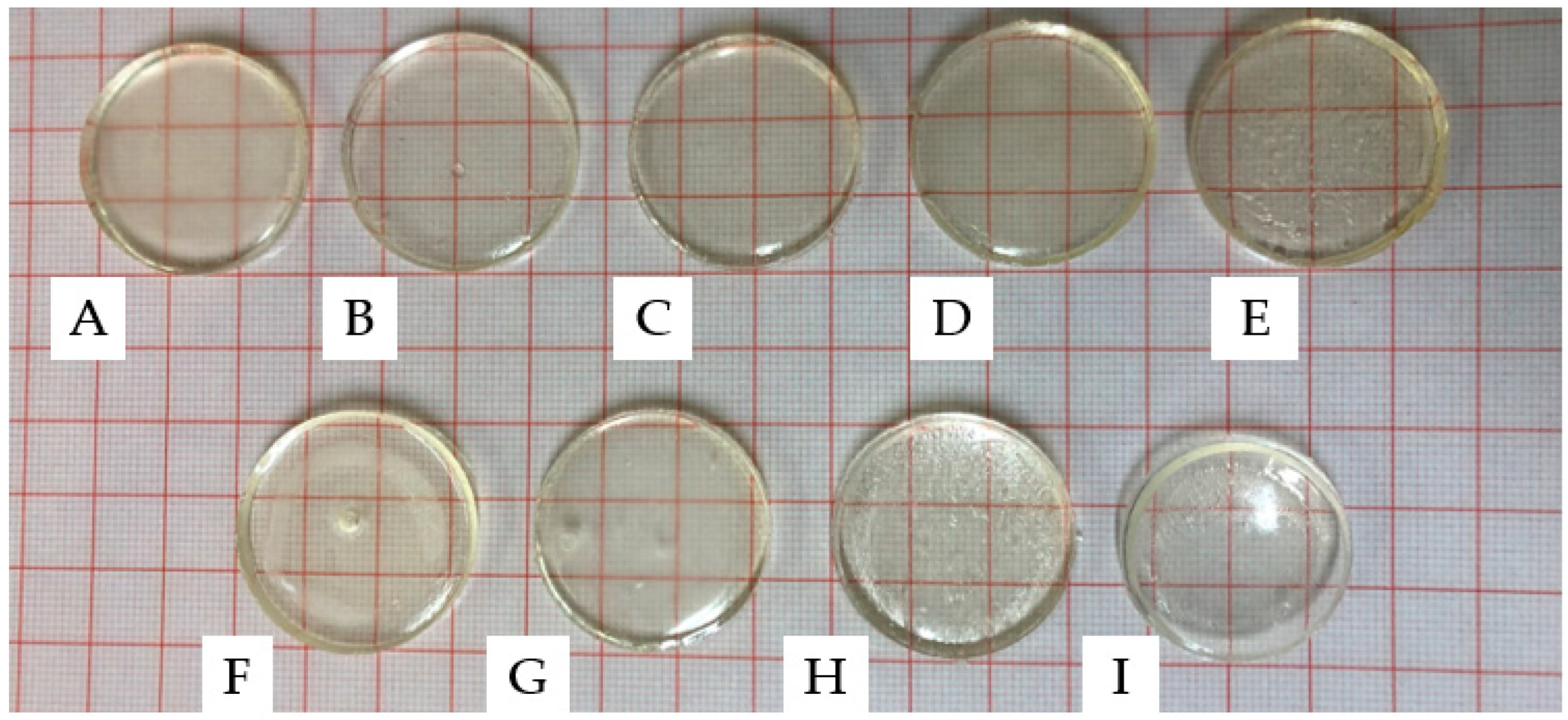
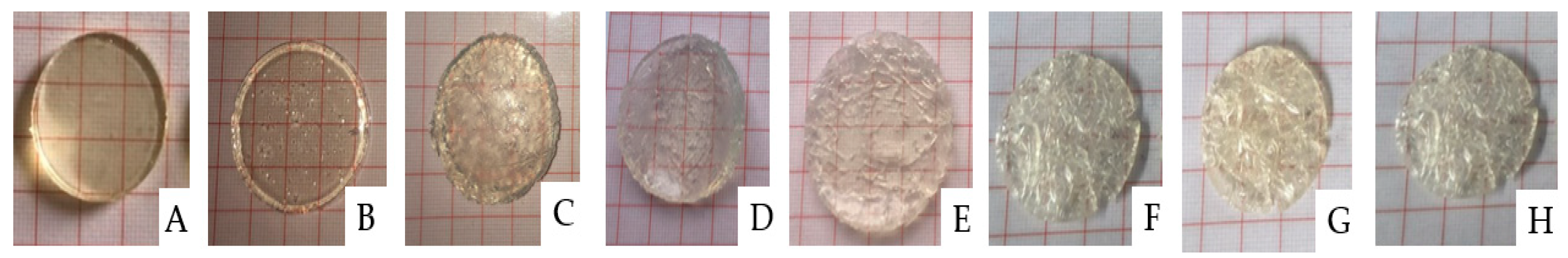
2.1. Photopolymerisation of PNVCL Hydrogels
2.2. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy
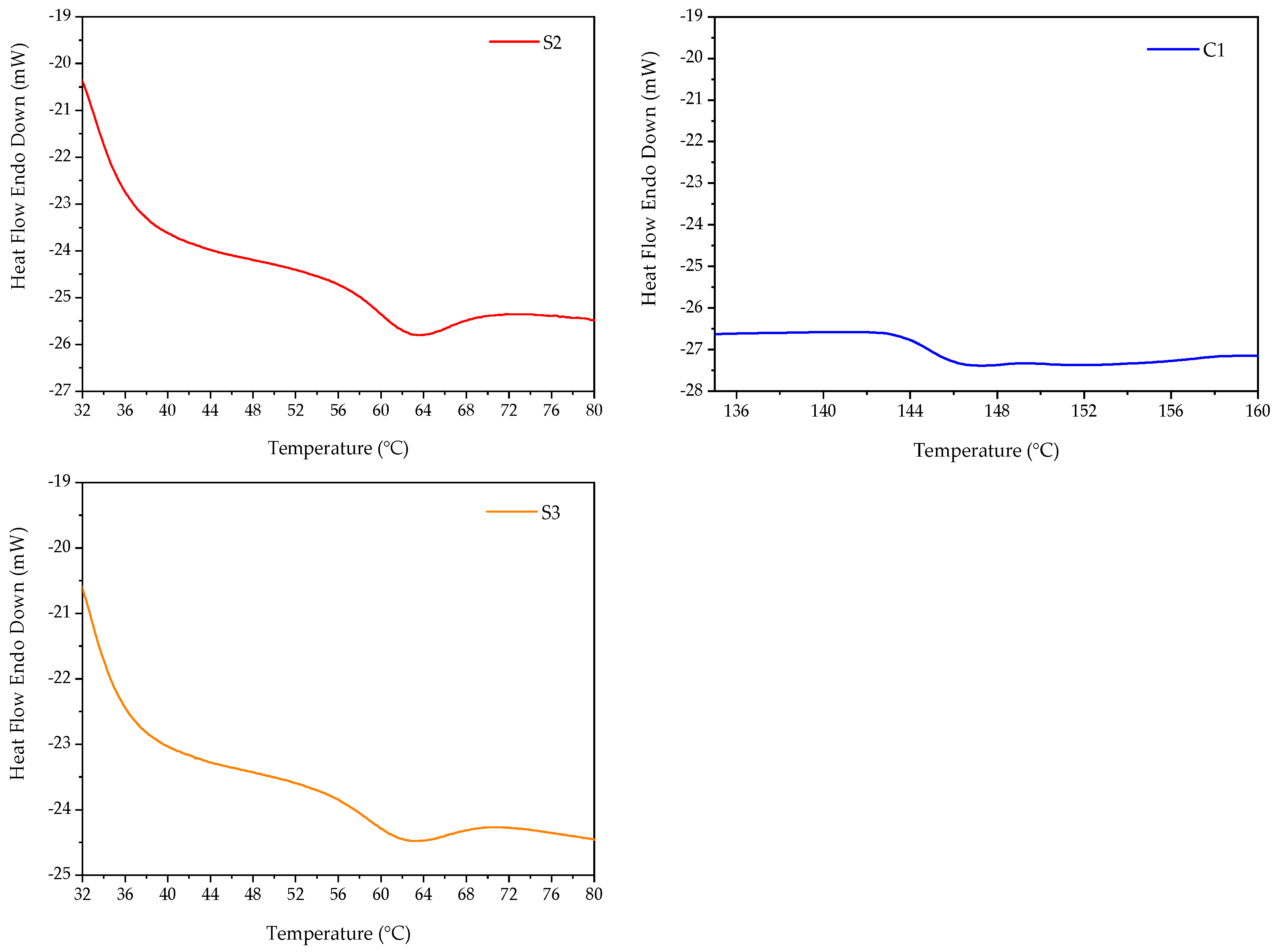
2.3. Differential Scanning Calorimetry
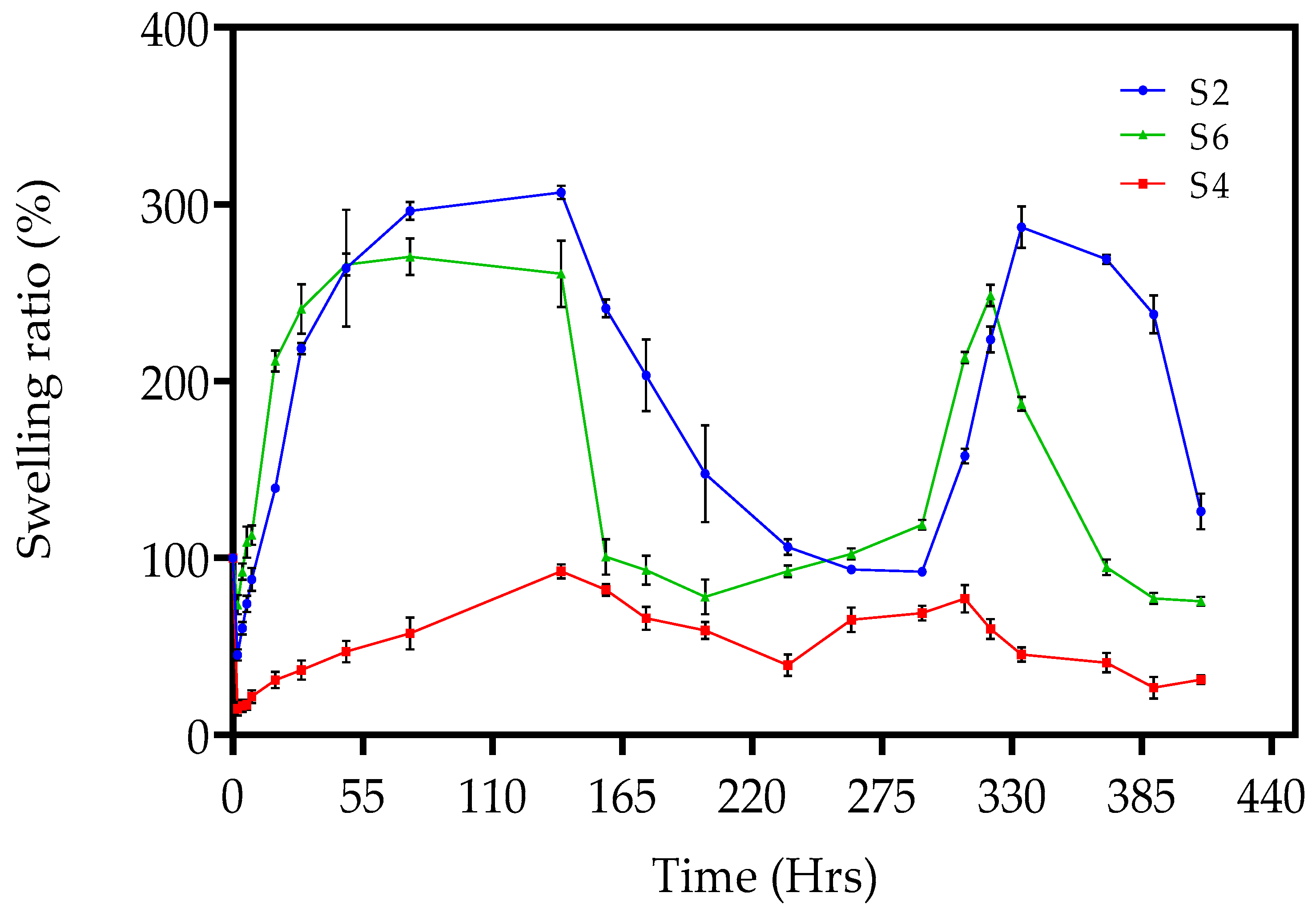
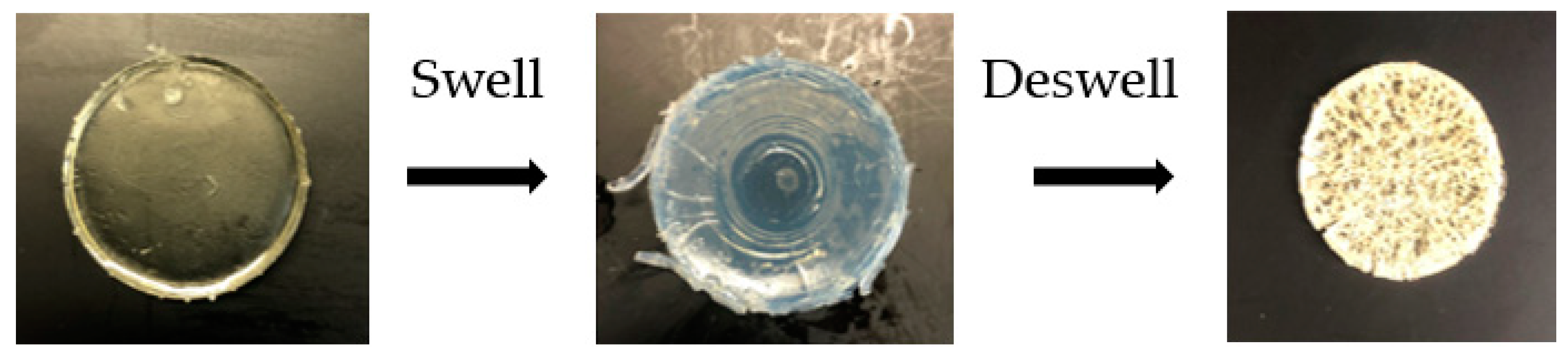
2.4. Pulsatile Swelling Studies
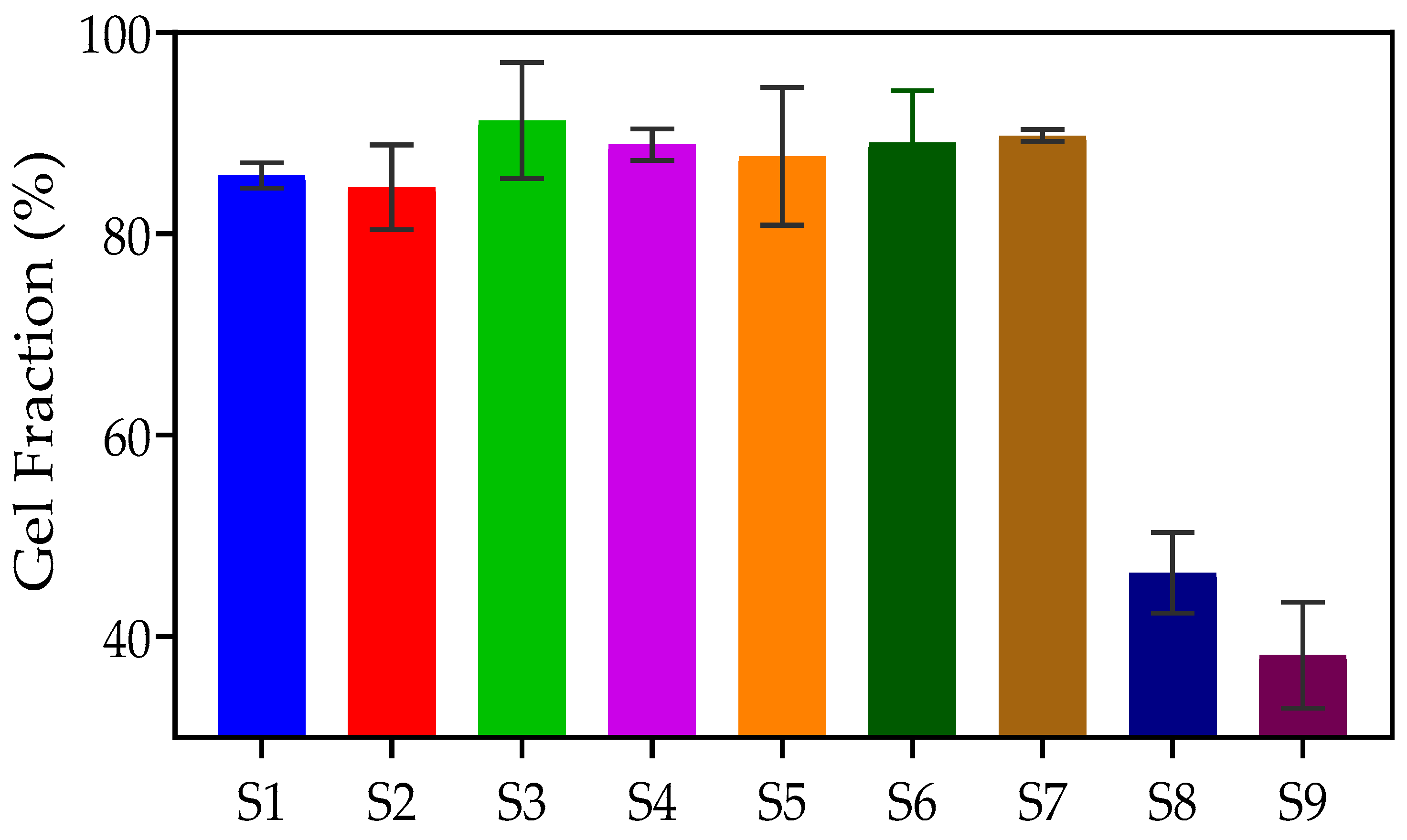
2.5. Gel Fraction Measurement
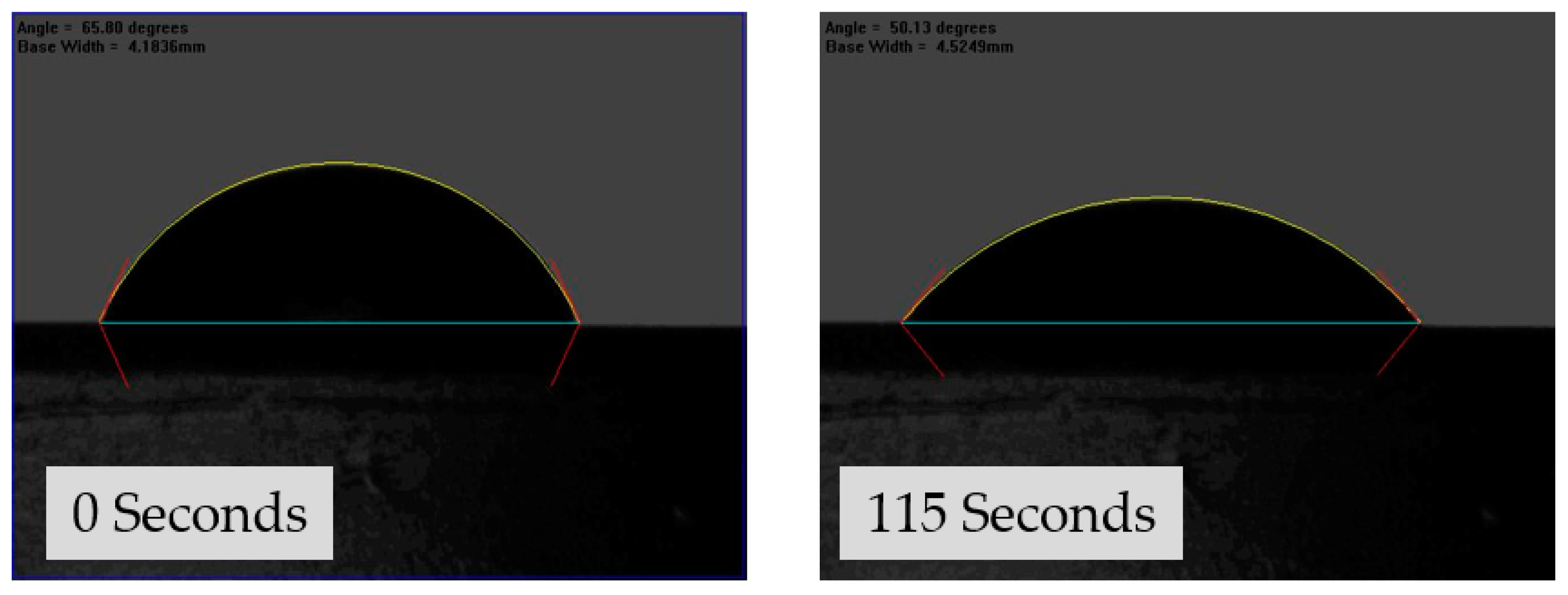
2.6. Goniometry
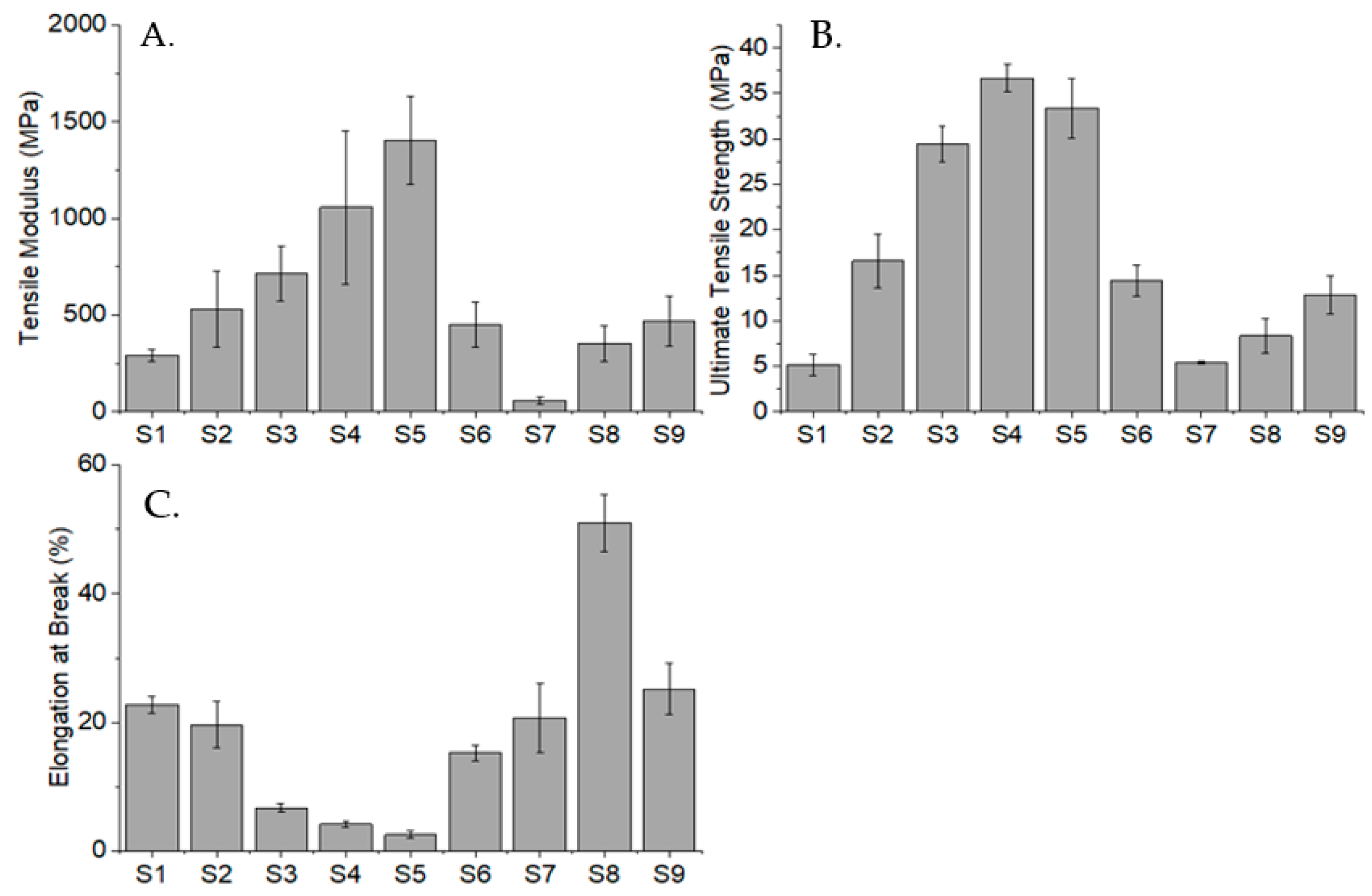
2.7. Tensile Testing
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Thermosensitive PNVCL Hydrogel
4.3. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy
4.4. Differential Scanning Calorimetry
4.5. Tensile Testing
4.6. Pulsatile Swelling Studies
4.7. Gel Fraction Measurement
4.8. Goniometry
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef]
- Bahram, M.; Mohseni, N. An Introduction to Hydrogels and Some Recent Applications; IntechOpen: New York, NY, USA, 2016. [Google Scholar]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; He, X.; Ji, X.; Hu, D.; Pan, M.; Zhang, M.; Qian, Z. Current research progress of photopolymerized hydrogels in tissue engineering. Chinese Chem. Lett. 2021, 32, 2117–2126. [Google Scholar] [CrossRef]
- Nicol, E. Photopolymerized Porous Hydrogels. Biomacromolecules 2021, 22, 1325–1345. [Google Scholar] [CrossRef]
- Swami, L.N.Ã.S.; Gordon-thomson, C. Hydrogels synthesised through photoinitiator-free photopolymerisation technique for delivering drugs including a tumour-tracing porphyrin. Radiat. Phys. Chem. 2006, 75, 604–612. [Google Scholar]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Keys, K.B.; Torres-lugo, M.; Lowman, A.M. Poly (ethylene glycol)-containing hydrogels in drug delivery. J. Control. Release 1999, 62, 81–87. [Google Scholar] [CrossRef]
- Schesny, M.K.; Monaghan, M.; Bindermann, A.H.; Freund, D.; Seifert, M.; Eble, J.A.; Vogel, S.; Gawaz, M.P.; Hinderer, S.; Schenke-Layland, K. Biomaterials Preserved bioactivity and tunable release of a SDF1-GPVI bi-speci fi c protein using photo-crosslinked PEGda hydrogels. Biomaterials 2014, 35, 7180–7187. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wai Lee, W.Y.; Feng, Q.; Xu, L.; Wang, B.; Wai Man, G.E.; Chen, Y.; Jiang, X.; Bian, L.; Cui, L.; et al. Synergistic effects on mesenchymal stem cell-based cartilage regeneration by chondrogenic preconditioning and mechanical stimulation. Stem Cell Res. Ther. 2017, 8, 221. [Google Scholar] [CrossRef]
- Tian, F.; Lyu, J.; Shi, J.; Tan, F.; Yang, M. Sensors and Actuators B: Chemical A polymeric microfluidic device integrated with nanoporous alumina membranes for simultaneous detection of multiple foodborne pathogens. Sens. Actuators B Chem. 2016, 225, 312–318. [Google Scholar] [CrossRef]
- Peiffer, R.W. Applications of Photopolymer Technology. Polymers 1997, 1, 1–14. [Google Scholar]
- Sikdar, P.; Uddin, M.; Dip, Y.M.; Islam, S.; Hoque, M.S.; Dhare, A.K.; Wua, S. Recent advances in the synthesis of smart hydrogels. Mater. Adv 2021, 2, 4532–4573. [Google Scholar] [CrossRef]
- Yu, Y.C.; Li, G.; Kim, J.; Youk, J.H. One-pot synthesis of poly(N-vinylcaprolactam)-based biocompatible block copolymers using a dual initiator for ROP and RAFT polymerization. Polymer 2013, 54, 6119–6124. [Google Scholar] [CrossRef]
- Medeiros, S.F.; Lopes, M.V.; Rossi-Bergmann, B.; Inês Ré, M.; Santos, A.M. Synthesis and characterization of poly(N-vinylcaprolactam)-based spray-dried microparticles exhibiting temperature and pH-sensitive properties for controlled release of ketoprofen. Drug Dev. Ind. Pharm. 2017, 43, 1519–1529. [Google Scholar] [CrossRef]
- Mohammed, M.N.; Yusoh, K.B.; Haslinda, J.; Haji, B. Polymer in novel drug delivery systems: A review. Int. J. Pharm. Sci. Rev. Res. 2018, 8, 21–34. [Google Scholar]
- Kozano, S.; Özdemir, T.; Usanmaz, A. Polymerization of N-vinylcaprolactam and characterization of poly(N-vinylcaprolactam). J. Macromol. Sci. Part A Pure Appl. Chem. 2011, 48, 467–477. [Google Scholar] [CrossRef]
- Cheng, R.; Jiang, J.; Hou, J.; Li, G.; Jiang, J.; Zhao, Y. Water-Soluble Copolymers and Their Hydrogels with pH-Tunable Diverse Thermoresponsive Behaviors Enabled by Hydrogen Bonding. Polym. Chem. 2022, 13, 1–8. [Google Scholar] [CrossRef]
- Cerda-Sumbarda, Y.D.; Domínguez-González, C.; Zizumbo-López, A.; Licea-Claverie, A. Thermoresponsive nanocomposite hydrogels with improved properties based on poly(N-vinylcaprolactam). Mater. Today Commun. 2020, 24, 101041. [Google Scholar] [CrossRef]
- Sala, R.L.; Kwon, M.Y.; Kim, M. Special focus: Strategic directions in musculoskeletal tissue engineering. Tissue Eng. Part A 2017, 23, 935–945. [Google Scholar] [CrossRef]
- Xu, X.D.; Zhang, X.Z.; Wang, B.; Cheng, S.X.; Zhuo, R.X.; Wang, Z.C. Fabrication of a novel temperature sensitive poly(N-isopropyl-3-butenamide) hydrogel. Colloids Surf. B Biointerfaces 2007, 59, 158–163. [Google Scholar] [CrossRef]
- Medeiros, S.F.; Santos, A.M.; Fessi, H.; Elaissari, A. Stimuli-responsive magnetic particles for biomedical applications. Int. J. Pharm. 2011, 403, 139–161. [Google Scholar] [CrossRef] [PubMed]
- Killion, J.A.; Kehoe, S.; Geever, L.M.; Devine, D.M.; Sheehan, E.; Boyd, D.; Higginbotham, C.L. Hydrogel/bioactive glass composites for bone regeneration applications: Synthesis and characterisation. Mater. Sci. Eng. C 2013, 33, 4203–4212. [Google Scholar] [CrossRef] [PubMed]
- Caballero, D.; Reis, R.L.; Kundu, S.C. Trends in biomaterials for three-dimensional cancer modeling. Mater. Today 2020, 2020, 3–41. [Google Scholar]
- Meng, W.; Gao, L.; Venkatesan, J.K.; Wang, G.; Madry, H.; Cucchiarini, M. Translational applications of photopolymerizable hydrogels for cartilage repair. J. Exp. Orthop. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Xu, R. Light scattering: A review of particle characterization applications. Particuology 2015, 18, 11–21. [Google Scholar] [CrossRef]
- Zhuo, S.; Geever, L.M.; Halligan, E.; Tie, B.S.H.; Breheny, C. A Development of New Material for 4D Printing and the Material Properties Comparison between the Conventional and Stereolithography Polymerised NVCL Hydrogels. J. Funct. Biomater. 2022, 13, 262. [Google Scholar] [CrossRef]
- Lin, Y.; Bilotti, E.; Bastiaansen, C.W.M.; Peijs, T. Transparent semi-crystalline polymeric materials and their nanocomposites: A review. Polym. Eng. Sci. 2020, 60, 2351–2376. [Google Scholar] [CrossRef]
- Shu, B.; Tie, H.; Halligan, E.; Zhuo, S.; Keane, G.; Geever, L. Synthesis of NVCL-NIPAM Hydrogels Using PEGDMA as a Chemical Crosslinker for Controlled Swelling Behaviours in Potential Shapeshifting Applications. Gels 2023, 9, 248. [Google Scholar]
- Madix, R.; Polymerizations, R.; Bourgeat-lami, E.; Santos, A.M. Synthesis of block copolymersvia the combination of RAFT and a macromolecular azo coupling reaction. Polym. Chem. 2020, 4, 402–406. [Google Scholar]
- Nathan, A.J.; Scobell, A. Polymerization and Characterization of N-Vinylcaprolactam. Master’s Thesis, Graduate School of Natural and Applied Sciences of Middle East Technical University, Ankara, Turkey, 2008. [Google Scholar]
- Yang, S.B.; Karim, M.R.; Lee, J.; Yeum, J.H.; Yeasmin, S. Alkaline Treatment Variables to Characterize Poly(Vinyl Alcohol)/Poly(Vinyl Butyral/Vinyl Alcohol) Blend Films. Polymers 2022, 14, 3916. [Google Scholar] [CrossRef]
- Şolpan, D.; Kolge, Z.; Torun, M. Preparation and characterization of poly(N-vinylpyrrolidoneco-methacrylic acid) hydrogels. J. Macromol. Sci.-Pure Appl. Chem. 2005, 42A, 705–721. [Google Scholar] [CrossRef]
- Loyer, F.; Combrisson, A.; Omer, K.; Moreno-Couranjou, M.; Choquet, P.; Boscher, N.D. Thermoresponsive Water-Soluble Polymer Layers and Water-Stable Copolymer Layers Synthesized by Atmospheric Plasma Initiated Chemical Vapor Deposition. ACS Appl. Mater. Interfaces 2019, 11, 1335–1343. [Google Scholar] [CrossRef]
- Halligan, S.C.; Dalton, M.B.; Murray, K.A.; Dong, Y.; Wang, W.; Lyons, J.G.; Geever, L.M. Synthesis, characterisation and phase transition behaviour of temperature-responsive physically crosslinked poly (N-vinylcaprolactam) based polymers for biomedical applications. Mater. Sci. Eng. C 2017, 79, 130–139. [Google Scholar] [CrossRef]
- Liu, J.; Detrembleur, C.; De Pauw-Gillet, M.C.; Mornet, S.; Duguet, E.; Jérôme, C. Gold nanorods coated with a thermo-responsive poly(ethylene glycol)-b-poly(N-vinylcaprolactam) corona as drug delivery systems for remotely near infrared-triggered release. Polym. Chem. 2014, 5, 799–813. [Google Scholar] [CrossRef]
- Fallon, M.; Halligan, S.; Pezzoli, R.; Geever, L.; Higginbotham, C. Synthesis and characterisation of novel temperature and pH sensitive physically cross-linked poly(N-vinylcaprolactam-co-itaconic acid) hydrogels for drug delivery. Gels 2019, 5, 41. [Google Scholar] [CrossRef]
- Lebedev, V.T.; Török, G.; Cser, L.; Káli, G.; Sibilev, A.I. Molecular dynamics of poly (N-vinylcaprolactam ) hydrate. Appl. Phys. A 2002, 74, s478–s480. [Google Scholar] [CrossRef]
- Motta, C. The effect of copolymerization on transition temperatures of polymeric materials. J. Therm. Anal. 1997, 49, 461–464. [Google Scholar] [CrossRef]
- Gaballa, H.A.; Geever, L.M.; Killion, J.A.; Higginbotham, C.L. Synthesis and characterization of physically crosslinked N-vinylcaprolactam, acrylic acid, methacrylic acid, and N,N-dimethylacrylamide hydrogels. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1555–1564. [Google Scholar] [CrossRef]
- Ii, W.W.; Web, T.P.; Polymerization, A.; Polymerization, C. Introduction to Plastics and Elastomers. In Chemical Resistance of Thermoplastics; ResearchGate: London, UK, 2012; pp. xxi–xxxiv. [Google Scholar]
- Kirsh, Y.E.; Yanul, N.A.; Kalninsh, K.K. Structural transformations and water associate interactions in poly-N-vinylcaprolactam-water system. Eur. Polym. J. 1999, 35, 305–316. [Google Scholar] [CrossRef]
- Ngadaonye, J.I.; Cloonan, M.O.; Geever, L.M.; Higginbotham, C.L. Synthesis and characterisation of thermo-sensitive terpolymer hydrogels for drug delivery applications. J. Polym. Res. 2011, 18, 2307–2324. [Google Scholar] [CrossRef]
- Schroeder, W.F.; Vallo, C.I. Effect of different photoinitiator systems on conversion profiles of a model unfilled light-cured resin. Dent. Mater. 2007, 23, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart hydrogels—Synthetic stimuli-responsive antitumor drug release systems. Int. J. Nanomed. 2020, 15, 4541–4572. [Google Scholar] [CrossRef] [PubMed]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Choong, Y.Y.C.; Maleksaeedi, S.; Eng, H.; Wei, J.; Su, P.C. 4D printing of high performance shape memory polymer using stereolithography. Mater. Des. 2017, 126, 219–225. [Google Scholar] [CrossRef]
- Holback, H.; Yeo, Y.; Park, K. Hydrogel Swelling Behavior and Its Biomedical Applications; Woodhead Publishing Limited: Cambridge, UK, 2011. [Google Scholar]
- Barleany, D.R.; Ananta, C.V.; Maulina, F.; Rochmat, A.; Alwan, H.; Erizal. Controlled release of metformin hydrogen chloride from stimuli-responsive hydrogel based on poly(N-Isopropylacrylamide)/Chitosan/Polyvinyl alcohol composite. Int. J. Technol. 2020, 11, 511–521. [Google Scholar] [CrossRef]
- Pantani, R.; Coccorullo, I.; Speranza, V.; Titomanlio, G. Morphology evolution during injection molding: Effect of packing pressure. Polymer 2007, 48, 2778–2790. [Google Scholar] [CrossRef]
- Halligan, S.; Murray, K.; Hopkins, M.; Rogers, I.; Lyons, J.; Vrain, O.; Geever, L. Enhancing and controlling the critical attributes of poly (N-vinylcaprolactam) through electron beam irradiation for biomedical applications. J. Appl. Polym. Sci. 2019, 137, 48639. [Google Scholar] [CrossRef]
- Mahltig, B. Smart Hydrophobic and Soil-Repellent Protective Composite Coatings for Textiles and Leather; Elsevier Ltd.: Amsterdam, The Netherlands, 2016. [Google Scholar]
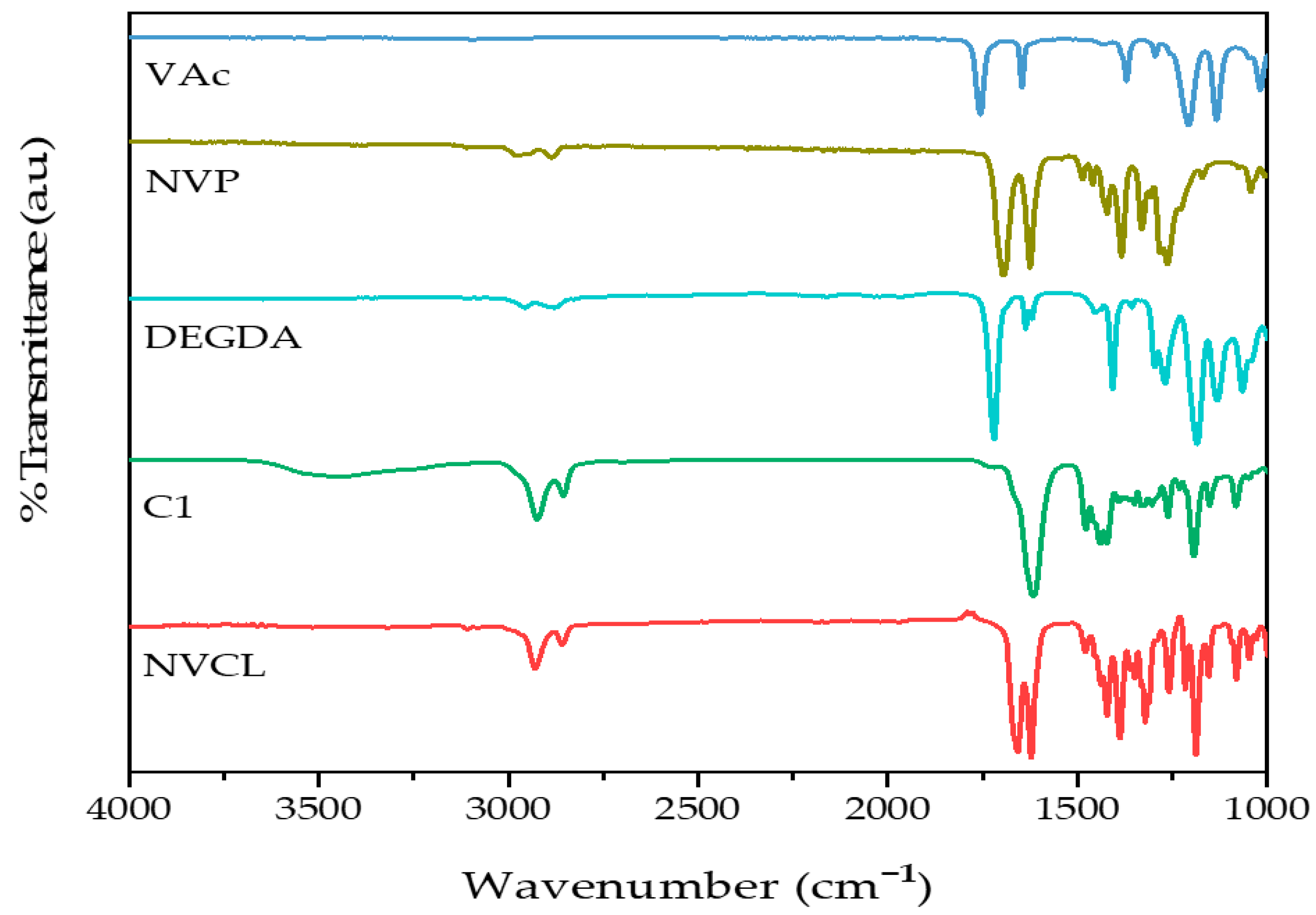
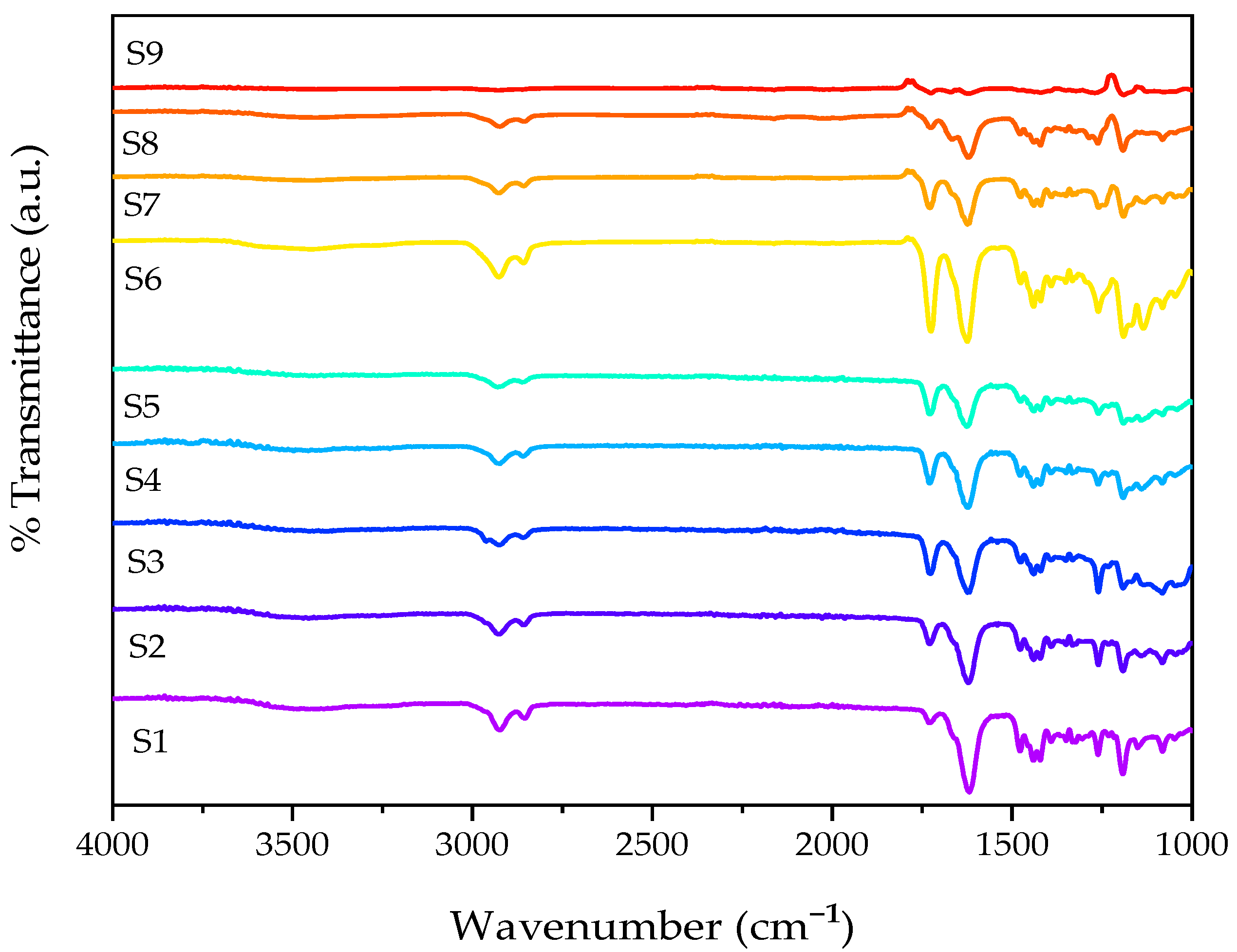

| Sample | Wavelength (cm−1) | Functional Group | |
|---|---|---|---|
| Monomers | NVCL | 1659 | C=O |
| 1625 | C=C | ||
| 2927, 2855 | C–H | ||
| DEGDA | 1720 | C=O | |
| VAc | 1648 | C=O | |
| 1740 | C–H | ||
| NVP | 1624 | C=O | |
| 1383 | C–N–C | ||
| Polymerised Samples | PNVCL | 1618 | C=O |
| 1196 | –CO–O- | ||
| 2919 | C–H | ||
| PNVCL/DEGDA | 1618 | C=C | |
| PNVCL/DEGDA/VAc | 2853 | C–H | |
| PNVCL/DEGDA/NVP | 1725 | C=O |
| ID Code | Gel Fraction (%) |
|---|---|
| S1 | 85 |
| S2 | 84 |
| S3 | 91 |
| S4 | 88 |
| S5 | 87 |
| S6 | 89 |
| S7 | 89 |
| S8 | 46 |
| S9 | 38 |
| Material | Chemical Structures |
|---|---|
| N-vinylcaprolatam (NVCL) | 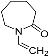 |
| Diphenyl(2,4,6-trimethylbenzoyl) phosphine oxide (TPO) | 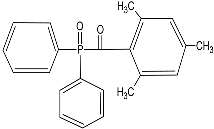 |
| Di(ethylene glycol) diacrylate (DEGDA) |  |
| Vinylacetate (VAc) | 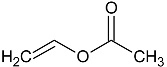 |
| N-vinylpyrrolidone (NVP) | 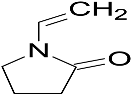 |
| ID CODE | Formulation | Photoinitiator | Monomer | Crosslinker | Comonomers | |
|---|---|---|---|---|---|---|
| TPO (wt%) | NVCL (wt%) | DEGDA (wt%) | VAc (wt%) | NVP (wt%) | ||
| C1 | P(NVCL100) | 1 | 100 | -- | -- | -- |
| S1 | P(NVCL95-DEGDA5) | 0.1 | 95 | 5 | -- | -- |
| S2 | P(NVCL90-DEGDA10) | 0.1 | 90 | 10 | -- | -- |
| S3 | P(NVCL80-DEGDA20) | 0.1 | 80 | 20 | -- | -- |
| S4 | P(NVCL70-DEGDA30) | 0.1 | 70 | 30 | -- | -- |
| S5 | P(NVCL60-DEGDA40) | 0.1 | 60 | 40 | -- | -- |
| S6 | P(NVCL70-DEGDA10-VAc20) | 0.1 | 70 | 10 | 20 | -- |
| S7 | P(NVCL50-DEGDA10-VAc40) | 0.1 | 50 | 10 | 40 | -- |
| S8 | P(NVCL70-DEGDA10-NVP20) | 0.1 | 70 | 10 | -- | 20 |
| S9 | P(NVCL50-DEGDA10-NVP40) | 0.1 | 50 | 10 | -- | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halligan, E.; Tie, B.S.H.; Colbert, D.M.; Alsaadi, M.; Zhuo, S.; Keane, G.; Geever, L.M. Synthesis and Characterisation of Hydrogels Based on Poly (N-Vinylcaprolactam) with Diethylene Glycol Diacrylate. Gels 2023, 9, 439. https://doi.org/10.3390/gels9060439
Halligan E, Tie BSH, Colbert DM, Alsaadi M, Zhuo S, Keane G, Geever LM. Synthesis and Characterisation of Hydrogels Based on Poly (N-Vinylcaprolactam) with Diethylene Glycol Diacrylate. Gels. 2023; 9(6):439. https://doi.org/10.3390/gels9060439
Chicago/Turabian StyleHalligan, Elaine, Billy Shu Hieng Tie, Declan Mary Colbert, Mohamad Alsaadi, Shuo Zhuo, Gavin Keane, and Luke M. Geever. 2023. "Synthesis and Characterisation of Hydrogels Based on Poly (N-Vinylcaprolactam) with Diethylene Glycol Diacrylate" Gels 9, no. 6: 439. https://doi.org/10.3390/gels9060439
APA StyleHalligan, E., Tie, B. S. H., Colbert, D. M., Alsaadi, M., Zhuo, S., Keane, G., & Geever, L. M. (2023). Synthesis and Characterisation of Hydrogels Based on Poly (N-Vinylcaprolactam) with Diethylene Glycol Diacrylate. Gels, 9(6), 439. https://doi.org/10.3390/gels9060439








